Abstract
Pulmonary sclerosing hemagioma (SH) is an uncommon tumor with malignance potential. Clinically this disease is regarded as benign but extremely rare cases can have lymph node metastasis. Up to date, there have been only very few reports concerning SH with lymph node metastasis. In this paper we reported one pulmonary SH case with lymph node metastasis and additionally overviewed the clinical and pathological features of SH. A young-aged female was found incidentally to have a nodule in the right upper lung. This patient presented no cough, no hemoptysis and chest pain. Computed tomography (CT) scan indicated a large mass in the right upper lung and enlarged lymph nodes in the right hilum. The patient underwent lobectomy of the right upper lung. Histologically, the tumor demonstrated typical features of SH and was consisted of angiomatoid areas, sclerosis, papillary structures lined with cuboidal cells and sheets of round to polygonal cells. Polygonal cells in some solid areas presented abnormal enlarged nuclei and increased karyoplasmic ratio; tumor giant cells were noted; whereas mitosis was not observed. One peribronchial lymph node was noted for SH metastasis and the metastatic tissue were consisted of polygonal cells. Immunohistochemistry (IHC) revealed that both surface-lining cuboidal and polygonal cells expressed EMA and thyroid transcription factor 1 (TTF-1), but were negative for CD34, VIII factor, CD68 and Claratinin. The polygonal cells showed relatively higher expression of Ki-67 and p53 than the surface-lining cells. Postoperatively, the patient received no chemotherapy or radiotherapy and no recurrence 2 years after surgery was noted.
Keywords: Sclerosing hemangioma (SH), pulmonary SH, lymph node metastasis
Introduction
Pulmonary sclerosing hemangioma (SH) is an uncommon tumor in the pulmonary parenchyma. This disease was first reported by Leibow and Hubbell in 1956 [1]. The tumor is mainly consisted of polygonal cells in the mesenchymal and cuboidal cells that line the papillary and cavity surface that form papillary, solid, angiomatoid and sclerotic patterns. Although there have been many reviews and reports demonstrating the pulmonary SH, cases with lymph node metastasis is extremely rare. Here we described one pulmonary SH with lymph node metastasis.
Clinical history
A 26-year-old female was referred to the hospital after health examination revealed a mass in the right upper lung. She demonstrated no cough, hemoptysis, chest pain or other pulmonary abnormalities. Chest CT indicated a nodule in the upper lobe of the right lung with enlarged right hilar lymph nodes. This pulmonary lesion was first considered as malignant tumor with lymph node metastasis. The patient underwent lobectomy of the right upper lung. Postoperatively, the patient received no chemotherapy or radiotherapy and no recurrence or metastasis 2 years after surgery was noted.
Pathologic findings
Grossly, the tumor was sharply demarcated from the surrounding lung tissue and was 9.7 × 8.3 × 7.5-cm in size. The cut surface of the lesion showed was dark red, faviform and with hemorrhage. Part of the tumor was grey, sclerotic and with partial calcification. Microscopically, the tumor was consisted of surface-lining and polygonal cells which were distributed in angiomatosis, solid, papillary and sclerotic patterns. The surface-lining cells were cuboidal with eosinophilic cytoplasms and presented small and dense nuclei. These cuboidal cells lined in the angiomatoid pattern or papillae surface. In addition, the angiomatoid cavities were often expanding and were rich in erythrocytes and albiminous exudates. The polygonal cells were similar in morphology and rich in light-stained eosinophilic cytoplasma (Figure 1A). The polygonal cells formed solid pattern or grew into the alveolar cavity to form papillary structure. Portion of the polygonal cells in the solid area presented enlarged nuclei and increased karyoplasmic ratio. Moreover, giant tumor cells were noted, but mitosis was not observed (Figure 1B). There were metastatic foci in one of the dissected peribronchial lymph nodes, which were consisted of mainly polygonal cells (Figure 1C).
Figure 1.
