Abstract
Epstein-Barr virus (EBV)-associated T-cell lymphoproliferative disease (LPD) is not uncommon in China, but gastrointestinal involvement is very rare. We report on an immunocompetent patient with EBV-associated T-cell LPD of the colon. The 26-year-old man was initially misdiagnosed with ulcerative colitis (UC). A colon biopsy revealed the presence of small to medium-sized lymphoid cells infiltrating the intestinal wall. The neoplastic cells expressed CD3, CD5, and granzyme B, not CD56. EBV-encoded small ribonucleic acid was detected in the tumor cells of the colon as well as the lymph node, and the T-cell receptor gene rearrangement result displayed δ gene monoclonal rearrangement. The patient died 2 moths after the diagnosis. The clinical course of EBV-associated T-cell LPD is aggressive and the prognosis is poor, the wrong diagnosis may delay treatment. Therefore, we should be very careful to prevent misdiagnosis. When patients have multiple intestinal ulcers that are not typical of UC and the clinical course is unusual, although morphology looks like inflammatory change, pathologist should consider the possibility of EBV-associated LPD. The treatment strategy and prognosis of these two diseases are different.
Keywords: Epstein-Barr virus, T cell, lymphoproliferative disease, ulcerative colitis
Introduction
The Epstein-Barr virus (EBV) is a ubiquitous virus that infects >90% of humans and persists for life [1]. EBV usually infects B cells, but it can also affect T and NK cells [2]. EBV-associated B cell diseases are relatively common in Western countries. In contrast, similar diseases with a higher morbidity and mortality have been reported in Asian countries, and they are usually associated with the proliferation of EBV-infected T or NK cells [3,4]. EBV-associated T/NK cell diseases are not uncommon, and there are various types in China. According to the course of the diseases that have been diagnosed, they can be divided into acute, subacute, and chronic, which include infectious mononucleosis (IM), LPD, and lymphoma. Herein, we describe the case of an immunocompetent patient with EBV-associated T-cell LPD of the colon who was initially misdiagnosed with UC.
Case report
Clinical history
A 26-year-old man with no personal or family history of immunodeficiency had an intermittently high fever for >3 months. The second month after the disease onset, he presented with diarrhea and hematochezia. The colonoscopy examination showed multiple colorectal ulcers, with the largest one about 3 cm. An inflammatory lesion was diagnosed on the basis of the rectal biopsy results. Anti-inflammation therapy was used to treat this patient, but the effect was not remarkable. The whole body positron emission tomography scan showed multiple high metabolisms of bone, hepatosplenomegaly, and multiple mesenteric and retroperitoneal lymphadenopathy. A bone marrow biopsy showed hemophagocytosis and pancytopenia. Laboratory tests showed a mild decrease in the leukocytes (2.8 × 109/L), anemia (87 g/L), and hypoalbuminemia (30 g/L), and elevations of the liver enzymes-aspartate aminotransferase (199 U/L) and alanine aminotransferase (477 U/L). A high EBV deoxyribonucleic acid (DNA) load was detected in the peripheral blood (9 × 104 copies/mL). The Epstein-Barr nuclear antigen-immunoglobulin (Ig) G, IgA/early antigen, IgA/viral capsid antigen (VCA), and IgG/VCA were all positive. The stool assays were negative. He underwent right hemicolectomy due to an active hemorrhage of the colonic ulcers. At a local hospital, he was diagnosed with chronic appendicitis and multiple colon ulcers with lymphocytes and plasma cell infiltration that may be related to a virus infection.
Morphology
Colonic mucosa manifested multifocal ulcers. A small number of pleomorphic lymphoid cells infiltrated in the mucosa, sub-mucosa, and muscularis (Figure 1A). Some of them were small to medium-sized cells with rounded or slightly irregular nucleus, loose chromatin, and less cytoplasm (Figure 1B). Nuclear fission was not easy to detect. Some histocytes, neutrophils, and plasma cells were diffused in the intestinal wall. The structure of the mesenteric lymph node was primarily reserved, and its paracortex zone was broadening with small to medium-sized mild atypical cells.
