Abstract
Chronic liver injury is an important clinical problem which eventually leads to cirrhosis, hepatocellular carcinoma and end-stage liver failure. It is well known that cell damage induced by reactive oxygen species (ROS) is an important mechanism of hepatocyte injure. N-acetylcysteine (NAC), a precursor of glutathione (GSH), is well-known role as the antidote to acetaminophen toxicity in clinic. NAC is now being utilized more widely in the clinical setting for non-acetaminophen (APAP) related causes of liver injure. However, the mechanisms underlying its beneficial effects are poorly defined. Thus, Aim of the present study was to investigate potential hepatic protective role of NAC and to delineate its mechanism of action against carbon tetrachloride (CCl4)-induced liver injury in models of rat. Our results showed that the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities as well as malondialdehyde (MDA) contents decreased significantly in CCl4-induced rats with NAC treatment. GSH content and superoxide dismutase (SOD) activities remarkably increased in the NAC groups compared with those in CCl4-induced group. Treatment with NAC had been shown to an increase in nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) mRNA levels. In conclusion, these results suggested that NAC upregulated HO-1 through the activation of Nrf2 pathway and protected rat against CCl4-induced liver injure. The results of this study provided pharmacological evidence to support the clinical application of NAC.
Keywords: N-acetylcysteine, liver injure, Nrf2/HO-1 pathway, carbon tetrachloride, oxidative stress
Introduction
Oxidative stress has been implicated in the pathogenesis and progression of various hepatic disorders. Reactive oxygen species (ROS) are normally produced by mitochondrial respiratory chain during metabolic reactions, and have important roles in cell signaling and homeostasis. However, ROS levels can increase dramatically during oxidative stress. Liver can maintain a dynamic equilibrium between production and elimination of ROS in normal conditions. This equilibrium is disrupted when organism is subjected to stress conditions. Excessive accumulation of ROS will result in hepatocyte injuries, including protein oxidation, lipid peroxidation, and DNA damage. Therefore, cellular antioxidant defense systems play important roles in counteracting these deleterious effects of ROS. Antioxidant enzymes, called phase II detoxifying enzymes, including heme oxygenase-1 (HO-1), NADPH quinone oxidoreductase 1 (NQO1) and glutathione S-transferase (GST), provide efficient cytoprotection by regulating the intracellular redox state. The nuclear factor erythroid 2-related factor 2 (Nrf2) is essential for the induction of such enzymes through the antioxidant response element (ARE) [1]. The Nrf2/ARE system has been proved to be the important regulator of cellular defense against oxidative stress. Recent study demonstrated that Nrf2/HO-1 signal pathway could represent an important target in protecting the liver from the damage induced by alcohol [2]. Several antioxidants are able to exert protective effects not only by free radical scavenging, but also by augment expression of cytoprotective and/or antioxidant genes via Nrf-2 signaling pathway [3].
N-acetylcysteine (NAC) is a thiol-containing compound that has been used for over 30 years as the antidote for acetaminophen (APAP) toxicity in man [4]. The clinical benefits of NAC are well documented. There are a wide range of therapeutic applications for NAC. For example, NAC is widely used to treat contrast-induced nephropathy, chronic obstructive pulmonary disorder and pulmonary fibrosis [5,6]. With ROS linked to carcinogenesis, recent reports indicate that NAC is beneficial to chemoprevention [7].
NAC also modulates inflammatory response through signaling pathways that control pro-inflammatory nuclear factor (NF)-κB activation [8,9]. These benefits attribute to NAC’s functionality as a precursor of the main cellular antioxidant glutathione (GSH), an antioxidant, a free radical scavenger, and to other as yet unidentified mechanisms [10]. Our previous study showed NAC raised the antioxidant capacity in hepatic tissue of rat administering alcohol [11]. In this paper we investigated the antioxidant activity of NAC in an animal model of liver injure induced by CCl4 and on the examination of selected parameters of oxidative stress in rats. NAC might upregulate phase II and antioxidant gene expression via activating Nrf2/HO-1 signaling pathway to protect CCl4-induced liver injury.
