Abstract
Expression of podoplanin in glial brain tumors is grade dependent. While serving as a marker for tumor progression and modulating invasion in various neoplasms, little is known about podoplanin function in gliomas. Therefore we stably transfected two human glioma cell lines (U373MG and U87MG) with expression plasmids encoding podoplanin. The efficacy of transfection was confirmed by FACS analysis, PCR and immunocytochemistry. Cells were then sorted for highly podoplanin expressing cells (U373Phigh/U87Phigh). Transfection did not influence the production of pro-angiogenic factors including VEGF, VEGF-C and D. Also, expression of VEGF receptors (VEGFR) remained unchanged except for U87Phigh, where a VEGFR3 expression was induced. U373Phigh showed significantly reduced proliferation as compared to mock transfected group. By contrast, podoplanin significantly increased migration and invasion into collagen matrix. Furthermore, conditioned media from Phigh glioma cells strongly induced tube formation on matrigel. In conclusion, podoplanin increased migration of tumor cells and enhanced tube formation activity in endothelial cells independent from VEGF. Thus, podoplanin expression may be an important step in tumor progression.
Keywords: Podoplanin, glioma, angiogenesis
Introduction
Podoplanin (PDPN), a transmembrane mucin-like protein, is broadly associated with lymphatic endothelium and lymphangiogenesis where it augments the separation of blood and lymphatic vessels during embryonic development. A more widespread PDPN expression has been described in various human tumor settings, where platelet aggregation by podoplanin through C-type lectin-like receptor-2 (CLEC-2) has been linked to the formation of metastasis [1] as well as PDPN has been linked to cytoskeleton regulation and thus increased migration and invasion.
In gliomas a grade dependent expression of podoplanin is usually found on tumor cells (apart from anaplastic oligodendrogliomas, where podoplanin is found also on tumor endothelium) [3]. Given the infiltrative and angiogen-ic behavior of these tumors and the strong expression of PDPN, we investigated its impact on glioma cell behavior and its potential effects on tumor-endothelial interaction in vitro.
Materials and methods
Cell lines and culture conditions
Human glioma cell lines U87MG and U373MG were purchased from ATCC. Human brain microvascular endothelial cells (HBMVEC) were purchased from, ScienCell Research Laboratories, (Provitro, Berlin, Germany). Human brain microvascular endothelial cells (HBMEC) were obtained from ScienCell Res. Labs. (Carlsbad, USA). Tumor cells were cultured at 37°C and 5% CO2 in DMEM containing Glutamine, 10% heat inactivated FCS and Streptomycin. HBMEC were cultured in endothelial cell medium (Promocell, Heidelberg).
Podoplanin transfection
For a stable transfection a cDNA fragment encoding human podoplanin was subcloned into the commercially available over-expression vector pcDNA3 (Invitrogen, Karlsruhe, Germany).
The cDNA resulted from a PCR using primer designed with the software HUSAR sequence analysis tool (DKFZ, Heidelberg, Germany) and synthesized by Metabion (Martinsried, Germany). To subclone the cDNA into the pcDNA3 vector enzymatic restriction was performed with the enzyme EcoR1 followed by ligation. The resulted vector (pcDNA3-Podoplanin) and empty vector controls (pcDNA3) were then stably transfected into the U87MG and U373MG glioma cell lines by utilizing the electroporation method.
For this method vectors were linearized by restriction with BglII, then isolated with QIAquick gel extraction kit (Qiagen, Hilden, Germany) and resuspended in 30 µl of sterile water. Together with 8×106 glioma cell lines these vectors were filled into electroporation cuvettes (4 mm, PeqLab) and the electroporation was performed at 230-260 mV and 960 µF.
Subsequently stably transfected glioma cells were selected by supplementation of 300 µg/ml G418 (Invitrogen, Karlsruhe, Germany) to the culture medium.
Resulting glioma cell lines overexpressing podoplanin were named U373Phigh, U87Phigh and the mock variants were named U373mock and U87mock.