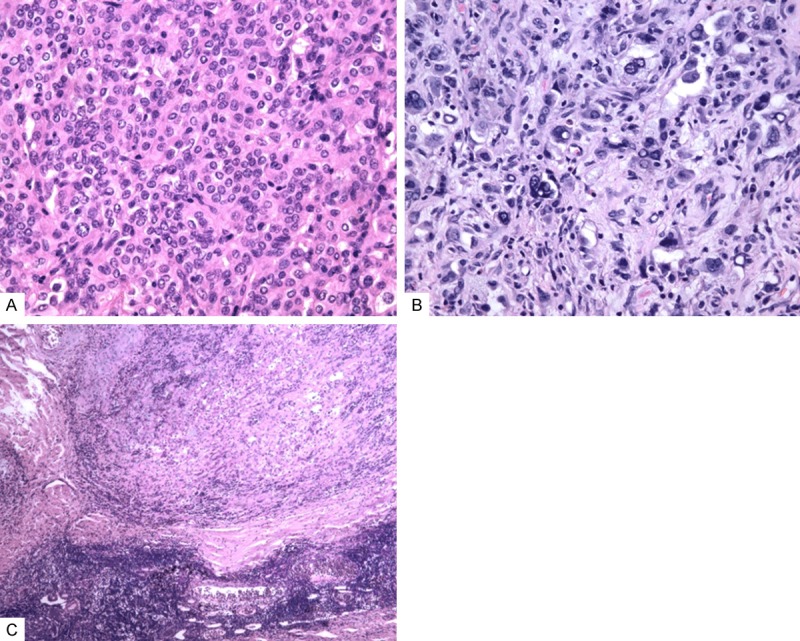
Histological findings. A: The polygonal cells were similar in morphology with light-stained eosinophilic cytoplasma (hematoxylin and eosin, × 200). B: The polygonal cells displayed strike pleomorphism, enlarged nuclei and increased karyoplasmic ratio. But mitosis was not observed (hematoxylin and eosin, × 400). C: Metastatic deposits in the lymph node were consisted of polygonal cells (hematoxylin and eosin, × 100).
In immunohistochemical studies, both the surface-lining and polygonal cells expressed EMA, TTF-1 (Figure 2A). The surface-lining cells were positive for pancytokeratin, napsin A and SP-A. The polygonal cells showed diffuse positivity for vimentin and focally positivity for Syn and CD56. The surface-lining and polygonal were all negative for CD34, VIII factor, CD68 and calratinin. Besides, the Ki-67 index was lower than 1% in surface-lining cells whereas the index of polygonal cells was higher (~10% in some foci) (Figure 2B). The polygonal cells also had higher positivity of p53 than the surface-lining cells.
Figure 2.
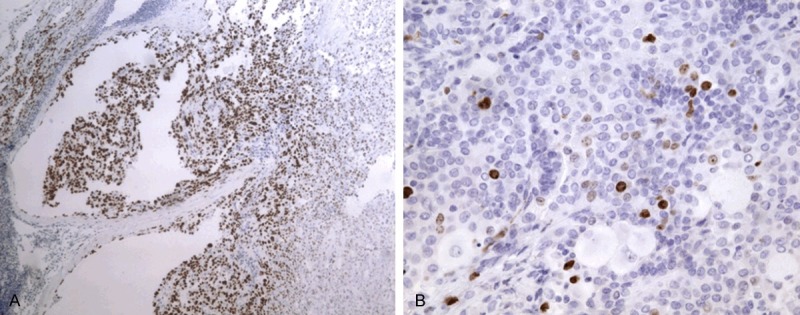
Immunophenotypic findings. A: The metastatic tumor cells were positive for TTF-1 (immunohistochemistry, × 100). B: The polygonal cells showed higher Ki-67 expression than surface-lining cells (immunohistochemistry, × 400).
Discussion
In our case, the tumor was composed of two cell types: the surface-lining cells and polygonal cells. IHC indicated that both cells expressed EMA and TTF-1. In addition, metastasis was detected in one peribrochial lymph node. According to the clinical and histological features, we considered this case as a pulmonary SH with lymph node metastasis.
Pulmonary SH is considered as a rare parenchyma tumor of the lung. Although this tumor has been proved not arise from vascular, but the world health organization (WHO) classification of pulmonary tumor still use this name and lists it as a mixed-origin tumor. Pulmonary SH is more common in adult female, with the male-to-female ratio 1:4. The occurrence of this disease covers large age-span with an average of 40 years. Generally the pulmonary SH is asymptomatic and only few cases are with cough, hemoptysis and chest pain. In most cases, the pulmonary SH is found incidentally in health examination. The chest X-ray and CT are two powerful tools for further diagnosis. Due to its low occurrence, many aspects of this tumor remain unclear, and in clinical pathologic diagnosis, pulmonary SH is usually misdiagnosed to inflammatory pseudotumor, lung adenocarcinoma or sclerosing neuroendocrine tumors.