Materials and methods
Chemical products
NAC was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Olive oil was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai China). Carbon tetrachloride was purchased from Shanghai Lingfeng Chemical Reagent Co., Ltd. (Shanghai China). All other chemicals were analytical grade products.
Animals and treatments
The animal experiment was conducted with approval from the Institutional Animal Care and Use Committee of the Zhejiang Academy of Medical Sciences. Ten-week old male. Sprague-Dawley rats weighing 223-246 g were housed in plastic cages on a bedding of wood chips maintained at 20-24°C, 50-60% relative humidity, a 12 h light-dark cycle. The animals were feed with standard lab chow, drinking water ad labium, and were acclimated for one week prior to the treatment. The rats were randomly divided into five groups: the control group (n=8 per group), CCl4-induced liver injure group, CCl4 induction with NAC of 50, 100 and 200 mg/kg body weight treatment groups. Rats were given subcutaneous injection of 25% CCl4 mixed with olive oil twice a week at a dose of 1 ml/kg for 4 weeks. The control mice received an equal volume of olive oil only. NAC was given by intraperitoneal (IP) injections for rats once a day with CCl4 induction. Animals were weighed once a week. Rats were general anesthesia with sodium pentobarbital at the fourth week and blood samples were collected from the inferior vena cava. Serum was obtained following blood clotting and centrifugation to analyze. At the same time, livers and other organs were excised, weighed, and further processed as described below. Portions of livers from control and NAC-treated rats were snap-frozen in liquid nitrogen and stored at -80°C for the further analysis.
Determination of ALT and AST production
The blood samples were centrifuged (Beckman GS-6R, Germany) at 4°C for 15 minutes to separate serum. Serum ALT and AST activity levels were measured according to the manufacturer’s protocols.
Measurement of GSH, MDA, SOD
The GSH levels were quantified using reduced glutathione assay kit (Jiancheng Biochemical Inc, Nanjing, China). The sulfhydryl group of GSH reacts with DTNB (5,5’-dithio-bis-2-nitrobenzoic acid) and yields a yellow colored chromophore 5-thio-2-nitrobenzoic acid (TNB). The rate of TNB production is proportional to this reaction which is in turn directly proportional to the concentration of GSH in the sample. Measurement of the absorbance of TNB at 412 nm provides an accurate estimation of GSH in the sample. The protein concentrations of the supernatant were determined by the bicinchoninic acid (BCA) protein assay kit (Jiancheng Biochemical Inc, Nanjing, China).
The level of lipid peroxidation in the tissues was determined by the thiobarbituric acid assay of MDA according to the method described by Ohkawa et al [12]. The method was based on the spectrophotometric measurement of the red color produced during the condensation reaction of MDA with thiobarbituric acid. The organic layer was transferred into a clear tube and was quantified by the absorbance of 532 nm. A standard curve was constructed extrapolating the amount to the measured absorbance. The content of MDA was expressed in terms of nmol/mg protein.
The activity of SOD was determined with a kit (Jiancheng Biochemical Inc, Nanjing, China). The method uses the SOD’s ability to inhibit WST formazan dye by superoxide anions which were produced from the xanthine-xanthine oxidase system. The reaction product level in samples was examined with a spectrophotometer at 550 nm. SOD activity was determined as the amount of enzyme ability that caused a 50% reduction and was expressed as per protein mg in liver tissue homogenates [13].
Hematoxylin and eosin staining
Hematoxylin and eosin (HE) staining was used to evaluate the degree of inflammation. The harvested liver samples were fixed in 10% (v/v) buffered formalin for 24 h. After dehydration with an ethanol solution and clearing with xylene, the liver tissues were embedded in paraffin and sliced into sections (5 μm thick). These sections were then stained with HE for histological assessment by two registered histopathologists unaware of the treatments.