FACS analysis
Flow cytometry was performed as described previously [4] using the FACS Calibur (BD Biosciences, Heidelberg, Germany). Podoplanin was detected with murine monoclonal antibodies directed against human podoplanin (mc, clone 18H5, Acris Antibodies, Hiddenhausen, Germany). For detection anti-mouse antibodies (PE, Dako, Glostrup, Denmark) were used, respectively.
Real-time PCR
RNA was isolated from human glioma specimens (glioblastoma (GBM) n=8, astrocytoma WHO°II (AII) n=5, non neoplastic brain (NNB) n=3), U373 and U87 (wt, mock, Phigh) using an RNA extraction kit used according to the manufacturer’s instructions (RNeasy Mini Kit, Qiagen, Hilden, Germany). TaqMan RT-PCR was performed as described [5]. Commercially available pre-developed TaqMan reagents were used for hVEGF-C, hVEGF-D, hVEGFR1, hVEGFR2, hVEGFR3, h-podoplanin, (Assay on Demand), and for hGAPDH and rRNA (PDAR). The following additional probe was generated: hVEGF-A sense primer GCCTTGCTGCTCTACCTCCAC, antisense primer ATGATTCTGCCCTCCTCCTTCT, probe: AAGTGGTCCCAGGCTGCACCCAT. All Taqman reagents were obtained from Applied Biosystems, Warrington, UK.
Proliferation assay
The colorimetric [3-(4,5-dimethylthiazol-2-yle)2,5-diphenyltetrazolium bromide] (MTT) assay was performed to quantify the cell proliferation. 2×103 cells per well were incubated in 96-well microplates (coated with collagen I, 4%) with DMEM (± 10% FCS) medium. After 24, 48 and 72 hours, medium was removed and replaced by 50 µl of 1 mg/ml MTT (Sigma Aldrich) in EC medium. Following 4 hrs incubation in this con-dition, 100 l DMSO solution was added and the plates were incubated for 60 minutes at 37°C to allow crystal solving. The optical density of each condition was determined using a Labsystems multiscan microplate reader (VWR, Dorset, UK) at a wavelength of 540 nm with a reference wavelength of 630 nm. Each experimental condition was repeated 3 times. All assays were done in triplicate.
Spheroid co-culture
HBMEC spheroids were also produced in hanging-drop technique. HBMEC and tumor cells were stained with CellTracker® Red and green respectively (Molecular Probes, Life Technology, Darmstadt, Germany). HBMEC spheroids were individually placed in agar-coated 96-well plates. Tumor spheroids of the same size were transferred to the wells with the brain aggregates, and 200 µl of medium was added. The spheroids were then placed against each other, using a sterile syringe. Co-cultures were photographically documented every 12 hours.
Collagen sprouting assay
Tumor cells were allowed to form spheroids using the “hanging drop” technique. 5000 cells were suspended in 50 µl medium and set on plastic petri wells, which were kept upside down in humid conditions. After 48 hours spheroids of identical size were harvested. For collagen gel assay a collagen (Cohesion, Palo Alto, USA), solution was made (7.5 ml collagen solution, 1 ml 10 x DMEM, 1.5 ml NaHCO3 [15.6 mg/ml]) under cold conditions. Wells from a 96-well plate were filled with 50 µl of solution and incubated at 37°C for three hours to allow geling. Spheroids were picked under a microscope and set in the centre of the well. A second amount of 30 µl collagen solution was added followed by another geling period. Medium was added (100 µl)/well, imag-es were taken under a Zeiss Axioskop 2 light microscope (Zeiss, Jena, Germany) microscope at time points 0, 24, 48, 72 hours. Area of migration was calculated by use of DatInf-Measure software (DatInf Inc., Tuebingen, Germany). All assays were done in triplicate.
