Abstract
A rare case is presented of a 62-year-old man with primary isolated cryptococcal femoral osteomyelitis. Magnetic resonance imaging (MRI) revealed osteolytic destruction of his left femur. Biopsy was performed firstly. Under microscope, the lesion was compose of numerous large mononuclear cells, scattered multinucleated giant cells, a few lymphocytes and neutrophils, necrosis with serious artificial deformation. By immunohistochemistry (IHC), only CD31 and CD68 were positive, while CK, CK8/18, EMA, P63, CK7, CK20, PSAP, PSA, CD34 negative. It was considered a low grade vascularsarcoma, but not confirmed. Then the operation was done. Surgical specimen showed a lot of red-sphere materials in most cells cytoplasm. The Gomorra methenamine silver staining and PAS revealed the mucopolysaccharide-containing capsule of the Cryptococcus. Laboratory culture of lesion liquid grew a kind of yeast at 37°C. Cryptococcal femoral osteomyelitis was diagnosed at last. The patient is good now after the thorough debridement and anti-fungal treatment.
Keywords: Femur, cryptococcus, osteomyelitis, cryptococcosis
Introduction
Cryptococcosis is a systemic mycosis caused by an encapsulated yeast-like fungus Cryptococcus neoformans. The principal source of this organism is avian excreta, particularly that of pigeons [1]. After inhalation, the organism may cause pulmonary disease or a systemic fungemia associated with numerous infections of extrapulmonary sites. 10% of disseminated cases involve bone tissues [2]. But isolated osteomyelitis is very uncommon [3]. Cryptococcal femoral osteomyelitis is extremely unusual [4,5].
This article describes an especial case of cryptococcal femoral osteomyelitis after two years of trauma.
Case report
The patient, male, 62-year-old, complained of pain on the left knee and hip for more than 4 months, which exacerbated 15 days ago and associated with prominent limp and swelling of the left knee and foot. He did not have any other symptoms, such as fever, chills, or loss of weight et al. Values for tumor markers, including CA 19-9 (15.8 units/mL), carcinoembryonic antigen (CEA; 1.53 ng/mL), alpha-fetoprotein (AFP; 2.99 ng/mL), and prostate-specific antigen (PSA; 0.21 ng/mL), were within the normal range. But on history, his left knee was injured in an accident in 2011, and treated with “patella neoplasty”.
The lesion was observed aggressive behavior and osteolytic destruction of the left femur, which associated with femoral head, neck and shaft under MRI. And the lesion broke through the bone cortex invading to soft tissues (Figure 1).
Figure 1.
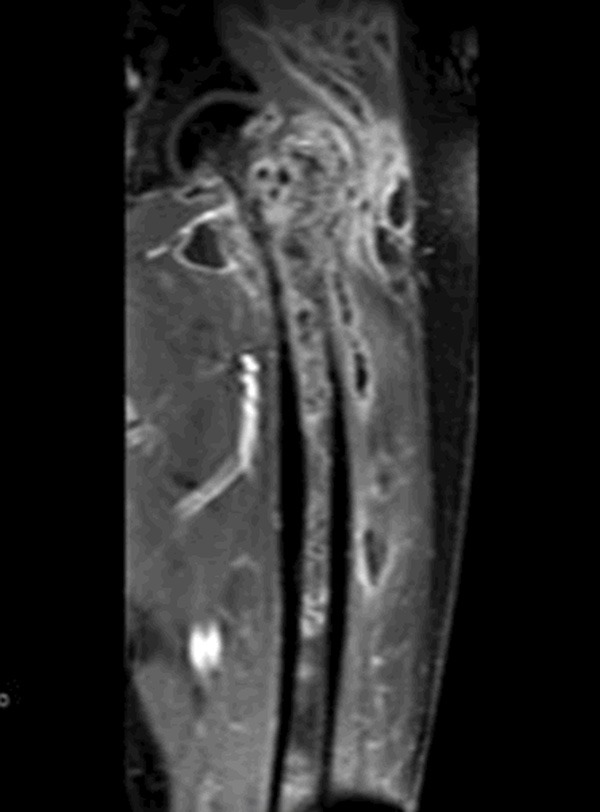
MRI of the left femur, showing osteolytic destruction.