Nrf2, HO-1 gene expression analysis
Expression of the tissue Nrf2 and HO-1 mRNA levels were examined by reverse transcription polymerase chain reaction (RT-PCR). Total RNA of livers was extracted using nucleic acid purification kit (Axygen Scientific Inc., USA) following the manufacturer’s directions. RNA concentration was determined by measuring the absorbance of a diluted sample at the 260 nm and 280 nm with a spectrophotometer (Shimadzu Co., Ltd., Tokyo, Japan). RNA was reversely transcribed into cDNA in a 20 μL reaction volume using PrimeScript RT reagent kit (TaKaRa Biotechnology Co., Ltd.) according to the manufacturer’s manuals. The synthesized cDNA was stored at -20°C until use. The primer sequences for tested genes were as follows: Nrf2, F: 5’-GCT ATT TTC CAT TCC CGA GTT AC-3’, R: 5’-CTG TCC ATC TCT GTC AG-3’, HO-1, F: 5’-CTT TCA GAA GGG TCA GGT GTC-3’, R: 5’-TGC TTG TTT CGC TCT ATC TCC-3’, GAPDH, F: 5’-TGT TGA AGT CGC AGG AGA CAA CCT-3’, R: 5’-AAC CTG CCA AGT ATG ACA TCA-3’. PCR reactions were performed using a real-time PCR system (Bio-Rad, Hercules, CA, USA). The cycling parameters were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, annealing at 57°C for 20 s, and extension at 72°C for 29 s. In addition, GAPDH was used as an internal control. The PCR signal intensities were calculated by scanning the gels using the StepOne Software.
Statistical analysis
The experimental data were expressed as the mean ± standard error. Statistical analysis was performed using the SPSS 16.00 statistical analysis package program (SPSS, Chicago, IL, USA). Statistical analyses were performed using one-way analysis of variance, followed by Tukey’s post hoc test for multiple comparisons. The P<0.05 value was considered as statistically significant.
Results
General characteristics of rats after CCl4 induction
No dead rat was observed during the 4 weeks treatment. The body weight of rats in each group was shown in Table 1. The body weight gain in the control group administrated with olive oil was the most obvious, but was small in the other groups. The weight of CCl4-treated group significantly reduced as compared with that of the control group for two weeks (P<0.05). The treatment with 50 mg/kg NAC produced a slight increase in body weight, but was not statistically significant (P>0.05). However, the body weight significantly increased administrated with 200 mg/kg NAC for three weeks. These results indicated that NAC intake may protect rats from CCl4-induced damage.
Table 1.
Changes in body weight (g) for each group during study period
| Baseline | 1 week | 2 weeks | 3 weeks | 4 weeks | |
|---|---|---|---|---|---|
| Control | 225±3 | 261±6 | 309±4 | 332±5 | 361±6 |
| CCl4 | 227±3 | 258±5 | 262±7a | 276±6a | 289±8a |
| NAC (50 mg/kg)+CCl4 | 230±6 | 269±4 | 278±6 | 294±7 | 297±6 |
| NAC (100 mg/kg)+CCl4 | 224±5 | 263±6 | 276±5 | 298±6 | 321±7b |
| NAC (200 mg/kg)+CCl4 | 225±4 | 264±6 | 283±7 | 309±5b | 337±8b |
NAC, N-acetylcysteine; CCl4, carbon tetrachloride. ALT, alanine aminotransferase; AST, aspartate aminotransferase. Data are expressed as means ± standard error (n=8).
P<0.05, vs. the control group.
P<0.05, vs. the CCl4-treated group.
Effects of NAC on hepatic damage induced by CCl4
To evaluate the effects of NAC on liver injure induced by CCl4 in rats, the serum levels of ALT and AST were measured. As shown in Figure 1, the levels of ALT and AST were significantly increased in the CCl4-treated group. After the administration of NAC, there were a significant reduction in ALT and AST (P<0.05) compared with the CCl4-induced group. Liver injure seemed to attenuate more frequently in rats treated with higher concentrations of NAC than that with a lower one.
Figure 1.