Immunohistochemistry
A series of 20 glioblastomas (GBM, WHO IV) and 20 low grade astrocytomas (WHO II) were stained for podoplanin expression. Specimens were fixed in 10% para-formaldehyde immediately after excision and embedded in paraffin. Remaining tissue from large specimens was snap frozen and stored in nitrogen for RNA and protein isolation. Tissue sections were deparaffined in xylol and re-hydrated in a graded series of ethanol. Endogenous peroxidase activity was eliminated by 0.3% methanolic hydrogen peroxide. Antigen retrieval was performed by sodium-citrate method and microwave heating for 20 minutes followed by a cooling down step of 30 minutes. After washing in TBS/0.5% TWEEN-20, slides were incubated for 30 minutes with a protein-block solution (DakoCytomation, Glostrup, Denmark). Then slides were incubated with mouse monoclonal (mc) antibody against human podoplanin [1:100] (Acris, Hiddenhausen, Germany, clone 18H5) for 30 minutes at room temperature. After thorough washing with TBS/0.5% TWEEN-20 three times between each step, sections were incubated with anti-rabbit/anti-mouse biotinylated linker and streptavidin-HRP (both from DakoCytomation, Glostrup, Denmark) subsequently. Visualization of the Biotin/Streptavidin complex was done by 0.05% diaminobenzidine dissolved in TBS containing 0.01% hydrogen peroxidase. Negative control was performed by use of non specific primary antibodies and omitting the primary antibodies.
Immunofluorescence and -cytochemistry
For staining, cells were plated on gelatin coated Lab-Tek chamber slides (Nalge Nunc Inc., Maperville, USA). After washing, cells were incubated with antibodies against podoplanin (clone 18H5, Acris Antibodies, Hiddenhausen, Germany [1:100]. Secondary stain was performed with either FITC-conjugated secondary antibodies [1:100] (both from DakoCytomation, Glostrup, Denmark). Nuclear counter-stain was performed with DAPI (Hoechst, Frankfurt, Germany). Images were taken by a Canon Powershot camera on a Zeiss immunofluorescence microscope (Axiovert 25, Zeiss, Jena, Germany). Negative control was performed by use of non specific primary antibodies and omitting the primary antibodies.
Tube formation assay
96 well plates (Nunc, Wiesbaden, Germany) were coated with growth factor reduced Matrigel (BD Biosciences, Heildelberg, Germany) 50 µl/well. Human brain microvascular (HBMEC) were stained with CellTracker® Red (Molecular Probes, Life Technology, Darmstadt, Germany) and were added to matrigel coated wells in different media in presence or absence of tumor cells. Images were taken 18 hours after plating the cells using a ZEISS Axioskop 2 light microscope (Zeiss, Jena, Germany). All assays were done in triplicate.
Statistical analysis
Data are expressed as mean ± standard derivation. Values were compared using Mann-Whitney-U-test. A two-sided P-value of < 0.05 was considered to indicate statistical significance.
Results
Podoplanin in GBM is expressed predominantly in cells scaffolding vascular structures
All GBM specimens showed a strong PDPN expression, which were mainly focused on areas of high vessel density with a strong signal on tumor cells adjacent to vessels. Endothelium itself did not show reactivity (Figure 1). Low grade gliomas did not show podoplanin expression (data not shown).
Figure 1.
