Abstract
Dendritic fibromyxolipoma is an uncommon benign soft tissue tumor. Here, we report a case in a 53-year-old man presenting a painless mass located deep in the latissimus dorsi of the right back. Microscopically, the tumor was mainly consisted of small spindle and stellate cells, abundant myxoid stroma, collagen bundles and mature adipose tissue. Immunohistochemical study showed the spindle and stellate cells were positive for CD34, Bcl-2 and Vimentim, but not for Keratin, EMA, SMA and Desmin. To date, one year after operation, the patient is well without evidence of recurrence or metastasis. The implication of this report is to provide insights into further understanding of this rare tumor with review of the literature.
Keywords: Dendritic fibromyxolipoma, latissimus dorsi, spindle cell lipoma, solitary fibrous tumor
Introduction
Dendritic fibromyxolipoma (DFML) is a rare benign soft tissue tumor, which is commonly located in the subcutis of the neck, back, chest wall and shoulders [1], occasionally in the muscle [2]. DFML often occurs in adult male patients, presenting as a slowly growing and well-demarcated mass. Clinical finding of DFML reported in English literature till date is presented in Table 1. Microscopically, the tumor is mainly consisted of small spindle and stellate cells, admixture of mature adipose tissue, and abundant myxoid stroma with prominent collagenization. Immunohistochemically, the spindle and stellate cells show positive for CD34, Bcl-2 and Vimentim, but negative for keratin, EMA, SMA and Desmin. Herein, we report a case of DFML in a 53-year-old man. Moreover, we review the available literature to gain more comprehension this uncommon tumor. To our best knowledge, such a presentation of DFML in the latissimus dorsi of the right back has not been reported before.
Table 1.
Clinical finding of dendritic myxofibrolipoma reported in English literature
| Author | Year | Cases | Sex | Age(year) | Tumor size (cm) | Location |
|---|---|---|---|---|---|---|
| Sister [1] | 1998 | 12 | 11/1 (M/F) | 33-81 (median, 64) | 2-11 (average, 6) | Neck (4 cases); Back (3 cases); Shoulder (2 cases); Chest wall (2 cases); Face right nasal area (1 case) |
| Kari [2] | 2003 | 1 | M | 73 | 13 | Between the infraspinatus and deltoid muscles |
| Al-Masker [3] | 2011 | 1 | F | 36 | 2 | Lower lip |
| Dahlia [4] | 2012 | 1 | F | 65 | 3.2 | Adherent to median nerve in the left forearm |
| Zhang XJ [5] | 2013 | 1 | F | 32 | 24 | Right inguinal and perineum regions |
| Han XC [6] | 2014 | 1 | M | 69 | 1 | Nasal tip |
| Wong YP [7] | 2014 | 1 | M | 67 | 7 | Shoulder |
| Xu X [8] | 2015 | 1 | M | 24 | 14 | Triceps brachii in the left shoulder region |
M, male; F, female.
Clinical data
A 53-year-old man noted a mass in his right back for one month and visited Futian District People’s Hospital, Shenzhen, China. He had no past medical history of disease. In physical examination, a well-demarcated and painless mass about 2 cm in diameter was palpable in the right back. The patient underwent a local excision and the mass was completely removed.
On gross examination, the mass was well-demarcated, measuring 2×1.5×1.5 cm in size and yellow-gray in color and myxoid on the cut surface. On microscopic examination of the HE sections, the lesion was mainly consisted of small spindle or stellate cells, abundant myxoid, and mature adipose tissue; the stroma was collagenized and vascular with thin-walled vessels (Figure 1A). Cytologically, small spindle or stellate cells were bland with thin, elongated cytoplasmic processes (Figure 1B). No atypical features and mitotic figures were found.
Figure 1.