Figure 1.
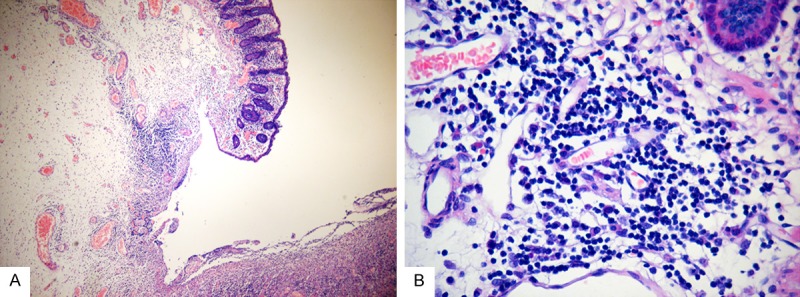
Imaging of the colonic mucosa multifocal ulcers. A. A small number of pleomorphic lymphoid cells are infiltrated in the mucosa, sub-mucosa, and muscularis (hematoxylin and eosin staining; original magnification, × 100). B. Cells were small tomedium-sized cells with rounded or slightly irregular nucleus, loose chromatin, and less cytoplasm, some plasma cells can be seen (original magnification, × 200).
Immunophenotype
The atypical lymphoid cells were CD3+ (Figure 2A), CD5+ (Figure 2B), CD20- (Figure 2C), and CD56- (Figure 2D). Some of the cells were TIA-1/granzyme B+. The histocytes were CD68+, and the plasma cells were CD138+. The Ki-67 index showed about 20-30% positive cells in the colon.
Figure 2.
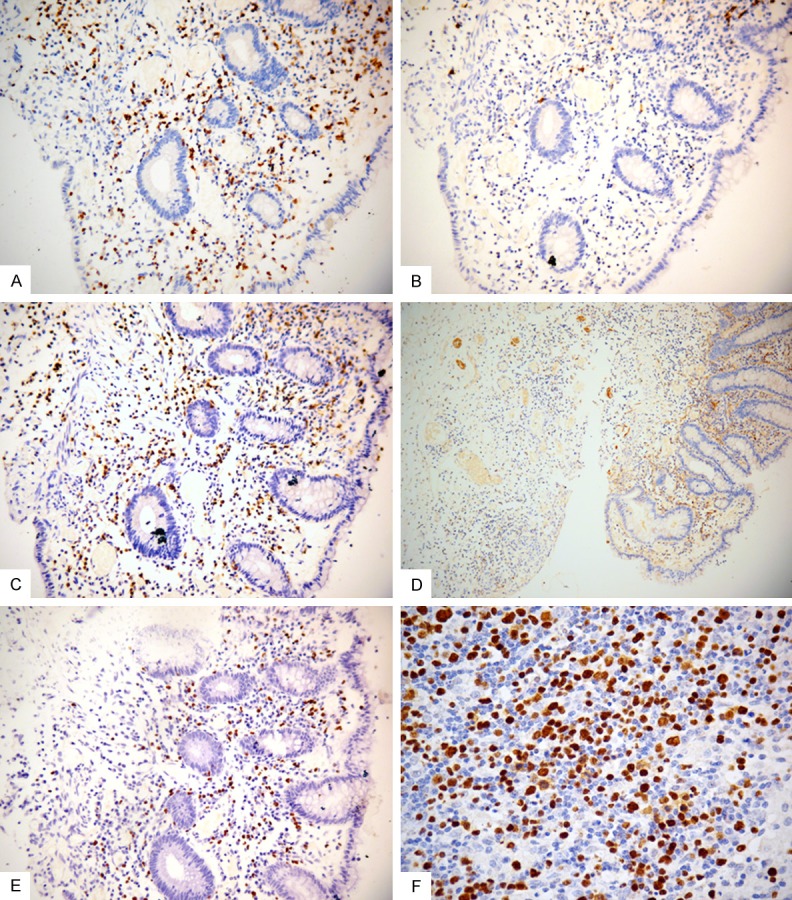
Immunohistochemical features of the case. A. Neoplastic cells in the mucosa are positive for CD3 (original magnification, × 200). B. Neoplastic cells in the mucosa are negative for CD20 (original magnification, × 200). C. Neoplastic cells in the mucosa are positive for CD5 (original magnification, × 200). D. Neoplastic cells in the mucosa are negative for CD56 (original magnification, ×100). E. The tumor cells are strongly positive for Epstein-Barr virus (EBV) by EBV-encoded small ribonucleic acid in-situ hybridization of the colon (original magnification, × 200). F. The tumor cells are strongly positive for Epstein-Barr virus (EBV) by EBV-encoded small ribonucleic acid in-situ hybridization of the lymph node (original magnification, × 400).