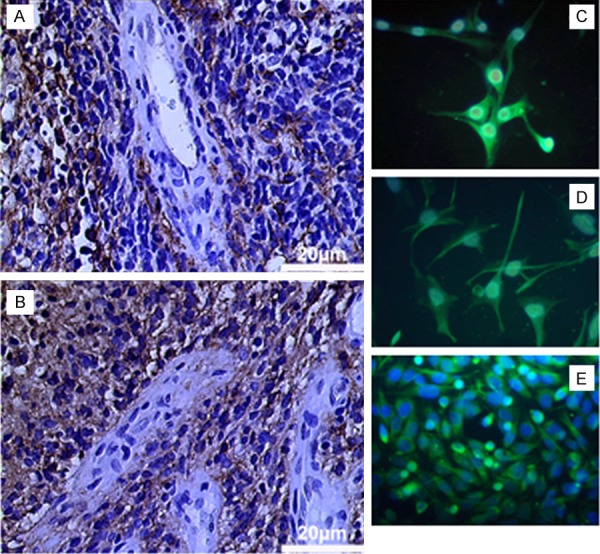
Podoplanin expression in human glioblastoma: tumor cells scaffolding vascular structures show strong expression of PDPN (A, B). Primary cultures show a stable expression of PDPN during cell culture (E, passage 6). Transfected U373Phigh (C) and U87Phigh (D) show strong expression of PDPN.
Primary cultures from four GBM showed strong immunoreactivity for podoplanin in vitro which remained stable during culture (Figure 1).
Human glioma cells stably tranfected for podoplanin
Wild type U373MG cells showed no endogenous expression of podoplanin, while wtU87 showed a weak basal expression. After transfection and selection of stably over expressing cells, a high expression of podoplanin was evidenced in both cell lines (U373Phigh and U87Phigh). Expression was documented by FACS, PCR and immunocytochemistry. Podoplaninhigh cells were separated by FACS sorting and cultured under standard medium conditions using G418 supplement (Figure 2).
Figure 2.
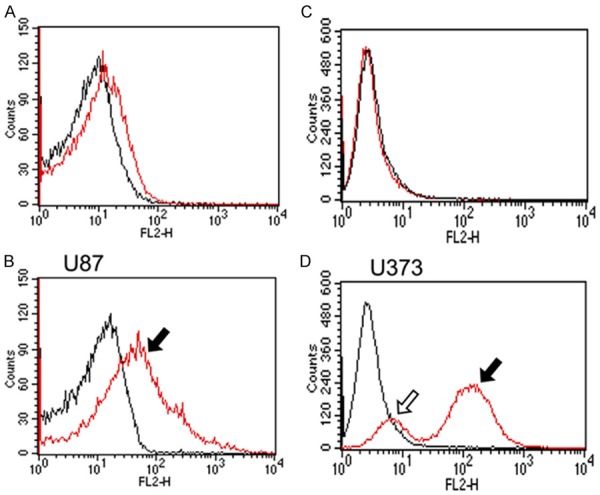
FACS analysis of transfected U373 and U87 cells. (red lines A & B: U373Pmock, U87Pmock, C & D: U373Phigh and U87podo). Wild type U87 (U87wt) showed a basal expression of PDPN, while U373wt and mock were negative. After transfection, PDPN high (filled arrows, U373Phigh, U87Phigh) and low (lined arrows) expressing cells could be distinguished.
Podoplanin increases invasion of tumor cells
U373Phigh and U87Phigh cells showed an increased invasion and migratory capacity compared to the mock transfected group. This effect was observed in three-dimensional collagen star bust assay. The effect was more pronounced in U373MG cells than in U87MG cells.
Podoplanin reduces tumor cell proliferatio
U373Phigh cells showed a significantly reduced proliferation after 96 hours in standard medium conditions as compared to wild type, and mock transfectants. In U87Phigh cell growth was unchanged as compared to control groups. Results were identical for serum dependent and independent growth (data shown for serum restricted conditions, Figure 3).
Figure 3.