Other findings: The leukocyte count was 7,440/cu mm. The chest x-ray was unremarkable.
The patient was treated with debridement and intravenous amphotericin B 40 mg daily for two weeks, and oral fluconazole 400 mg daily for six weeks, followed by oral fluconazole 200 mg daily for 6 months. On follow up for one year, it was not recurrent.
Pathologic findings
With so destructive lesions under MRI, before determination, the clinician predicted it was a metastatic carcinoma, for elevated serum alkaline phosphatase. A core needle biopsy was proceeded. Under microscope, the lesion showed numerous large mononuclear cells, scattered multinucleated giant cells, a few lymphocytes and neutrophils, necrosis with serious artificial deformation (Figure 2). Immunohistochemical stains showed CK, CK8/18, EMA, P63, CK7, CK20, PSAP, PSA, CD34 negative, and only CD31 and CD68 positive (Figure 3). It was presumed low-grade malignant vascular tumors (as epithelioid hemangioendothelioma), but not concluded, because of little tissues, artificial deformation, and negative CD34. Then the operation was done. The specimens were present about 6×4×3 cm white-yellow soft fragmentary tissues with broken bones. All H & E slides showed cytoplasm of the large mononuclear and multinucleated cells were vacuolated, containing lightly eosin-stained, variably sized, round structures surrounded by a clear zone (Figure 4). These structures were clearly outlined by Gomorra methenamine silver staining and PAS (Figure 5). With these stains, themucopolysaccharide-containing capsule of the Cryptococcus was clearly defined. At the same time, Laboratory culture of lesion liquid grew a kind of yeast at 37°C that had a capsule. Finally, according to H & E, special staining and laboratory culture, it was demonstrated cryptococcal infection of femoral bone.
Figure 2.
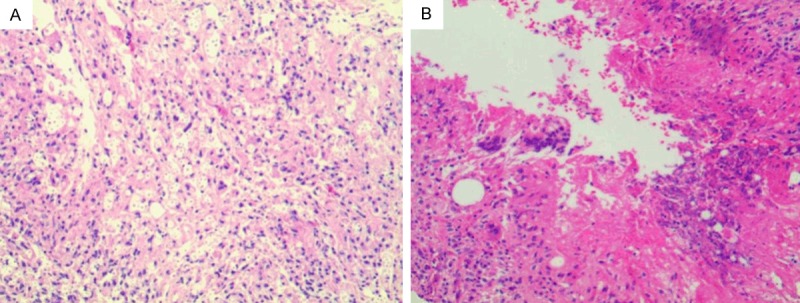
Numerous mononuclear, multinucleated giant cells. A. lymphocytes and neutrophils, necrosis. B. (H & E 100×).
Figure 3.
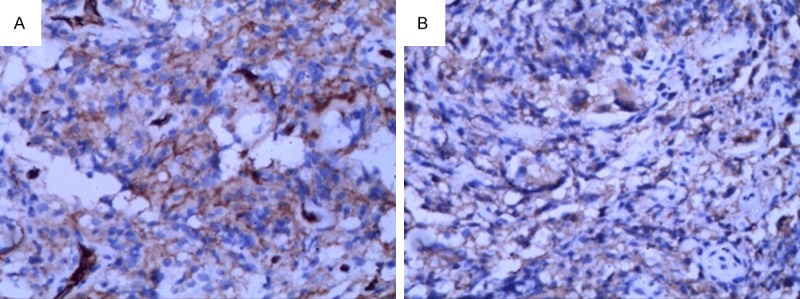
A. Vascellum and some mononuclear cells expressed CD31. B. Mononuclear, multinucleated giant cells expressed CD68. (IHC 200×).
Figure 4.