The debate for the origin of SH has been last for a long time. Initially the tumor cell was believed to originate from the vascular endothelial cell. But later other different hypothesis of mesothelial origin, neuroendocrine origin and epithelial origin arose. With the development of electron microscope, IHC and molecular biology, study of the SH origin goes deeper. Wang proposed that the two types of SH cells were not arising from the same origin. The surface-lining cells were originating from the type II alveolus cells and were due to reactive hyperplasia. Whereas the polygonal cells in the mesenchyme were the parenchymal cells and most likely arose from the multipotential primitive respiratory epithelial cells [2]. The latter opinion is now preferred by most researchers.
In the past, the pulmonary SH was generally regarded as benign tumor, but with increasing reports of pulmonary SH with lymph node metastasis, now most researchers classify this disease into tumor type with malignance potential. Searching the literatures, we found 18 cases (14 in English and 4 in Chinese) about pulmonary SH with lymph node metastasis (Table 1) [3-13]. In summary, the age range was between 10 and 70 years, with an average of 38.3 and medium of 40 years. Males accounted for 6/18 cases (33.3%) and females 12/18 cases (66.7%). The male-to-female ratio was 1:2. And 8/18 (44.4%) of the primary tumors were noted in the left lower lobe, 1/18 (5.6%) in the left upper lobe, 5/18 (27.8%) in the right lower lobe, 1/18 (5.6%) in the right middle lobe and 3/18 (16.7%) in the right upper lobe. The size of the primary tumors ranged from 10 to 100 mm with the average of 52 mm. 5 cases had mediastinal or supraclavicular lymph node metastasis whereas the metastatic area of left 13 cases all restricted in the hilus of the lung.
Table 1.
Review of pulmonary sh cases with lymph node metastasis
| Author (Year) | Age | Gender | Primary tumor location | Primary tumor size (mm) | Metastatic lymph node, n | Site of Metastatic lymph node |
|---|---|---|---|---|---|---|
| Tanaka I (1986) | 22 | Male | Right lower lobe | 50 | 1 | Hilum |
| Chan AC (2000) | 48 | Male | Right lower lobe | 80 | 2 | Hilum |
| Devouassoux-Shisheboran M (2000) | 18 | Female | Left lower lobe | 35 | 2 | Hilum |
| Yano M (2002) | 67 | Female | Right lower lobe | 90 | 5 | Hilum |
| Miyagawa-Hayashino A (2003) | 10 | Female | Right middle lobe | 47 | 1 | Regional |
| Miyagawa-Hayashino A (2003) | 45 | Female | Right upper lobe | 25 | 3 | Hilum |
| Miyagawa-Hayashino A (2003) | 45 | Male | Left lower lobe | 37 | 1 | Mediastinum |
| Miyagawa-Hayashino A (2003) | 50 | Female | Left lower lobe | 15 | 1 | Intralobular |
| Chan NG (2003) | 19 | Male | Left upper lobe | 30 | Not described | Intralobular |
| Kim KH (2003) | 19 | Female | Left lower lobe | 100 | 11 | Hilum, intralobular |
| Kim GY (2003) | 37 | Female | Left lower lobe | 20 | 1 | Supraclavicular |
| Katakura H (2005) | 35 | Male | Left lower lobe | Unknown | 1 | Mediastinum |
| Vaideeswar P (2009) | 23 | Male | Right upper lobe | 90 | Several | Hilum |
| Adachi Y (2014) | 40 | Female | Left lower lobe | 10 | 1 | Mediastinum |
| Jiang ZN (2007) | 59 | Female | Right lower lobe | 65 | 1 | Hilum |
| Li JC (2006) | 70 | Female | Left lower lobe | 30 | 1 | Mediastinum |
| Wang L (2005) | 56 | Female | Right lower lobe | 65 | Not described | Hilum |
| Present case | 26 | Female | Right upper lobe | 97 | 1 | Hilum |
Previously, Devouassoux-Shisheboran et al carried out a detailed analysis of 100 SH cases [5]. In these 100 cases: 1) the patients’ age ranged between 16 and 76 years with an average of 46 years. 2) 17 male patients and 83 female patients with the male-to-female ration of 1:5. 3) 46% tumors were found in the left lung (17% in the left upper lobe, 25% in the left lower lobe, 1% in the fissure between the upper and lower lobe and the 3% cases with unknown specific site), and left 54% cases were in the right lung (9% in the right upper lobe, 17% in the right middle lobe, 22% in the right lower lobe, 4% in the fissure between the middle and upper lobe, 1% in the fissure between the middle and lower lobe and 1% in unknown specific site). 4) The size of tumors ranged between 3 and 70 mm with an average of 26 mm.