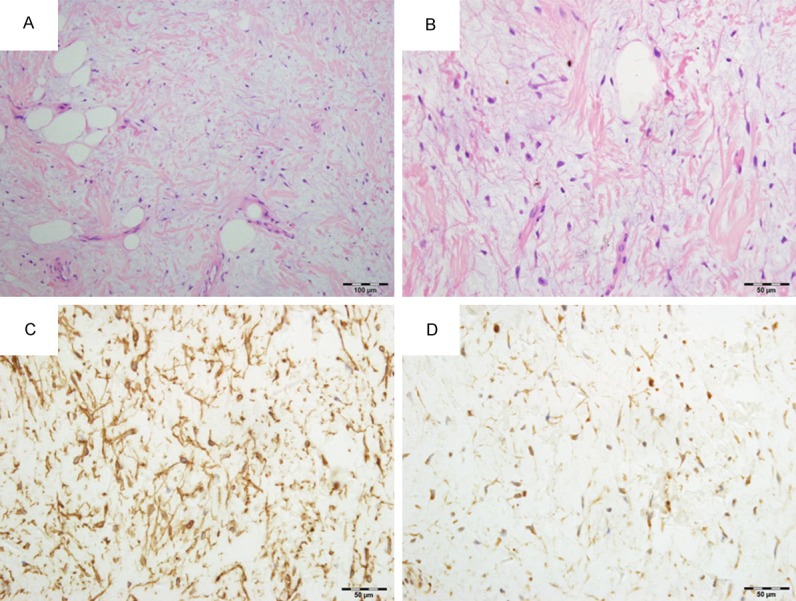
A. Hematoxylin and eosin (H&E)-stained section showed the tumor was consisted of small spindle or stellate cells, abundant myxoid, and mature adipose tissue; the stroma was collagenized and vascular with thin-walled vessels. (HE 200×). B. H&E-stained section showed small spindle and stellate cells were bland embedded within an abundant myxoid (HE 400×). C. Immunohistochemical stain for CD34 showed spindle and stellate cells with many elongated cytoplasmic processes (400×). D. Immunohistochemical stain showed Bcl-2 proteins expression was positive in spindle and stellate cells (400×).
Immunohistochemical study revealed that the spindle and stellate cells were positive for CD34 (Figure 1C), Bcl-2 (Figure 1D) and vimentin, but not for keratin, EMA, SMA and desmin. The adipocytes were positive for S-100 protein. Ki-67 showed low proliferative activity.
To date, one year after operation, the patient is well without evidence of recurrence or metastasis.
Discussion
Dendritic myxofibrolipoma is a benign soft tissue tumor. Suster et al firstly reported DFML and indicated it may be mistaken for a sarcoma. Since then, only 19 cases of DFML from 8 reports have been reported in the English literature and cited in PUBMED. A review of these cases shows that the patients were 24 to 81 years old at the time of diagnosis (median, 66 years); 15 were male and 4 were female. The most common tumor locations were the subcutis of the neck, back, chest wall and shoulder, other rare sites including muscle, lip [3], median nerve [4] and nasal tip [6] etc. Tumor size varied from 1 to 24 cm (average, 7.1 cm). Local excision is the most effective treatment for DFML. All patients have benign clinical course.
The mass of our case was located deep in the latissimus dorsi of the right back. To our best knowledge, only two cases were reported occurring in the intramuscular location. One was located between the infraspinatus and deltoid muscles [2] and the other in the triceps brachii of the left shoulder region [8]. Like previous intramuscular DFMLs, our case presented expansive growth with focally involved skeletal muscle. DFML is usually larger than 2 cm in greatest diameter at the time of diagnosis. The reason is that most patients have no obvious symptoms and the lesion is commonly located in the subcutis resulting in difficulty of early detection. Considering the mass of our case was only 2 cm in size, we believe that high alert for signs of the lesion and self examination of the patient may be play an important role in finding such a small tumor.
DFML has its unique and characteristic morphological features. It is predominantly consisted of abundant myxoid and collagenized stroma, spindle or stellate cells, and mature adipose tissues. Elongated cytoplasmic processes are the main characteristic of the spindle cells, which can be revealed by the CD34 and vimentin immunohistochemical stains, as indicated in our study. The histological nature of this tumor has not yet been determined. DFML is not specifically mentioned in most of the literatures of soft tissue pathology. In fact, the tumor has combined features of spindle cell lipoma (SCL) and solitary fibrous tumor (SFT). Although some features such as the dendritic nature of the spindle cells, the plexiform vascular pattern, and the abundance of keloidal collagen bundles can be used to distinguish between DFML and SCL, there is still a view argued that DFML was an unusual variant of myxoid spindle cell lipoma (SCL), because of the similarities in their clinical characteristics, pathological features and immunophenotype [1]. DFML also has the same features with SFT, such as a strong positivity for CD34 and BCl-2. However, SFT often contains “hemangiopericytoma-like” vascular pattern and has no mature adipose tissue component [9]. For these reasons, another view proposed that DFML represents an intermediate form between SCL and SFT [2].