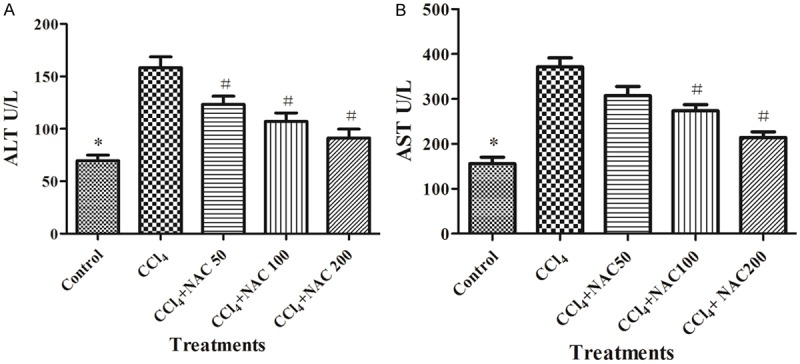
Effect of NAC on CCl4-induced elevations in serum liver enzyme activities in rats. A. ALT in CCl4-treated rats with different doses of NAC for four weeks. B. AST in CCl4-treated rats with different doses of NAC for four weeks. The data were expressed as means ± standard error of the mean of eight rats in each group. *P<0.05, vs. the control group, #P<0.05, vs. the CCl4 group, as determined by one-way analysis of variance and Dunnett’s test. NAC, N-acetylcysteine; CCl4, carbon tetrachloride; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Effects of NAC on CCl4-induced histopathological alterations
Paraffin sections were utilized to investigate the protective effect of NAC in the present experiment. The results were presented in Figure 2. The structure of the liver tissue was normal in the control group, with hepatocytes in ordered arrangement and no pathological changes area. In contrast, hepatocytes in CCl4 treatment were characterized by hepatic lobule impairment and inflammatory infiltration. Interestingly, NAC treatment significantly reduced the percentage of necrotic area in the liver tissue, which the improved conditions were involved in hepatic cell increment, cytoarchitecture repair and inflammatory reduction. Compared with the CCl4-treated group, no areas of cell necrosis were observed in the high-dose group. This indicated that NAC attenuated the intensity of CCl4-induced liver damage.
Figure 2.
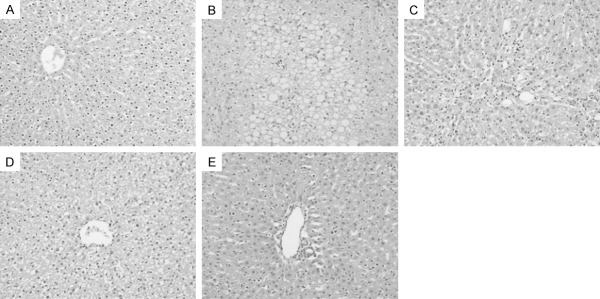
Effects of NAC on CCl4-induced histopathological alterations (HE staining, ×100). A. The control group; B. The CCl4-induced group; C. 50 mg/kg NAC treatment group; D. 100 mg/kg NAC treatment group; E. 200 mg/kg NAC treatment group. NAC, N-acetylcysteine; CCl4, carbon tetrachloride; HE, Hematoxylin and eosin.
Effects of NAC on CCl4-induced hepatic oxidative stress
To evaluate the effects of NAC on CCl4-induced hepatic oxidative stress in rats, the contents of MDA and GSH were determined, as well as the activity of SOD. As shown in Table 2, a significant increase was observed in the level of lipid peroxidation in rats exposed to CCl4 as compared to control rats. Interestingly, NAC treatment significantly decreased MDA levels compared to rats treated with CCl4 alone.
Table 2.
