Abstract
It is well known that taste perception influences food intake. After ingestion, gustatory receptors relay sensory signals to the brain, which segregates, evaluates, and distinguishes the stimuli, leading to the experience known as “flavor.” It is well accepted that five taste qualities – sweet, salty, bitter, sour, and umami – can be perceived by animals. In this review, the anatomy and physiology of human taste buds, the hormonal modulation of taste function, the importance of genetic chemosensory variation, and the influence of gustatory functioning on macronutrient selection and eating behavior are discussed. Individual genotypic variation results in specific phenotypes of food preference and nutrient intake. Understanding the role of taste in food selection and ingestive behavior is important for expanding our understanding of the factors involved in body weight maintenance and the risk of chronic diseases including obesity, atherosclerosis, cancer, diabetes, liver disease, and hypertension.
Keywords: chemosensation, gustatory system, taste perception
INTRODUCTION
Ingestive behavior is influenced by taste perception. Humans tend to favor pleasurable tastes and avoid those that are perceived as unpleasant. In this review, the anatomy and physiology of taste buds, the hormonal modulation of taste function, the importance of genetic chemosensory variation, and the influence of gustatory functioning on macronutrient selection and eating behavior are discussed.
The gustatory system acts as a sentinel, enabling humans to recognize and “evaluate” external chemical stimuli that enter the alimentary canal. Together with receptors of the olfactory and somatosensory systems, gustatory receptors recognize distinct characteristics of many chemicals that comprise ingested foods. Receptors relay sensory signals to the brain, which segregates, evaluates, and distinguishes the stimuli, leading to the experience known as “flavor.”1–3 The complexity of gustatory processing is now appreciated and has become an area of much interest and research. The processing of taste information is essential for mediating food preference and, as a result, weight maintenance.4 It is well accepted that five taste qualities – sweet, salty, bitter, sour, and umami – can be perceived by animals and humans. These qualities likely have an evolutionary role in nutrition and avoidance of hazardous compounds: sweet for carbohydrate source of calories, salty for minerals, bitter for harmful compounds, sour for spoiled foods, and umami for protein and amino acid content.5,6 Additionally, there is emerging evidence that lipids can be detected by fatty acid receptors on taste cells, leading to the development of a sixth taste quality for fat.7–11 The hormonal modulation of the output from taste cells can also change an animal’s relative sensitivity to tastants and possibly modify food intake. More recently, evidence has suggested that macronutrient choice and regulation of gastrointestinal function may also be affected by the oral sensory system. Understanding the role of taste in food selection and ingestive behavior is important for expanding our understanding of the factors involved in body weight maintenance and the risk of chronic diseases including obesity, atherosclerosis, cancer, diabetes, liver disease, and hypertension.
TASTE BUD ANATOMY AND PHYSIOLOGY
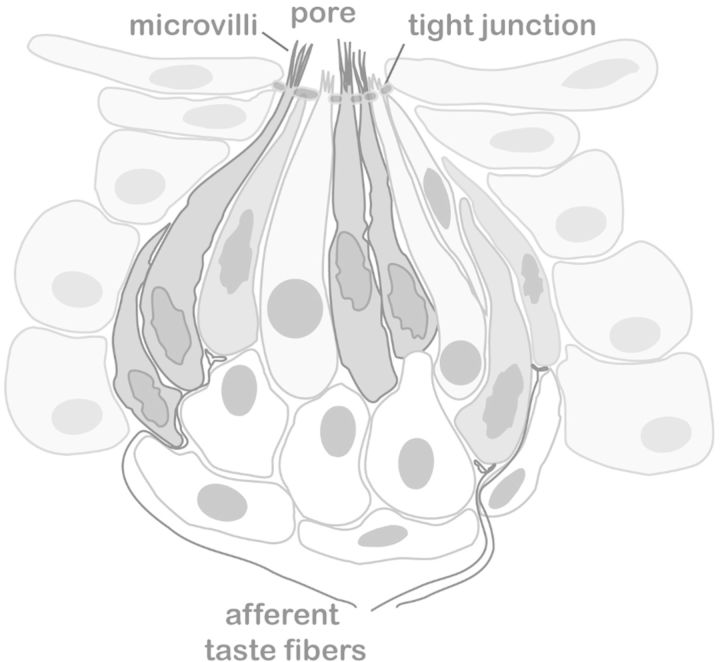
Taste buds present in the oral cavity initiate gustatory signaling. Although extreme variation can exist,12,13 humans normally have between 5,000 and 10,000 taste buds, the majority of which are distributed on the tongue surface, palate, and epiglottis.14,15 Each taste bud is, in fact, a group of between 50 and 100 neuroepithelial cells contained within the epithelium of the oral cavity (Figure 1).14–16 Individual cells are further categorized into one of three established cell types based on morphological features, protein expression, and signaling.
