Abstract
Fat-forming solitary fibrous tumor (SFT) is a rare soft tissue tumor. Herein, we reported a 30-year-old woman was found to have a solid mass measuring 60×45 mm in the right kidney on an abdominal computed tomography scan. The tumor was well-circumscribed and composed of cellular nodules with the classic SFT admixed with clusters and lobules of mature adipocytes. Immunohistochemistry staining showed that the tumor cells were diffusely and strongly positive for CD34 and Bcl-2, focally and weakly positive for CD99 and EMA. Mature adipocytes were positive for S-100 protein. Ki-67 expression was found in approximately 2% of tumor cells. However, tumor cells were negative for cytokeratin, S-100 protein, HMB-45, Melan-A, SMA, and CD117. We made the pathological diagnosis of fat-forming SFT of the right kidney. The differential diagnosis includes angiomyolipoma, liposarcoma, spindle cell lipoma, sarcomatoid renal cell carcinoma, synovial sarcoma, and gastrointestinal stromal tumor. The patient was alive and well without evidence of recurrence or metastasis at 19 months after tumor resection.
Keywords: Fat-forming solitary fibrous tumor, kidney
Introduction
Fat-forming solitary fibrous tumor (fat-forming SFT) previously known as lipomatous haemangiopericytoma is a recently recognized rare variant of SFT. Fat-forming SFT usually affects middle-aged adults and occur mostly in the deep soft tissues of the lower extremities and retroperitoneum [1]. Fat-forming SFT arising in the kidney is extremely rare [2,3]. Herein, we present one case of fat-forming SFT in the kidney and analyzed its clinicopathological features and reviewed the literature.
Materials and methods
Tumor specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissues were cut into 4-μm thick sections and stained with hematoxylin-eosin staining. Immunohistochemistry staining was carried out on formalin-fixed, paraffin-embedded tissue using an EnVision kit (Dako, Carpinteria, CA). The following primary antibodies purchased from Dako Corporation were used: cytokeratin, EMA, S-100 protein, HMB45, Melan-A, SMA, CD34, CD117, bcl-2, CD99, and Ki-67. Positive and negative control slides were employed.
Results
Clinical findings
The patient was a 30-year-old woman with dull pain in her right lower back for one month. Physical examination revealed a palpable, ill-defined mass in the right flank. Abdominal computed tomography (CT) scan showed a well-delineated heterogeneous mass measuring 60×45 mm at the hilar region of the right kidney with scattered low-attenuating adipose areas. The solid areas within the mass demonstrated post-contrast enhancement (Figure 1A). Angiography demonstrated a hypervascular mass fed by a small branch of the right renal artery. The mass was initially diagnosed as angiomyolipoma of the right kidney. Laboratory findings were within normal limits. The patient was performed surgical excision of the mass in our Hospital without further chemotherapy or radiation therapy.
Figure 1.
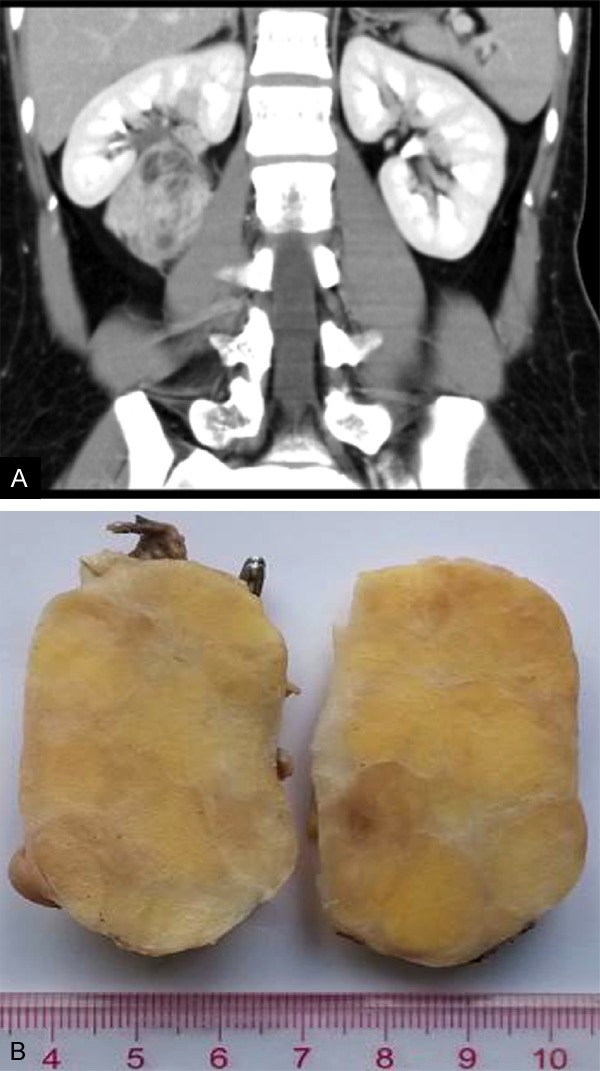
A. Computed tomography showed an enhanced well-circumscribed mass measuring 60 mm×45 mm at the hilar region of the right kidney; B. Grossly, the tumor appeared well-encapsulated with a smooth surface, measuring 60×47×46 mm in size. On cut surface, the tumor was firm and white to yellowish, with a vaguely lobular configuration.