Effects of NAC on CCl4-induced hepatic oxidative stress
| MDA (nmol/mg protein) | SOD (U/mg protein) | GSH (nmol/mg protein) | |
|---|---|---|---|
| Control | 3.25±0.45 | 278.7±19.7 | 17.7±1.2 |
| CCl4 | 7.49±0.58a | 156.3±12.4a | 6.3±0.5a |
| NAC (50 mg/kg)+CCl4 | 6.72±0.48 | 175.8±14.5b | 7.9±0.6b |
| NAC (100 mg/kg)+CCl4 | 6.12±0.52b | 190.4±17.6b | 8.7±0.8b |
| NAC (200 mg/kg)+CCl4 | 5.37±0.41b | 217.1±19.2b | 10.6±0.8b |
MDA, malondialdehyde; SOD, superoxide dismutase; GSH, glutathione. Data are expressed as means ± standard error (n=8).
P<0.05, vs. the control group.
P<0.05, vs. the CCl4-treated group.
The levels of GSH and SOD were significantly decreased in the liver of rats exposed to CCl4. NAC administration rats treated with CCl4 significantly increased the activities of antioxidant enzyme and GSH content in the liver as compared to CCl4 induction rats. The effects exhibited dose-dependent relationships. These results suggested that NAC may be associated with decreased oxidative stress damage in rats treated with CCl4.
Effects of NAC on CCl4-induced hepatic Nrf2/HO-1 pathway
To determine whether the Nrf2 and HO-1 mRNA were upregulted in NAC treatment rats induced by CCl4, RT-PCR analysis was carried out to examine the expression in different groups. The results were showed in Figure 3. Compared with the control group, the expression levels of Nrf2 and HO-1 mRNA were significantly lower in rats induced by CCl4. Strikingly, with the treatments of NAC, Nrf2 and HO-1 mRNA expressions were increased in the liver. The effects of NAC (50, 100 and 200 mg/kg) treatments exhibited a dose-effect relationship. These data suggested that NAC upregulated the antioxidant defense genes through the activation of the Nrf2 pathway.
Figure 3.
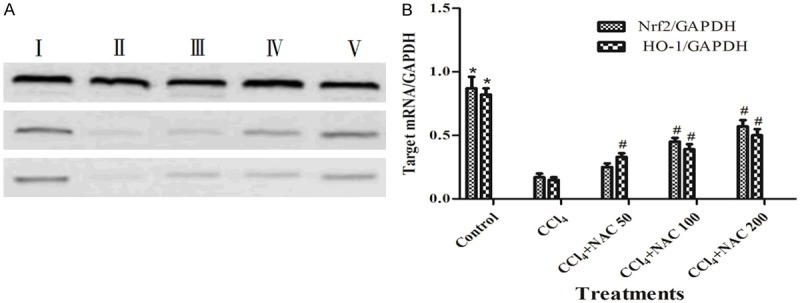
Effects of NAC on CCl4-induced hepatic Nrf2/HO-1 pathway. A. Nrf2, HO-1 and GAPDH mRNA expression in rat livers. B. Assessment of the results was done by measuring the levels of Nrf2, HO-1 and GAPDH mRNA expression. I, The control group; II, The CCl4-induced group; III, 50 mg/kg NAC treatment group; IV, 100 mg/kg NAC treatment group; V, 200 mg/kg NAC treatment group. The results were analyzed using one-way analysis of variance and Dunnett’s test and the data were expressed as means ± standard error of the mean of 3 mice in each group. *P<0.05, vs. the control group, #P<0.05, vs. the CCl4 group, as determined by one-way analysis of variance and Dunnett’s test. NAC, N-acetylcysteine; CCl4, carbon tetrachloride; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; GAPDH, glyceraldehydes phosphate dehydrogenase.
Discussion
The liver is an important organ which plays a central role in the metabolism, detoxification and excretion of various endogenous and exogenous substances such as xenobiotics. Liver injury can be caused by bacterial and viral infections, alcohol abuse, environmental pollutants, and several other factors [13]. Despite different etiology, liver injuries are frequently associated with excess oxidative stress. In recent decades, the pharmaceutical application potential of NAC supplement has attracted much interest from researchers in treating liver damage. NAC exerts its antioxidant action by facilitating glutathione biosynthesis and scavenging the ROS formed during oxidative stress [14]. In this study, we used CCl4 induction rat’s model to study the effect and mechanism of NAC.