Figure 1.
Illustration of a taste bud. A taste bud is composed of taste receptor cells, embedded into the lingual epithelia. Gustatory transduction takes place in taste receptor cells that lie in taste buds, which are located predominantly on the tongue and soft palate. Taste receptor cells are small bipolar cells with no axon. The taste receptors are located on microvilli at the apical surface of taste receptor cells. These microvilli gain access to the oral cavity through a taste pore.
Type I cells, which are the most abundant subtype, possess cytoplasmic lamellae that envelop other taste cells.17–19 These cells express a plasma membrane-bound nucleotidase, which degrades extracellular adenosine triphosphate (ATP) and restricts neurotransmitter spread.20,21 Regulation of the extracellular ionic environment within the taste bud seems to be a key function.22 Because of their role in terminating synaptic transmission, these cells are considered to play a supporting, glial-like function within the taste bud.14 Recently, it has been suggested that type I cells may be responsible for the mediation of sodium transduction. The cellular basis for the taste of salt in mice has been linked to epithelial sodium channels, which may be expressed on type I taste cells.23–25 However, further research is needed to characterize the efficacy of this pathway in humans.
Type II cells express plasma membrane G protein–coupled receptors that bind compounds, giving rise to sweet, bitter, and umami taste perception.18,26 Although some evidence exists to the contrary,27 it has been generally accepted that individual type II receptor cells only express receptors for a specific taste quality — sweet, umami, or bitter (i.e., these three classes of taste stimuli are detected by nonoverlapping populations of taste cells within the taste bud). Sweet tastants, including artificial sweeteners, bind to a heterodimer of the G protein–coupled receptors, T1R2 and T1R3.28–33 Similarly, umami tastants bind to a dimer of T1R1 and T1R3.34,35 Stimuli that give rise to bitter taste perception are known to bind with members from the T2R family of receptor proteins.36,37 Activation of these G protein–coupled receptors results in the digestion of plasma membrane phospholipids into diacylglycerol and inositol triphosphate, which in turn mobilizes intracellular calcium.38–40 Increased intracellular calcium generated in response to tastant binding initiates membrane depolarization and the release of ATP via Calcium homeostasis modulator 1 (CALHM1) ion channels.41,42 Type II cells also express voltage-gated sodium and potassium channels that mediate the secretion of ATP as a function of action potential firing rate.43 Upon secretion, ATP acts as a neurotransmitter on nearby sensory afferent fibers44,45; ATP also acts as a paracrine/autocrine hormone, binding with receptors expressed on neighboring taste receptor cells.46–50
Type III cells release serotonin, γ-amino butyric acid, and norepinephrine and are most notable for possessing synapses.51–54 These cells, also termed presynaptic cells, express voltage-gated calcium channels associated with neurotransmitter release, enzymes for serotonin, and γ-amino butyric acid, as well as uptake transporters for biogenic amines.18 In addition to their neuronal actions, type III cells also respond to sour taste and carbonation.55–58 Interestingly, data from functional magnetic resonance imaging in humans suggest carbonation attenuates brain activity in gustatory brain regions, thus reducing sweetness perception.59 Attenuated sweetness perception may trigger sweet-seeking behaviors in some individuals and may potentially increase the risk of overconsumption of high-energy foods and development of obesity.
HORMONAL MODULATION OF TASTE FUNCTION
The complexity of the peripheral gustatory system is further enhanced by endocrine and paracrine modulation. Hormones that bind to receptors on taste cells alter the palatability of food and, therefore, intake. Current knowledge of the hormonal modulation of taste function is summarized in Table 160–77 and described in greater detail below.
Table 1.
Summary of hormonal modulation of taste function.