In this paper, cases of the pulmonary SH with lymph node metastasis we summarized were compared with the cases of Devouassoux-Shisheboran et al reviewed. We found that young male patients of pulmonary SH tend to have lymph node metastasis. Primary tumor in the left lower lobe also tended to has higher occurrence of lymph node metastasis. Furthermore, the mean size of lymph node metastatic pulmonary SH tumor was much larger than the non-metastatic SH which indicated the metastasis potential may correlate with the primary tumor size.
In conclusion, SH is the tumor that has malignance potency since several cases reported lymph node metastasis, pleura invasion and reoccurrence. Based on literature review and comparison, SHs with lymph node metastasis may possible correlate with age, gender, primary tumor site and size. Due to the lack of cases report with lymph node metastatic, the determination of pathologic or biologic behavior of SHs still requires additional clinical documents. Even though SHs can have lymph node metastasis, the prognosis is not strongly influenced. Moreover, until now there has been no death report of SH. The patient we reported in this paper was found only having bronchial lymph node metastasis and she received no chemotherapy after the operation. In the two-year follow up, this patient remained healthy. Currently the complete resection of the SH tumor is the only effective way for treating SH and further investigation is needed to elucidate the mechanism of lymph node metastatic SHs.
Disclosure of conflict of interest
None.
References
- 1.Liebow A, Hubbell DS. Sclerosing hemangioma (histocytoma, xarthoma) of the lung. Cancer. 1956;9:53–75. doi: 10.1002/1097-0142(195601/02)9:1<53::aid-cncr2820090104>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang EH, Wu GP, Zhang ZK, Lin D. Pulmonary sclerosing hemangioma immunochemical markers and ultra-structure study: different progenitors of SH. Chinese Journal of Lung Cancer. 2003;6:89–93. [Google Scholar]
- 3.Jiang ZN, Zhu T, Jin M, Wang LB. Case report: Pulmonary sclerosing hemangioma with lymph node metastasis. Chinese Journal of Pathology. 2007;36:282–283. [PubMed] [Google Scholar]
- 4.Chan AC, Chan JK. Pulmonary sclerosing hemangioma consistently expresses thyroid transcription factor-1 (TTF-1): a new clue to its histogenesis. Am J Surg Pathol. 2000;24:1531–1536. doi: 10.1097/00000478-200011000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000;24:906–916. doi: 10.1097/00000478-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Yano M, Yamakawa Y, Kiriyama M, Hara M, Murase T. Sclerosing hemangioma with metastases to multiple nodal stations. Ann Thorac Surg. 2002;73:981–983. doi: 10.1016/s0003-4975(01)03122-8. [DOI] [PubMed] [Google Scholar]
- 7.Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med. 2003;127:321–325. doi: 10.5858/2003-127-0321-PSHWLN. [DOI] [PubMed] [Google Scholar]
- 8.Chan NG, Melega DE, Inculet RI, Shepherd JG. Pulmonary sclerosing hemangioma with lymph node metastases. Can Respir J. 2003;10:391–392. doi: 10.1155/2003/534147. [DOI] [PubMed] [Google Scholar]
- 9.Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J. 2003;44:150–154. doi: 10.3349/ymj.2003.44.1.150. [DOI] [PubMed] [Google Scholar]
- 10.Kim GY, Kim J, Choi YS, Kim HJ, Ahn G, Han J. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci. 2004;19:352–358. doi: 10.3346/jkms.2004.19.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katakura H, Sato M, Tanaka F, Sakai H, Bando T, Hasegawa S, Nakashima Y, Wada H. Pulmonary sclerosing hemangioma with metastasis to the mediastinal lymph node. Ann Thorac Surg. 2005;80:2351–2353. doi: 10.1016/j.athoracsur.2004.06.099. [DOI] [PubMed] [Google Scholar]
- 12.Vaideeswar P. Sclerosing hemangioma with lymph nodal metastases. Indian J Pathol Microbiol. 2009;52:392–394. doi: 10.4103/0377-4929.55004. [DOI] [PubMed] [Google Scholar]
- 13.Adachi Y, Tsuta K, Hirano R, Tanaka J, Minamino K, Shimo T, Ikehara S. Pulmonary sclerosing hemangioma with lymph node metastasis: A case report and literature review. Oncol Lett. 2014;7:997–1000. doi: 10.3892/ol.2014.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]