Due to the presence of abundant myxoid matrix and proliferation of capillaries, DFML can be misdiagnosed as myxoid liposarcoma, myxofibrosarcoma and low-grade fibromyxoid sarcoma. Carefully microscopic examination and a panel of immunohistichemistry can lead to a correct diagnosis. Myxoid liposarcoma often occurs in the deep soft tissue, especially in lower extremities and retroperitoneum. The tumor is characterized by the presence of lipoblasts and invasive growth. Immunohistochemically, most of the tumor cells are positive for S-100 protein, while negative for CD34 protein. Moreover, almost all (>95%) myxoid liposarcomas involve chromosomal translocations of t(12;16)(q13;p11) and t(12;22)(q13;q12), rendering gene fusions of DDIT3 with FUS and EWSR1 [10]. DDIT3 (12q13) dual-color break-apart rearrangement probe for fluorescence in situ hybridization has been used in diagnosis of myxoid liposarcoma [11]. Myxofibrosarcoma is another myxoid spindle cell tumor should be considered. It often has hyperchromatic and irregular nuclei, variable pleomorphism, scattered giant cells and curvilinear vessels [12]. Low-grade fibromyxoid sarcoma typically shows alternating myxoid and dense fibrous areas, moderate to low cellularity, bland spindle cells with no or slight nuclear pleomorphism and rare mitotic figures [13]. The whorled or swirling growth pattern is an important histological feature for distinguishing low-grade fibromyxoid sarcoma from DFML. Sometimes, the distinction between the tumors may be difficult only by clinical presentation and pathologic features. Therefore, long-term follow-up is necessary for the correct diagnosis.
DFML in the latissimus dorsi has never been described. The present study reported a case in an unusual location with rather small in size. A diagnosis of DFML was made based on the typical morphological and immunohistochemical features. It is important for clinicians to be aware of characteristics of this tumor. This will aid clinicians to avoid misdiagnosis and confusion with other more aggressive neoplasms such as myxoid liposarcoma, myxofibrosarcoma and low-grade fibromyxoid sarcoma.
Acknowledgements
We thank the patients for giving us written consent for publishing their details.
Disclosure of conflict of interest
None.
References
- 1.Suster S, Fisher C, Moran CA. Dendritic fibromyxolipoma: clinicopathologic study of a distinctive benign soft tissue lesion that may be mistaken for a sarcoma. Ann Diagn Pathol. 1998;2:111–120. doi: 10.1016/s1092-9134(98)80047-6. [DOI] [PubMed] [Google Scholar]
- 2.Karim RZ, McCarthy SW, Palmer AA, Bonar SF, Scolyer RA. Intramuscular dendritic fibromyxolipoma: myxoid variant of spindle cell lipoma? Pathol Int. 2003;53:252–258. doi: 10.1046/j.1320-5463.2003.01464.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Maskery AY, Al-Sidairy SM, Al-Hamadani AS. Dendritic myxofibrolipoma: often misdiagnosed as sarcoma. Craniomaxillofac Trauma Reconstr. 2011;4:171–174. doi: 10.1055/s-0031-1286122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlin LB, Ljungberg O. Dendritic fibromyxolipoma adherent to the median nerve in the forearm. J Plast Surg Hand Surg. 2012;46:120–123. doi: 10.3109/02844311.2010.503083. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XJ, Zhou S, Nie K, Chen da F, Kui GJ, Zhang XH. Dendritic fibromyxolipoma in the right inguinal and perineum regions: a case report and review of the literature. Diagn Pathol. 2013;8:157. doi: 10.1186/1746-1596-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han XC, Zheng LQ, Shang XL. Dendritic fibromyxolipoma on the nasal tip in an old patient. Int J Clin Exp Pathol. 2014;7:7064–7067. [PMC free article] [PubMed] [Google Scholar]
- 7.Wong YP, Chia WK, Low SF, Mohamed-Haflah NH, Sharifah NA. Dendritic fibromyxolipoma: a variant of spindle cell lipoma with extensive myxoid change, with cytogenetic evidence. Pathol Int. 2014;64:346–351. doi: 10.1111/pin.12176. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Xiong W, Zheng L, Yu J. Intramuscular dendritic fibromyxolipoma in a 24-year-old male: A case report and review of the literature. Oncol Lett. 2015;9:583–586. doi: 10.3892/ol.2014.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdag G, Qureshi HS, Patterson JW, Wick MR. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007;34:844–850. doi: 10.1111/j.1600-0560.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 10.Powers MP, Wang WL, Hernandez VS, Patel KS, Lev DC, Lazar AJ, Lopez-Terrada DH. Detection of myxoid liposarcoma-associated FUS-DDIT3 rearrangement variants including a newly identified breakpoint using an optimized RT-PCR assay. Mod Pathol. 2010;23:1307–1315. doi: 10.1038/modpathol.2010.118. [DOI] [PubMed] [Google Scholar]
- 11.Narendra S, Valente A, Tull J, Zhang S. DDIT3 gene break-apart as a molecular marker for diagnosis of myxoid liposarcoma--assay validation and clinical experience. Diagn Mol Pathol. 2011;20:218–224. doi: 10.1097/PDM.0b013e3182107eb9. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LE, Zhang PJ, Crawford GH, Elenitsas R. Myxofibrosarcoma in the skin. J Cutan Pathol. 2008;35:935–940. doi: 10.1111/j.1600-0560.2007.00922.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans HL. Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol. 2011;35:1450–1462. doi: 10.1097/PAS.0b013e31822b3687. [DOI] [PubMed] [Google Scholar]