In-situ hybridization for EBER
The EBV infection was detected in many lymphoid cells of the colon and lymph node by in-situ hybridization (ISH) for EBER (Figure 2E, 2F).
Molecular analysis
DNA was abstracted from the lymph node for TCR gene rearrangement according to the BIOMED-2 PCR protocols, and the result displayed the T-cell receptor delta (Vδ+Dδ+Jδ) monoclonal rearrangement (Figure 3).
Figure 3.
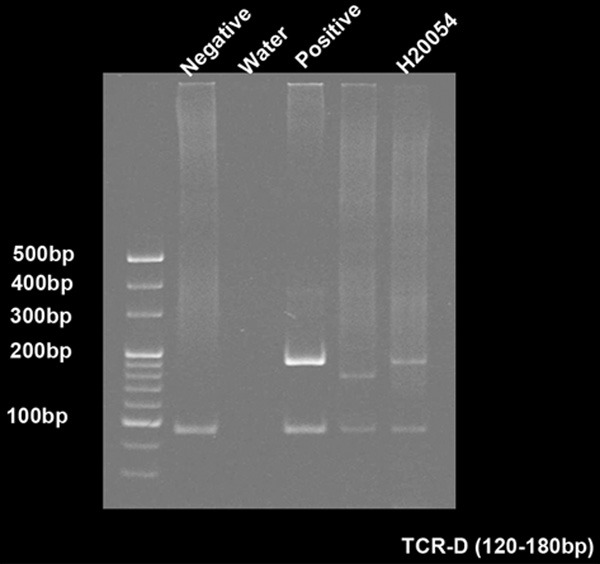
T-cell receptor delta (TCRD; Vδ+Dδ+Jδ) monoclonal rearrangement. TCR gene rearrangement according to the BIOMED-2 PCR protocols.
Diagnosis
Based on the morphology, immunohistochemistry, ISH for EBER, and TCR gene rearrangement, the patient was diagnosed with EBV+ T-cell LPD, grade II (borderline lesions) of the colon ascendens and lymph node.
Follow-up
After diagnosis, the patient’s medical condition worsened. He had a fever twice a day, up to 40°C. The symptoms of hepatosplenomegaly, lymphadenopathy, and liver dysfunction continued. The patient received antiviral and hormonal therapy, but the effect was unremarkable. Two months after the right colon resection was performed, he died from bleeding caused by disseminated intravascular coagulation.