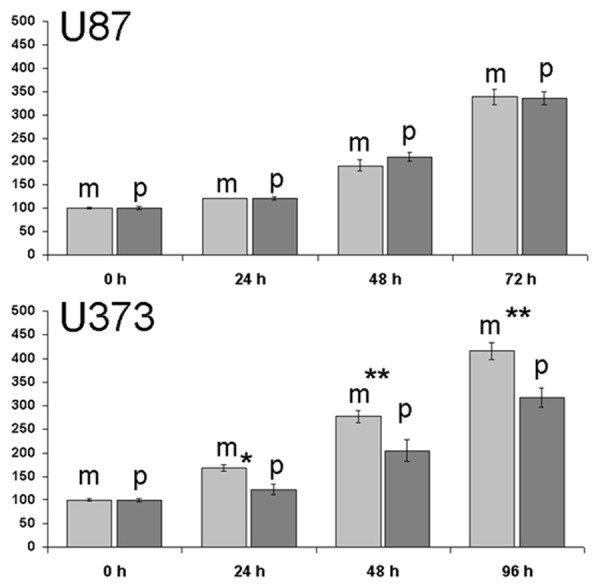
Proliferation assay showing reduced cell growth of U373Phigh compared to mock transfectants while no difference was seen for U87. Data were shown for serum restricted conditions.
Growth patterns in monolayer culture did not show any differences in the groups compared in terms of cell shape or growth pattern (data not shown).
Podoplaninhigh glioma cells enhance tube formation in human endothelial cells independent from VEGF
Tube formation on matrigel was assessed in human brain microvascular endothelial cells. All cells showed tube formation after 24 hours under standard condition, which could be further stimulated by addition of VEGF. Tumor conditioned media from U373mock and U87mock did have a stimulating effect upon formation activity compared to control group. Addition of conditioned media from U373Phigh further resulted in a significant increase of tube formation. In U87MG a similar response was seen, however, this difference did not reach statistical significance (Figure 4).
Figure 4.
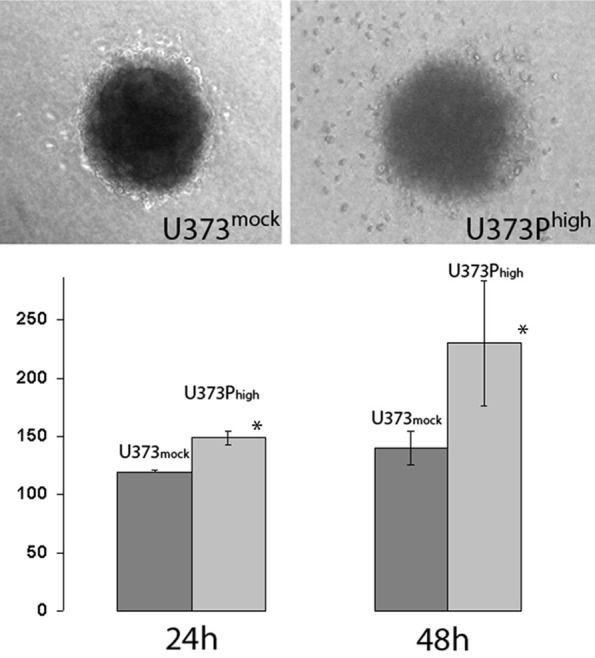
Star bust assay in collagen gel. U373Phigh showed increased migration compared to mock transfected cells. U373mock cells at time point 0 hours was set as 100% (*P < 0.03).
On the mRNA level, podoplanin did not alter the expression of VEGFR 1-3 in U373Phigh but increased VEGFR3 expression in U87Phigh (Figure 6). Levels of VEGF-A, -C or -D remained unchanged in both cell lines.
Figure 6.
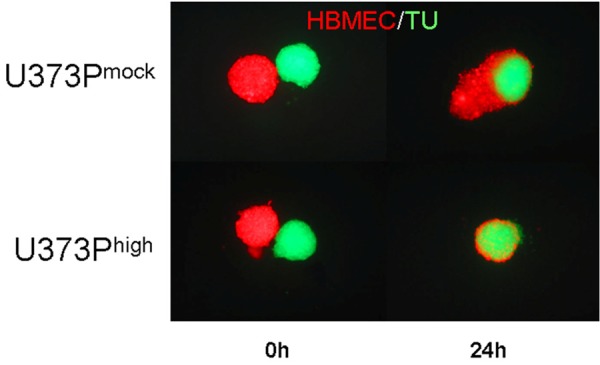
Co-culture of U373Pmock (upper panel) and U373Phigh (lower panel) spheroids with HBMEC spheroids at time point 0 (left images) and 24 hrs.