Hepatic injury induced by CCl4, a classic experimental model, has been extensively used for evaluation of hepatoprotective activity. The mechanism is involved in free radicals which are generated during CCl4 metabolism by hepatic cellular cytochrome P450, including trichloromethyl and oxygen-centered lipid radicals, lipid peroxidation, mitochondrial damage, DNA modification and even cell death in organisms. In the present study, the results showed that body weight of CCl4 intoxicated rats decreased compared to the control group. NAC treatment for 4 weeks with CCl4 injection significantly prevented body weight reduces in a dose-dependent manner.
The levels of ALT and AST in the serum, commonly referred as liver enzymes which are released from damaged hepatocytes, have been widely recognized as useful quantitative markers to study the extent and type of hepatocellular damage. In our experiment, the results showed that CCl4 induced severe hepatic damage as represented by the significantly elevated levels of the hepatic enzymes and by the marked histopathological alterations. However, Administration of NAC along with CCl4 markedly restored levels of the hepatic enzymes and significantly improved the histopathology of the liver, in accordance with previous study the report demonstrating that NAC attenuates liver injure in rats induced by CCl4, dimethylnitrosamine and trichloroethylene [15,16]. NAC has also been shown to offer protection against liver damage induced by various chemical hepatotoxins such as ethanol [17] and methanol [18]. NAC significantly attenuated the elevated serum levels of ALT and AST indicating that the proportion of damaged hepatocytes was reduced as a direct result of NAC administration.
The enzymatic antioxidant defense system is the nature protector against excessive free radicals. SOD, a scavenger of superoxide, acts as a key ROS scavenge that prevents generation of hydroxyl radical and protects the cellular constituent from oxidative damage [19]. GSH is the important scavenger to protect against oxidative stress in the liver, and its depletion in hepatocytes could endanger the antioxidant defense system, leading to accumulation of ROS [20]. Previous studies have indicated that CCl4 decreased activities of antioxidant enzymes [21-23]. In this study, the levers of SOD and GSH in the liver of CCl4-induced group rats were significantly lower than those in the normal control group, suggesting that the decrease in antioxidant scavenging capacity occurred in CCl4-treated severe stress injury. NAC markedly elevated the levels of SOD and GSH, indicating that inhibition of the oxidative cascading stress was one of the main mechanisms in CCl4-induced rats. Elevated levels of MDA reflect an enhanced lipid peroxidation leading to liver tissue damage and failure of antioxidant defense mechanisms [24]. A significant increase in MDA level was observed in the CCl4-treated rats compared with the control group. However, NAC treatments significantly decreased the level of lipid peroxidation induced by CCl4, indicating that the protective effect of NAC on CCl4-induced liver injury were related to the alleviated lipid peroxidation. The results are in accordance with those of our earlier study that NAC treatment was able to ameliorate oxidative stress and cytotoxicity in HepG2 cells [25]. Antioxidants play an important role in protecting against liver injury induced by CCl4. NAC, an excellent source of sulfhydryl compound, is actively transported into hepatocytes and converted into metabolites capable of stimulating GSH synthesis. NAC treatment provides sufficient cysteine to promote detoxification and eliminates directly reactive oxygen species.
Oxidative stress is an important contributing factor in the pathogenesis of CCl4-induced liver injure, suggesting that therapeutic strategies directed against ROS might be particularly valuable for the prevention of liver injure. There is increasing evidence showing that induction of the endogenous Nrf2/HO-1 antioxidant pathway can confer protection against liver injury. Targeting the Nrf2 pathway presents an attractive opportunity since this intrinsic cellular pathway can be dynamically modulated.