Pathological findings
Grossly, the tumor appeared well-encapsulated with a smooth surface, measuring 60×47×46 mm in size. On cut surface, the tumor was firm and white to yellowish, with a vaguely lobular configuration (Figure 1B). Necrosis, hemorrhage, and calcifications were not present.
Microscopically, the tumor was well-circumscribed mass with a fibrous capsule and was composed of cellular nodules with the classic appearance of SFT admixed with clusters or lobules of mature adipose tissue. The typical SFT component showed a patternless architecture characterized by a mixture of hypercellular and hypocellular areas separated by thick bands of collagen. Dilated and branching haemangiopericytoma-like vessels were frequently observed, particularly evident in the hypercellular area. The spindle tumor cells had pale eosinophilic cytoplasm with spindled nuclei and small nucleoli. Numerous mast cells and occasional lymphocytes were scattered throughout the tumor. Mitotic figures were rarely observed (≤2/10 HPF). No hemorrhage and necrosis were found in the tumor. The tumor cells were diffusely and strongly positive for CD34 and Bcl-2, focally and weakly positive for CD99 and EMA. Ki-67 expression was found in approximately 2% of the tumor cells (Figure 2). The mature adipocytes were positive for S-100 protein. Tumor cells were negative for cytokeratin, S-100 protein, HMB-45, Melan-A, SMA, and CD117 by immunohistochemistry staining.
Figure 2.
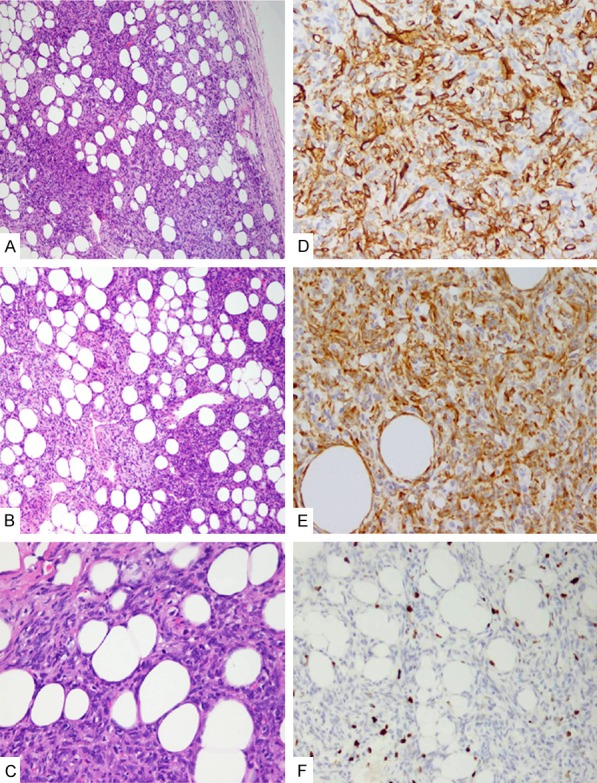
Histological features of fat-forming solitary fibrous tumor (SFT) in the kidney. A. The tumor appeared as a well-circumscribed mass with a fibrous capsule, HE×100; B. The classic appearance of SFT with hemangiopericytoma-like vasculature admixed with clusters or lobules of mature adipose tissue, HE×100; C. Mitotic figure in spindle tumor cells, HE×200; D. Tumor cells were positive for CD34, IHC×200; E. Tumor cells were positive for bcl-2, IHC×200; F. Ki-67 expression was approximately 2% of tumors, IHC×200).