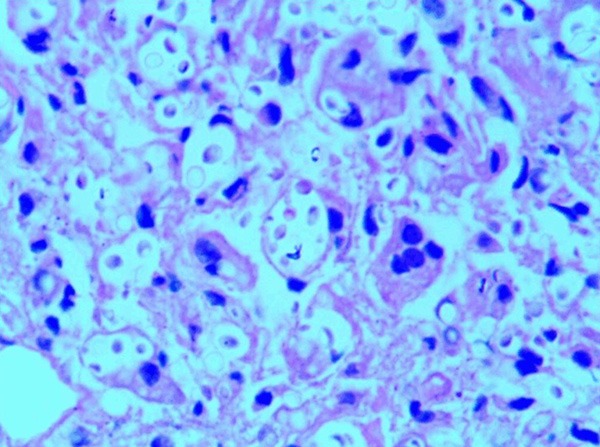
Magnification of area (A) of Figure 2, illustrating giant cells containing well-defined organisms and vacuolated appearance (H & E 400×).
Figure 5.
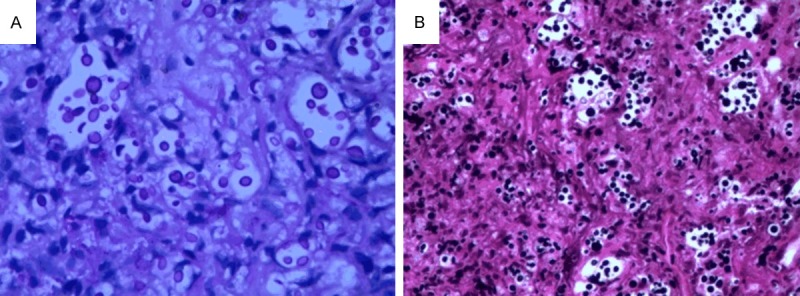
Numerous cryptococci in stroma, mononuclear cells, and giant cells are made obvious by PAS (A 400×) and Gomorra methenamine silver staining (B 200×).
Discussion
In most situations, cryptococcosis involves many organ systems. But on few occasion, a single organ may be the only target. A prime and not infrequent example of such limited involvement is the central nervous system. On the other hand, the skeletal system is rare. Sites of bony predilection have been reported including the humerus, skull, and spine [6-8]. Evaluation of the primary cryptococcal osteomyelitis should exclude involvements of other sites in whole body. Chest X-ray is useful for lung lesion. Clinical features and spinal fluid culture are good for central nervous system. Histological examination and special stain is very useful for cryptococcosis diagnosis. But cultural and biochemical characteristics of the organism are essential for species identification. Other features of cryptococcus infection are absent, including an elevated white cell count. Isolated single focus of skeletal disease easily may be confused with a neoplastic process because of its rarity, such as what is recorded in this paper. It is critical to prepare microbiological cultures at the time of biopsy. The routine use of frozen sections is helpful not only for determining the lesion malignant or not, but also alerting the clinician proceeding microbiological cultures when infective lesions are discovered unexpectedly [9]. On the other hand, the lesions in the bones usually are purely osteolytic with little or no periosteal reaction on imageology. In this case, the patient tested negative for human immunodeficiency virus (HIV), had no prior medical history, and denied any direct contact with birds. So it was thought that the infective lesions may be associated with “patella neoplasty” in 2011.
CD31 positive staining has confused pathologists in this case. CD31 is well known to stain vessel endothelial cell. But CD31 is also expressed in a subset of histiocytes and some histiocytic lesions [10-13], plasma cells and plasmacytic/plasmablastic lesions [14]. CD31-positive expression was also reported in leiomyosarcoma, cutaneous juga fibrosarcoma and melanoma [15]. In summary, CD31 alone is not enough for distinction between vascular neoplasms and other lesions.
The treatment of skeletal cryptococcosis should include both thorough debridement and appropriate chemotherapeutic agents. The patients are usually treated with amphotericin B and 5-flucytosine or fluconazole. Because even with treatment, not all patients are cured. Radiological improvement is consistent with cryptococcal antigen drop in the serum, which can be used as a reliable indicator for monitoring condition changes [16]. The patient of this case is good now after the thorough debridement and anti-fungal treatment.