Discussion
EBV-associated LPD was defined at an international meeting as EBV+B cell LPD or EBV+T/NK cell LPD [5]. EBV+T/NK cell LPD is characterized as persistent or recurring IM-like symptoms, including fever, hepatosplenomegaly, lymphadenopathy, liver dysfunction, and a high EBV-DNA load in the peripheral blood for at least 3 months. EBV-infected individuals are without apparent immunodeficiency [5]. EBV and EBV-infected cells usually have polyclonal proliferation and oligoclonal proliferation to monoclonal proliferation. Therefore, it may be a spectrum, encompassing benign, borderline, and malignant lesions. One Japanese study proposed a clinicopathological categorization of EBV+T/NK LPD based on a pathological evaluation and the molecular data as follows: (i) category A1, polymorphic LPD without the clonal proliferation of EBV-infected cells; (ii) category A2, polymorphic LPD with clonality; (iii) category A3, monomorphic LPD (T-cell or NK cell lymphoma) with clonality; and (iv) category B, monomorphic LPD (T-cell lymphoma) with clonality and fulminant course. Categories A1, A2, and A3 possibly constitute a continuous spectrum and are equivalent to chronic active EBV infection. Category B is equivalent of infantile fulminant EBV-associated T-cell LPD [6].
Organs that are most frequently involved in EBV-associated LPD are the lymph nodes, skin, liver, spleen, and bone marrow [7]. Gastrointestinal involvement is very rare, and to our knowledge, only several prior cases in immunocompetent hosts have been reported [8-10]. In our case, histologically, there were some small to medium-sized lymphoid cells with slight atypia infiltrated in the mucosa, submucosal, and muscularis, and several acute and chronic inflammatory cells were also found; thus, the lesion may be easily misdiagnosed as an inflammatory disease. In addition, the patient was a young male, conforming to the epidemiology of inflammatory bowel disease (IBD). The age of onset for IBD is usually before 30 years, predominantly in male patients [11]. However, the clinical manifestation in our patient was not typical of IBD. Abdominal pain and bloody diarrhea are the primary presenting symptoms in most patients with UC, and some have coexisting disorders such as hyperlipidemia, high blood pressure, diabetes, and autoimmune hepatitis [11], in the absence of systemic symptoms such as fever, hepatosplenomegaly, and lymphadenopathy. Our patient’s clinical data showed a high EBV-DNA load, so ISH for EBER was performed. Amazingly, there were a large number of EBV positive cells, and they were confirmed as T-cells. Therefore, we diagnosed him with EBV+ T-cell LPD. Since the treatment strategy and prognosis of patients with EBV-associated LPD differ markedly from those with UC, it is essential to make a proper differential diagnosis. The clinical information is important. In patients who are characterized by inflammation histologically but do not have typical clinical features of IBD, we should use ISH for EBER to rule out EBV associated diseases. EBV+ T-cell LPD has an aggressive clinical course and worse prognosis, and the treatment effect is unsatisfactory. Therefore, we should be very careful to prevent misdiagnosis. In addition to UC, this disease should be distinguished from extranodal NK/T-cell lymphoma (ENKTL) involving the gastrointestinal tract. ENKTL almost always has an extranodal presentation. The upper aerodigestive tract (e.g., the nasal cavity, nasopharynx, and paranasal sinuses) is most commonly involved. Preferential sites of extranasal involvement include the skin, soft tissue, and gastrointestinal tract. The most typical immunophenotypes of ENKTL are CD3ε+, CD56+, and TIA-1/granzymeB+. Lymphomas that demonstrate a CD3ε+ and CD56- immunophenotype are also classified as ENKTL if both the cytotoxic molecules and EBV are positive [12,13]. This case conformed to the diagnostic criteria for ENKTL immunophenotypically. However, there are still some differences between these two diseases. Patients with ENKTL commonly have a high-stage disease on presentation with involvement of multiple extranodal sites that usually occur as nasal lesions, except for intestinal lesions. Intestinal lesions often manifest as perforations, and the histological features are aggressive. In most cases, the mucosal glands become widely spaced or are lost and are filled with many irregular cells. An angiodestructive growth pattern and coagulative necrosis are frequently present. Although a few cases, particularly in those predominated by small or mixed cell populations, are accompanied by a heavy admixture of inflammatory cells that may mimic the inflammatory process. CD5 are usually negative; however, cytotoxic molecules are positive for many tumor cells [12,13]. Furthermore, T-cell receptor and immunoglobulin genes are in germline configuration in most cases. In a very small proportion of cases, the T-cell receptor genes show clonal rearrangement [14]. Therefore, we diagnosed this patient with EBV+ T-cell LPD rather than ENKTL involving the intestinal tract.