Base on the above histological features and immunophenotype, we made a diagnosis of fat-forming SFT of the right kidney. The patient was alive and well without evidence of recurrence or metastasis at 19 months after tumor resection.
Discussion
Nielsen et al. reported three soft tissue tumors composed of haemangiopericytoma-like areas and mature adipose tissue, for which they proposed the term “lipomatous hemangiopericytoma” [4]. Guillou et al. reported that lipomatous haemangiopericytoma and SFT shared similar clinical, pathological, immunohistochemical, and ultrastructural features except for the presence of mature adipocytes, and suggested that lipomatous haemangiopericytoma was likely to represent a fat-containing variant of SFT [1]. Now, fat-forming SFT is a distinct variant of extrapleural SFT in 2013 WHO classification of tumors of soft tissue and bone tumors [5].
Fat-forming SFT affects mainly middle-aged adults, with a male predilection. The common sites for fat-forming SFT affected were the deep soft tissues of the lower extremities and retroperitoneum [1]. Other sites included orbit, pleura, perineum, spine, and mediastinum [6-10]. Up to now, only 2 cases of fat-forming SFT in the kidney have been reported in the English literature [2,3]. Histologically, most tumors in the reported cases resemble the cellular form SFT except for the presence of a variable number of mature adiopocytes. The nonadipocytic tumor cells were consistently positive for CD99, and frequently for CD34 (75%) and Bcl-2 (60%), as in our case. The adipocytic component of the fat-forming SFTs reported in the literature was mostly benign mature adipocytes, as in our case. Of note, multivacuolated lipoblasts and/or atypical lipomatous tumor-like areas can be observed in some cases, closely mimicking well-differentiated liposarcoma [11,12]. But the origin of the adipocytic components remains unknown. Ceballos et al. suggested that the adipocytic components of fat-forming SFT most likely arose from the population of “pericyte” cells [12].
The definitive site of SFT in the kidney may arise from the renal capsule, pelvis, and peripelvic connective tissue, but rarely invade the kidney, even in cases of larger masses. In our case, angiography confirmed that the tumor was fed by a small branch of the right renal artery, indicating a primary tumor of the kidney. However, the tumor was located at the hilar region of the kidney, and did not involve the renal parenchyma and pelvis, suggesting the possibility of hilar soft tissue origin of the tumor. However, one SFT of the kidney showed an intrarenal growth pattern without connection with the renal capsule or renal pelvis [13].
Considering the unusual histological appearance of fat-forming SFT in the kidney, the differential diagnosis includes angiomyolipoma, well-differentiated liposarcoma, spindle cell lipoma, sarcomatoid renal cell carcinoma, monophasic synovial sarcoma, and gastrointestinal stromal tumor (GIST). In fact, our current case was initially considered as angiomyolipoma by CT scan. Angiomyolipoma typically consists of variable amounts of fat, thick-walled vessels, and smooth muscle components which are immunoreactive for SMA, HMB-45, and Melan-A, but negative for CD34. Well differentiated liposarcoma usually exhibits infitrative growth, unlike the well-encapsulated fat-forming SFT. The fat component in fat-forming SFT is mature without atypical cells as seen in well-differentiated liposarcoma. In addition, MDM2 and CDK4 were positive in tumor cells of well-differentiated liposarcoma by immunohistochemistry staining and fluorescence in situ hybridization (FISH) analysis, respectively. Spindle cell lipoma usually develops in the subcutaneous tissue of the neck and upper back of male patients and contains short bundles of wiry collagen admixed with the spindle cells. The thick bands of hyalinized collagen and hemangiopericytoma-like vasculature, which are the features of fat-forming SFT, are not found in spindle cell lipoma. Monophasic synovial sarcoma may exhibit alternating hypercellular and hypocellular areas with hemangiopericytoma-like vasculature and tumor cells are immunoreactive for bcl-2 and CD99. However, synovial sarcoma cells display nuclear pleomorphism, atypical mitoses, and immunoreactive for cytokeratin and EMA, but negative for CD34. In addition, synovial sarcoma is specific for the presence of chromosomal translocation t(X; 18) by FISH analysis. Sarcomatoid renal cell carcinoma appears dramatically atypical, nuclear pleomorphism and atypical mitoses, and immunorective for epithelial markers. GIST does not contain the mature adipocytes which are characteristic of fat-forming SFT. In addition, GIST is positive for CD117, DOG-1, and CD34.