Disclosure of conflict of interest
None.
References
- 1.Littman ML, Walter JE. Cryptococcosis: Current status. Am J Med. 1968;45:922–932. doi: 10.1016/0002-9343(68)90190-3. [DOI] [PubMed] [Google Scholar]
- 2.McCullough NB, Louria DB, Hilbish TF, et al. Cryptococcus clinical staff conference at the National Institutes of Health. Ann Intern Med. 1958;49:642–61. doi: 10.7326/0003-4819-49-3-642. [DOI] [PubMed] [Google Scholar]
- 3.Rocco A, Fleckenstein JM, Brandvold B, Ondra SL. Cryptococcal Skull Infection: A case report with review of the literature. Neurosurgery. 1993;32:1034–1036. doi: 10.1227/00006123-199306000-00028. [DOI] [PubMed] [Google Scholar]
- 4.BUBB H. Cryptococcus neoformans infection in Bone. S Afr Med J. 1955;29:1259–61. [PubMed] [Google Scholar]
- 5.Zainal AI, Wong SL, Pan KL, Wong OL, Tzar MN. Cryptococcal osteomyelitis of the femur: A case report and review of literature. Tropical Biomedicine. 2011;28:444–449. [PubMed] [Google Scholar]
- 6.Goldshteyn N, Zanchi A, Cooke K, Agha R. Cryptococcal osteomyelitis of the humeral head initially diagnosed as avascular necrosis. South Med J. 2006;99:1140–1. doi: 10.1097/01.smj.0000224744.75040.13. [DOI] [PubMed] [Google Scholar]
- 7.Burch KH, Fine G, Quinn EL, Eisses JF. Cryptococcus neoformans as a Cause of Lytic Bone Lesions. JAMA. 1975;231:1057–1059. [PubMed] [Google Scholar]
- 8.Bryan CS. Vertebral osteomyelitis due to Cryptococcus neoformans. Case report. J Bone Joint Surg Am. 1977;59:275–6. [PubMed] [Google Scholar]
- 9.Dahlin DC. Bone tumors. In: Thomas CC, editor. General aspects and data on 6,221 cases. Springfield: Illinois: 1978. p. 5. [Google Scholar]
- 10.Mckenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25:1167–1173. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Akiba J, Harada H, Kawahara A, Arakawa F, Mihashi H, Mihashi R, Ohshima K, Yano H. Histiocytic sarcoma of the parotid gland region. Pathol Int. 2011;61:373–376. doi: 10.1111/j.1440-1827.2011.02671.x. [DOI] [PubMed] [Google Scholar]
- 12.Tidwell WJ, Googe PB. Tissue histiocyte reactivity with CD31 is comparable to CD68 and CD163 in common skin lesions. J Cutan Pathol. 2014;41:489–493. doi: 10.1111/cup.12327. [DOI] [PubMed] [Google Scholar]
- 13.Slone SP, Fleming DR, Buchino JJ. Sinus Histiocytosis With Massive Lymphadenopathy and Langerhans Cell Histiocytosis Express the Cellular Adhesion Molecule CD31. Arch Pathol Lab Med. 2003;127:341–344. doi: 10.5858/2003-127-0341-SHWMLA. [DOI] [PubMed] [Google Scholar]
- 14.Plocharczyk E, Wakely PE Jr. CD31 expression in plasmacytic/plasmablastic lesions. Ann Diagn Pathol. 2013;17:498–501. doi: 10.1016/j.anndiagpath.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Hongying Z, Wenbin H. The value of ERG protein in vascular tumors. J Clin Exp Pathol. 2014;30:1263–1265. [Google Scholar]
- 16.Chang WC, Tzao C, Hsu HH, Lee SC, Huang KL, Tung HJ, Chen CY. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunoeompromised patients. Chest. 2006;129:333–340. doi: 10.1378/chest.129.2.333. [DOI] [PubMed] [Google Scholar]