To date, the most common and effective therapy for EBV+ T-cell LPD is hematopoietic stem cell transplantation. This treatment has been used for several years in Japan, even though some cases have not yet progressed to lymphoma [15]. Further studies are needed to clarify the pathogenesis of EBV+ T-cell LPD and to facilitate the development of effective treatments.
Acknowledgements
It was supported by China National Natural Science Foundation (Grant No. 81272633).
Disclosure of conflict of interest
None.
References
- 1.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–92. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 2.Rezk SA, Weiss LM. Epstein-Barr virus-associated lymphoproliferative disorders. Hum Pathol. 2007;38:1293–304. doi: 10.1016/j.humpath.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Kimura H, Hoshino Y, Hara S. Differences between T-cell type and natural killer cell-type chronic active Epstein-Barr virus infection. J Infect Dis. 2005;191:531–5. doi: 10.1086/427239. [DOI] [PubMed] [Google Scholar]
- 4.Isobe Y, Sugimoto K, Yang L. Epstein-Barr virus infection of human natural killer cell lines and peripheral blood natural killer cells. Cancer Res. 2004;64:2167–74. doi: 10.1158/0008-5472.can-03-1562. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20:1472–82. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohshima K, Kimura H, Yoshino T. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008;58:209–17. doi: 10.1111/j.1440-1827.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 7.Quintanilla-Martinez L, Kimura H, Jaffe E. EBV-positive T-cell lymphoproliferative disorders of childhood. In: Swerdlow S, Campo E, Harris N, editors. International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th edition. Geneva, Switzerland: World Health Organization; 2008. pp. 278–80. [Google Scholar]
- 8.Clayton RA, Malcomson RD, Gilmour HM, Crawford DH, Parks RW. Profuse gastrointestinal haemorrhage due to delayed primary Epstein-Barr virus infection in an immunocompetent adult. Histopathology. 2005;47:439–41. doi: 10.1111/j.1365-2559.2005.02130.x. [DOI] [PubMed] [Google Scholar]
- 9.Lavín AC, Román JG, Zárate SA, Porras MC, Caviedes RS. Acute upper gastrointestinal bleeding associated with Epstein-Barr virus reactivation in an immunocompetent patient. Am J Gastroenterol. 2009;104:253–4. doi: 10.1038/ajg.2008.25. [DOI] [PubMed] [Google Scholar]
- 10.Karlitz JJ, Li ST, Holman RP, Rice MC. EBV-associated colitis mimicking IBD in an immunocompetent individual. Nat Rev Gastroenterol Hepatol. 2011;8:50–4. doi: 10.1038/nrgastro.2010.192. [DOI] [PubMed] [Google Scholar]
- 11.Shirazi KM, Somi MH, Bafandeh Y. Epidemiological and clinical characteristics of inflammatory bowel disease in patients from northwestern Iran. Middle East J Dig Dis. 2013;5:86–92. [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JK. Natural killer cell neoplasms. Anat Pathol. 1998;3:77–145. [PubMed] [Google Scholar]
- 13.Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127:860–8. doi: 10.1309/2F39NX1AL3L54WU8. [DOI] [PubMed] [Google Scholar]
- 14.Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS, Kim SW. Clinicopathologic and genotypic study of extranodal nasal-type natural killer/T-cell lymphoma and natural killer precursor lymphoma among Koreans. Cancer. 2000;89:2106–16. doi: 10.1002/1097-0142(20001115)89:10<2106::aid-cncr11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Arai A, Imadome K, Wang L. Recurrence of chronic active Epstein-Barr virus infection from donor cells after achieving complete response through allogeneic bone marrow transplantation. Intern Med. 2012;51:777–82. doi: 10.2169/internalmedicine.51.6769. [DOI] [PubMed] [Google Scholar]