Most fat-forming SFT showed benign histology and followed an indolent clinical course. As our case, the patient was alive and well without evidence of recurrence or metastasis at 19 months after tumor resection. Very few cases with malignant histological features of fat-forming SFT have been reported [11,14,15].
Disclosure of conflict of interest
None.
References
- 1.Guillou L, Gebhard S, Coindre JM. Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohistochemical and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol. 2000;31:1108–15. doi: 10.1053/hupa.2000.9777. [DOI] [PubMed] [Google Scholar]
- 2.Cortes LG, Caserta NM, Billis A. Fat-forming solitary fibrous tumor of the kidney: a case report. Anal Quant Cytopathol Histpathol. 2014;36:295–98. [PubMed] [Google Scholar]
- 3.Yamaguchi T, Takimoto T, Yamashita T, Kitahara S, Omura M, Ueda Y. Fat-containing variant of solitary fibrous tumor (lipomatous hemangiopericytoma) arising on surface of kidney. Urology. 2005;65:175. doi: 10.1016/j.urology.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen GP, Dickersin GR, Provenzal JM, Rosenberg AE. Lipomatous hemangiopericytoma. A histologic, ultrastructural and immunohistochemical study of a unique variant of hemangiopericytoma. Am J Surg Pathol. 1995;19:748–56. doi: 10.1097/00000478-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. pp. 306–09. [Google Scholar]
- 6.Park CY, Rho JY, Yoo SM, Jung HK. Fat-forming variant of solitary fibrous tumour of the pleura: CT findings. Br J Radiol. 2011;84:e203–05. doi: 10.1259/bjr/68692634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aftab S, Casey A, Tirabosco R, Kabir SR, Saifuddin A. Fat-forming solitary fibrous tumour (lipomatous haemangiopericytoma) of the spine: case report and literature review. Skeletal Radiol. 2010;39:1039–42. doi: 10.1007/s00256-010-0991-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim MY, Rha SE, Oh SN, Lee YJ, Byun JY, Jung CK, Kang WK. Case report. Lipomatous haemangiopericytoma (fat-forming solitary fibrous tumour) involving the perineum: CT and MRI findings and pathological correlation. Br J Radiol. 2009;82:e23–26. doi: 10.1259/bjr/26727658. [DOI] [PubMed] [Google Scholar]
- 9.Pitchamuthu H, Gonzalez P, Kyle P, Roberts F. Fat-forming variant of solitary fibrous tumour of the orbit: the entity previously known as lipomatous haemangiopericytoma. Eye (Lond) 2009;23:1479–81. doi: 10.1038/eye.2008.215. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Zhang HY, Bu H, Meng GZ, Zhang Z, Ke Q. Fat-forming variant of solitary fibrous tumor of the mediastinum. Chin Med J (Engl) 2007;120:1029–32. [PubMed] [Google Scholar]
- 11.Lee JC, Fletcher CD. Malignant fat-forming solitary fibrous tumor (so-called “lipomatous hemangiopericytoma”): clinicopathologic analysis of 14 cases. Am J Surg Pathol. 2011;35:1177–85. doi: 10.1097/PAS.0b013e318219cd0b. [DOI] [PubMed] [Google Scholar]
- 12.Ceballos KM, Munk PL, Masri BA, O'Connell JX. Lipomatous hemangiopericytoma: a morphologically distinct soft tissue tumor. Arch Pathol Lab Med. 1999;123:941–45. doi: 10.5858/1999-123-0941-LH. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Arber DA, Frankel K, Weiss LM. Large solitary fibrous tumor of the kidney: report of two cases and review of the literature. Am J Surg Pathol. 2001;25:1194–99. doi: 10.1097/00000478-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Noh SJ, Jang KY. Malignant fat-forming solitary fibrous tumor of the thigh. Korean J Pathol. 2014;48:69–72. doi: 10.4132/KoreanJPathol.2014.48.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carvalho AD, Abrahao-Machado LF, Viana CR, de Castro CR, Mamere AE. Malignant fat-forming solitary fibrous tumor (lipomatous hemangiopericytoma) in the neck: Imaging and histopathological findings of a case. J Radiol Case Rep. 2013;7:1–7. doi: 10.3941/jrcr.v7i3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]