Several studies have highlighted the ability of antioxidants to protect against liver damage via activation of the Nrf2 defense pathway [26-28]. NAC is often referred to as a powerful antioxidant which elevates the synthesis of intracellular GSH. In this study, we have demonstrated that NAC can activate the Nrf2 pathway protect liver against CCl4-induced damage. HO-1 belongs to a family of cytoprotective and detoxification genes that possess AREs in their regulatory regions. HO-1 enzyme is well known for its key role in maintaining antioxidant homeostasis during cellular stress [27]. Nrf-2 is a transcription factor and its activation can coordinately enhanced expression of several antioxidative enzymes such as quinone oxidoreductase [NQO1], γ-glutamatecysteine ligase (γ-GCS) and HO-1 [29]. As compared to rats of the control group, the expression of HO-1 mRNA was significantly downregulated in rats induced by CCl4 alone. The co-administration of NAC with CCl4 resulted in a significant increase in the expression of HO-1 mRNA. The protective mechanism by NAC is believed to be attributable to its ability to regenerate GSH stores due to its capacity to increase in dissociation cysteine concentrations. This study provides the experimental evidence that NAC affords protection against liver damage via activation of the Nrf2/HO-1 pathway. Our observation supports a previous report demonstrating that NAC attenuated dimethylnitrosamine induced oxidative damage through the Nrf2/ARE signaling pathway in rats [15]. Further extensive study is highly warranted to disclose this mechanism.
In conclusion, our present study shows that NAC possessed beneficial anti-oxidative effects that could attenuate the severity of liver injury treated by CCl4 via activation of the Nrf2/HO-1 pathway.
Acknowledgements
This work was supported by funding from the Science and Technology Program of Hangzhou (grant no. 20140633B28, grant no. 20130733Q30), the Health Science and Technology Program of Hangzhou (grant no. 2014A44).
Disclosure of conflict of interest
None.
References
- 1.Boettler U, Volz N, Teller N, Haupt LM, Bakuradze T, Eisenbrand G, Bytof G, Lantz I, Griffiths LR, Marko D. Induction of antioxidative Nrf2 gene transcription by coffee in humans: depending on genotype? Mol Biol Rep. 2012;39:7155–7162. doi: 10.1007/s11033-012-1547-6. [DOI] [PubMed] [Google Scholar]
- 2.Senthil-Kumar KJ, Liao JW, Xiao JH, Gokila-Vani M, Wang SY. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidantgenes. Toxicol In Vitro. 2012;26:700–708. doi: 10.1016/j.tiv.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–778. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 4.Prescott LF, Ballantyne A, Proudfoot AT, Park J, Driaenssens PA. Treatment of paracetamol (acetaminophen) poisoning with N-acetylcysteine. Lancet. 1977;310:432–434. doi: 10.1016/s0140-6736(77)90612-2. [DOI] [PubMed] [Google Scholar]
- 5.Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine-a cytochrome-P450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corcoran GB, Todd EL, Racz WJ, Hughes H, Smith CV, Mitchell JR. Effects of N-acetylcysteine on the disposition and metabolism of acetaminophen in mice. J Pharmacol Exp Ther. 1985;232:857–863. [PubMed] [Google Scholar]
- 7.Balansky R, Ganchev G, Iltcheva M, Steele VE, De-Flora S. Prevention of cigarette smoke-induced lung tumors in mice by budesonide, phenethyl isothiocyanate and N-acetylcysteine. Int J Cancer. 2010;126:1047–1054. doi: 10.1002/ijc.24942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansour HH, Hafez HF, Fahmy NM, Hanafi N. Protective effect of N-acetylcysteine against radiation induced DNA damage and hepatic toxicity in rats. Biochem Pharmacol. 2008;75:773–780. doi: 10.1016/j.bcp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Slim R, Toborek M, Robertson LW, Lehmler HJ. Cellular glutathione status modulates polychlorinated biphenyl-induced stress response and apoptosis in vascular endothelial cells. Toxicol Appl Pharmacol. 2000;166:36–42. doi: 10.1006/taap.2000.8944. [DOI] [PubMed] [Google Scholar]