In ELISA VEGF protein expression was not influenced by podoplanin transfection (data not shown).
Podoplanin increases attraction and inclusion of HBMEC in tumor spheroids
U373Phigh and U87Phigh cells showed an increased attraction of endothelial cells compared to the mock transfected group. After 24 hours spheroids of U373Phigh cells were completely embraced and pervaded by HBMEC whilst U373Pmock showed merely an increased contact area compared to start point. The effect was more pronounced in U373MG cells than in U87MG cells (results shown for U373MG) (Figure 5).
Figure 5.
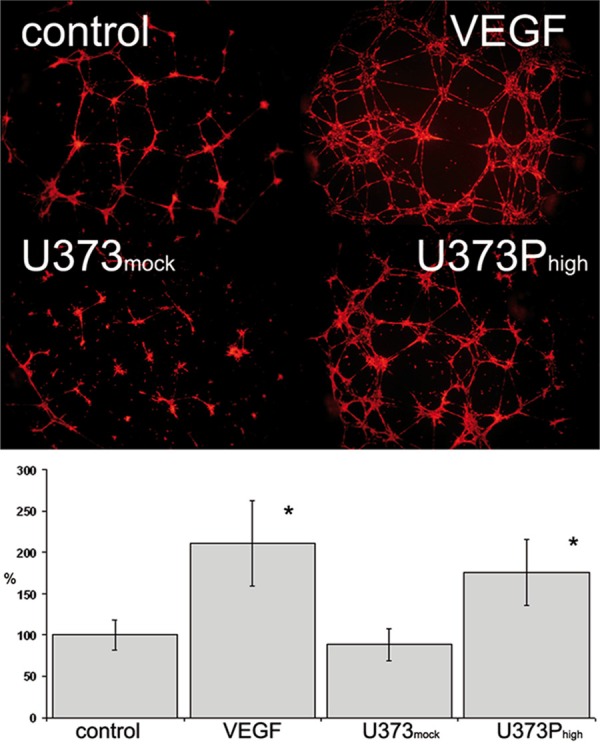
Tube formation of human brain microvascular endothelial cells on growth factor reduced matrigel. Incubation with conditioned media from U373Phigh induced tube formation significantly, no effect was seen for mock transfected (*P < 0.05).
Discussion
In contrast to several other neoplasms, podoplanin is not expressed on glioma associated endothelium but on the tumor cells themselves (with the exception of some anaplastic oligodendrogliomas) [3]. Furthermore, gliomas do not show metastatic spread, thus the functional importance of podoplanin expression by these tumors is still under discussion.
In our studies a grade dependent expression could be confirmed with all glioblastomas being positive for PDPN. In all our specimens, expression was focused on areas of high angiogenic activity with PDPN positive cells scaffolding vascular structures. Considering the importance of PDPN as a lymphatic marker, these findings might suggest a potential association of PDPN with glioma angiogenesis.
Therefore, we transfected the PDPN negative glioma cell line U373 and the line U87, which showed a weak basal expression with expression plasmids for human PDPN under the controlof the CMV promoter.
Interestingly, significant changes of cellular parameters were observed only in the U373MG cells, which basically did not express podoplanin. U87MG, which already showed a constitutive podoplanin expression, did not show significant changes induced by further expression of podoplanin. This suggests that the impact of podoplanin expression may be more related to a question of absence vs. presence rather than the degree of expression. PDPN expression did not change cellular shape or growth patterns of the tranfectants in vitro. Though PDPN expression is linked to tumor progression, PDPM expression resulted in a significantly reduced proliferation rate in U373MG cells. On the other hand, U373Phigh showed an enhanced invasive behavior in 3D collagen gels as compared to wild type and mock transfectants. This supports observations of PDPN being up-regulated at the rim of invasive tumors[9]. This invasive behavior might be based on downregulation of specific miRNAs as well as STAT3 activity [10,11].