| Hormone | Anatomic source | Receptor | Effect on taste | Reference |
|---|---|---|---|---|
| Leptin | Adipose tissue | Ob-R | ↓ sensitivity to sweet tastants | Nakamura et al. (2008)60 |
| Di Marzo et al. (2001)61 | ||||
| Endocannabinoids | Anandamide: ubiquitous | CB1 | ↑ sensitivity to sweet tastants | Yoshida et al. (2013)62 |
| 2-arachidonoyl glycerol: CNS | ||||
| GLP-1 | Intestinal L cell | GLP-1R | ↑ sensitivity to sweet tastants | Shin et al. (2008)63 |
| ↓ sensitivity to umami tastants | Martin et al. (2012)64 | |||
| Cholecystokinin | Intestinal I cells | Cholecystokinin -A | Largely unknown but possible effect on bitter taste | Herness et al. (2002)65 |
| Lu et al. (2003)66 | ||||
| Vasoactive intestinal polypeptide | Duodenum | VPAC1/2 | Modulates sweet, bitter, sour tastants | Martin et al. (2010)67 |
| CNS/PNS | ||||
| Peptide YY | Intestinal L cells | NPYR | ↑ response to bitter tastants and fat | La Sala et al. (2013)68 |
| Hurtado et al. (2012)69 | ||||
| Neuropeptide Y | CNS/PNS | NPYR | Largely unknown but possible effect on bitter taste | Herness and Zhao. (2009)70 |
| Zhao et al. (2005)71 | ||||
| Oxytocin | Posterior pituitary gland | OXTR | Largely unknown but possible effect on sweet and salty taste | Sinclair et al. (2010)72 |
| Sclafani et al. (2007)73 | ||||
| Puryear et al. (2001)74 | ||||
| Insulin | Pancreatic beta cells | IR | ↑ response to salty tastants | Baquero and Gilbertson (2011)75 |
| Ghrelin | Stomach | G protein–coupled growth hormone secretagogue receptor | ↑ response to salty and sour tastants | Shin et al. (2010)76 |
| Galanin | CNS and gastrointestinal tract | GALR2 | Unknown | Seta et al. (2006)77 |
Abbreviations: ↓, decreases; ↑, increases; CNS, central nervous system; GALR2, G protein-coupled galanin receptor 2; GLP-1, glucagon-like peptide 1; IR, insulin receptor; NPYR, neuro peptide Y receptor; OXTR, oxytocin receptor; PNS, peripheral nervous system; VPAC, Vasoactive intestinal peptide receptor; OB-R leptin receptor; CB1, Cannabinoid receptor type 1.
Leptin
Largely produced by adipose tissue, leptin is a hormone that binds to a specific receptor (Ob-R) and is known to reduce food intake by upregulating anorexigenic neuropeptides such as α-melanocyte-stimulating hormone78 and downregulating orexigenic factors such as neuropeptide Y4.79 While leptin’s principal influence on appetite suppression is through central hypothalamic receptors, taste buds are now known to be a peripheral target of the hormone, resulting in inhibition of sweet taste. Studies have demonstrated that leptin increases potassium release from taste bud cells, reducing cell excitability. Injection of leptin into lean mice resulted in suppressed response to sweet tastants in peripheral taste nerves without effect on other taste qualities.80 Shigemura et al.81 demonstrated that the functional leptin receptor Ob-Rb messenger RNA is expressed in the fungiform and circumvallate taste buds in mice and that the presence or absence of Ob-Rb was associated with differences in leptin's effect on behavioral response to sweet substances. Leptin receptor–deficient obese mice showed increased gustatory neural response to sweet compounds. In human studies, the recognition thresholds for sweet compounds exhibited a diurnal variation from 8:00 am to 10:00 pm that parallels variation for leptin levels, with the lowest thresholds in the morning and the highest thresholds at night.60 Together, these data confirm that leptin activation of taste cells inhibits gustatory response to sweet compounds.
Endocannabinoids
Endocannabinoids, including anandamide and 2-arachidonoyl glycerol, induce appetite and food intake by binding to CB1 receptors in the hypothalamus and limbic forebrain. Studies have elucidated that endocannabinoid release is inhibited in the presence of leptin and contributes to increased food intake and, therefore, development of obesity. Di Marzo et al.61 demonstrated that defective leptin signaling is associated with elevated levels of endocannabinoids in the hypothalamus in obese mice and that acute leptin treatment reduces anandamide and 2-arachidonoyl glycerol levels. Endocannabinoids have been shown to oppose the action of leptin on peripheral taste buds as well, enhancing sweet taste. Yoshida et al.62 demonstrated that sweet taste responses are selectively enhanced by administration of endocannabinoids and that the sweet-enhancing effect of endocannabinoids is mediated by CB1 receptors, which are coexpressed in taste cells with the sweet receptor component T1R3. Also, administration of anandamide or 2-arachidonoyl glycerol increases gustatory nerve responses to sweeteners in a concentration-dependent manner without affecting responses to salty (sodium chloride), sour (hydrogen chloride), bitter (quinine), and umami (monosodium glutamate) compounds.