- 10.Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002;7:22–44. [PubMed] [Google Scholar]
- 11.Wang FG, Xi JJ. The influence of acetylcysteine on cytokines of alcoholic liver injury in rat. Chin J Public Health. 2009;25:336–337. [Google Scholar]
- 12.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford A, Chung RT. Acute liver failure: mechanisms of hepatocyte injury and regeneration. Semin Liver Dis. 2008;28:167–74. doi: 10.1055/s-2008-1073116. [DOI] [PubMed] [Google Scholar]
- 14.Ocal K, Avlan D, Cinel I, Unlu A, Ozturk C, Yaylaka F, Dirlika M, Camdevirenf H, Aydina S. N-acetylcysteine on oxidative stress in intestine and bacterial translocation after thermal injury. Burns. 2004;30:778–784. doi: 10.1016/j.burns.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Priya S, Vijayalakshmi P, Vivekanandan P, Karthikeyan S. N-acetylcysteine attenuates dimethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 2011;654:181–186. doi: 10.1016/j.ejphar.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 16.Wong CK, C-Ooi VE, Wong CK. Protective effect of N-acetylcysteine against carbon tetrachloride and trichloroethylene induced poisoning in rats. Environ Toxicol Pharmcol. 2003;14:109–116. doi: 10.1016/S1382-6689(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 17.Ronis MJJ, Butura A, Sampey BP, Shankar K, Prior RL, Korouriane S, Albanof E, Ingelman-Sundbergc M, Petersend DR, Badger TM. Effects of N-acetylcysteine on ethanol induced hepatotoxicity in rats fed via total enteral nutrition. Free Radic Biol Med. 2005;39:619–630. doi: 10.1016/j.freeradbiomed.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raza M, Ahmad M, Gado A, Alshabanah OA. A comparison of hepatoprotective activities of aminoguanidine and N-acetylcysteine in rat against the toxicdamage induced by azathioprine. Comp Biochem Physiol. 2003;134:451–456. doi: 10.1016/s1532-0456(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 19.Nishino Y, Takemura S, Minamiyama Y, Kazuhiro H, Tetsuya O, Inoue M, Okada S, Kinoshita H. Targeting superoxide dismutase to renal proximal tubule cells attenuates vancomycin-induced nephrotoxicity in rats. Free Radic Res. 2003;37:373–379. doi: 10.1080/1071576031000061002. [DOI] [PubMed] [Google Scholar]
- 20.Antunes LM, Darin JD, Bianchi NL. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001;43:145–50. doi: 10.1006/phrs.2000.0724. [DOI] [PubMed] [Google Scholar]
- 21.Ye JF, Zhu H, Zhou ZF, Xiong RB, Wang XW, Su LX, Lou BD. Protective mechanism of andrographolide against carbon tetrachloride-induced acute liver injury in mice. Biol Pharm Bull. 2011;34:1666–1670. doi: 10.1248/bpb.34.1666. [DOI] [PubMed] [Google Scholar]
- 22.Ilavenil S, Kaleeswaran B, Ravikumar S. Protective effects of lycorine against carbon tetrachloride induced hepatotoxicity in Swiss albino mice. Fundam Clin Pharmacol. 2012;26:393–401. doi: 10.1111/j.1472-8206.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Gao M, Sun S, Bi A, Xin Y, Han X, Wang L, Yin Z, Lou L. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem Biophys Res Commun. 2012;422:344–350. doi: 10.1016/j.bbrc.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Termini J. Hydroperoxide-induced DNA damage and mutations. Mutat Res. 2000;450:107–124. doi: 10.1016/s0027-5107(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang FG, Liu SR, Shen YQ, Zhuang RX, Xi JJ, Fang HY, Pan XW, Sun JJ, Cai ZB. Protective effects of N-acetylcysteine on cisplatin- induced oxidative stress and DNA damage in HepG2 cells. Exp Ther Med. 2014;8:1939–1945. doi: 10.3892/etm.2014.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 27.Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatoma cells from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf-2 pathway. J Hepatol. 2007;47:253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Patel R, Maru G. Polymeric black tea polyphenols induce phase II enzymes via Nrf2 in mouse liver and lungs. Free Radic Biol Med. 2008;44:1897–1911. doi: 10.1016/j.freeradbiomed.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in gingko biloba (EGB 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