In organ specific endothelial cells, tube formation was inducible by both VEGF and the addition of U373mock/U87mock conditioned media. This effect was significantly boosted by addition of conditioned media from U373Phigh cells.
As VEGF expression was unchanged and due to high expression of PDPN in lymphatic endothelium, we also excluded increased levels of VEGF-C and -D, both of which are highly expressed in malignant gliomas [12]. Thus, dif-ferent humoral factors may be responsible for the observed response by HBMEC. Such factors may also explain the observed attraction of HBMEC in spheroid co-cultures, which was strongly increased in U373Phigh. An additional direct cell-cell-interaction mediated by PDPN can be discussed, a fortiori an increased integration of tumor cells into vessel-like tube structures on Matrigel was observed in podoplaninhigh transfectants, however, these differences did not reach significance (data were not shown).
Our observations support a role for podoplanin not only in tumor invasion but also in angiogenic processes, which corresponds to immunohistochemical observations, where podoplanin positive cells are scaffolding tumor vessels.
Disclosure of conflict of interest
None.
References
- 1.Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, Matsuura N, Hasegawa Y, Suzuki-Inoue K, Inoue O, Ozaki Y, Narimatsu H. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99:54–61. doi: 10.1111/j.1349-7006.2007.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 3.Grau SJ, Trillsch F, von LI, Nelson PJ, Herms J, Tonn JC, Goldbrunner RH. Lymphatic phenotype of tumor vessels in malignant gliomas. Neuropathol Appl Neurobiol. 2008;34:675–679. doi: 10.1111/j.1365-2990.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- 4.Tabatabai G, Herrmann C, von KG, Mittelbronn M, Grau S, Frank B, Mohle R, Weller M, Wick W. VEGF-dependent induction of CD62E on endothelial cells mediates glioma tropism of adult haematopoietic progenitor cells. Brain. 2008;131:2579–2595. doi: 10.1093/brain/awn182. [DOI] [PubMed] [Google Scholar]
- 5.Cohen CD, Frach K, Schlondorff D, Kretzler M. Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int. 2002;61:133–140. doi: 10.1046/j.1523-1755.2002.00113.x. [DOI] [PubMed] [Google Scholar]
- 6.Mishima K, Kato Y, Kaneko MK, Nishikawa R, Hirose T, Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 2006;111:483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 7.Birner P, Pusch S, Christov C, Mihaylova S, Toumangelova-Uzeir K, Natchev S, Schoppmann SF, Tchorbanov A, Streubel B, Tuettenberg J, Guentchev M. Mutant IDH1 inhibits PI3K/Akt signaling in human glioma. Cancer. 2014;120:2440–2447. doi: 10.1002/cncr.28732. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 9.Wicki A, Christofori G. The potential role of podoplanin in tumor invasion. Br J Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priester M, Copanaki E, Vafaizadeh V, Hensel S, Bernreuther C, Glatzel M, Seifert V, Groner B, Kogel D, Weissenberger J. STAT3 silencing inhibits glioma single cell infiltration and tumor growth. Neuro Oncol. 2013;15:840–852. doi: 10.1093/neuonc/not025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez MA, Nicoloso MS, Shimizu M, Rossi S, Gopisetty G, Molina JR, Carlotti C Jr, Tirapelli D, Neder L, Brassesco MS, Scrideli CA, Tone LG, Georgescu MM, Zhang W, Puduvalli V, Calin GA. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes Cancer. 2010;49:981–990. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grau SJ, Trillsch F, Herms J, Thon N, Nelson PJ, Tonn JC, Goldbrunner R. Expression of VEGFR3 in glioma endothelium correlates with tumor grade. J Neurooncol. 2007;82:141–150. doi: 10.1007/s11060-006-9272-4. [DOI] [PubMed] [Google Scholar]