Glucagon-like peptide 1
Glucagon-like peptide 1 (GLP-1) is a hormone produced by the enteroendocrine L cell in response to food intake. GLP-1 enhances satiety by stimulating insulin secretion and inhibiting glucagon secretion, limiting postprandial glucose excursions, and decreasing gastrointestinal motility.82 The discovery that secretion of GLP-1 from intestinal cells is mediated, in part, by the taste receptor subunit T1R383 led to investigation of the role of GLP-1 as a paracrine hormone in gustatory function. Shin et al.63 first showed GLP-1 expression in two groups of taste cells in the mouse circumvallate papillae: α-gustducin-expressing/T1R3-expressing cells and serotonergic cells. Coexpression of GLP-1 and T1R3 suggested a potential role of GLP-1 signaling in sweet and umami taste function. GLP1-R knockout mice exhibited reduced sensitivity to both nutritive and nonnutritive sweeteners,63,64 suggesting that type II and/or type III cells may provide distinct sites for modulation of sweet taste.
Cholecystokinin
Located mainly in the I cells of the duodenal and jejunal mucosa, cholecystokinin (CCK) is a hormone released in response to food ingestion; it is mediated by a “luminal CCK-releasing factor” that is secreted from the duodenal mucosa and pancreatic cells. Physiological effects of CCK include stimulation of gastric acid, gallbladder and pancreatic secretion, decreased gastric motility, and suppression of energy intake.84 CCK is also highly expressed in the brain.85,86 Research suggests that centrally expressed CCK may also contribute to the sensation of satiety.87–89 Herness et al.65 were the first to localize the peptide CCK and its receptor, CCK-A, to subsets of taste receptor cells. It was determined that the CCK receptor is completely coexpressed in CCK-positive taste cells, suggesting an autocrine mechanism of CCK action in taste cells. Activation of the CCK-A receptor by CCK leads to inhibition of outward potassium current, inhibition of inwardly rectifying potassium current, and increased intracellular calcium. Although the exact impact of CCK signaling in the taste bud remains unknown, Lu et al.66 showed that a large fraction of CCK-responsive cells were also responsive to bitter stimuli, suggesting a role of CCK in the transduction and processing of ligands that give rise to bitter taste perception.
Vasoactive intestinal peptide
Belonging to the secretin–glucagon superfamily of peptides, vasoactive intestinal polypeptide (VIP) was first discovered in the duodenum and is widely expressed in both the peripheral and central nervous systems. VIP acts by binding to one of two G protein–coupled receptors: Vasoactive intestinal peptide receptor subtype 1 (VPAC1) or vasoactive intestinal peptide receptor subtype 2 (VPAC2); its effects include suppression of food intake and stimulation of insulin secretion in pancreatic islet cells.90 In taste cells, VIP localizes with T1R2 as well as α-gustducin91 and VPAC1/2, which are primarily expressed in type II cells of the circumvallate papillae.67 Other research suggests that VIP is almost exclusively coexpressed with CCK.70 Martin et al.67 showed that VIP knockout mice displayed altered concentration-dependent licking of sucrose, denatonium benzoate, and citric acid, validating a functional role of VIP in modulating perception of sweet, bitter, and sour tastants. VIP knockout mice also showed decreased leptin receptor expression and increased GLP-1 expression, which may explain sweet preference.
Peptide YY
Peptide YY (PYY) is a gut hormone that belongs to the pancreatic polypeptide family; it is released mainly by the L cells of the gastrointestinal tract.92,93 PYY is released in response to food ingestion. In humans, peripheral infusion of PYY at dose-mimicking normal-postprandial concentrations significantly decreased appetite and reduced food intake by 33% over a 24-h period, improving satiation.94,95 Immunohistochemistry studies have shown that PYY and all four neuropeptide Y (NPY) receptors are expressed in taste receptor cells in the circumvallate and fungiform papillae or in the oral mucosa.68,69 Disruption of PYY signaling decreases behavioral response to bitter compounds (denatonium benzoate and quinine hydrochloride) as well as corn oil fat emulsions.68 Decreased fat responsiveness would likely result in consumption of more fatty foods and suggests that PYY may be a therapeutic target for decreasing food intake.
Neuropeptide Y
NPY, like PYY, is also a member of the pancreatic polypeptide family. NPY is a principal neurotransmitter found in the central and peripheral nervous systems, predominantly in sympathetic neurons. NPY expression in the taste bud has been extensively characterized. NPY is thought to be expressed in type II taste cells in the specific subset that coexpresses T2R.70 Its expression has been shown to coincide with VIP and CCK expression (i.e., approximately 95% of taste cells that express NPY also express VIP and CCK).70,71 However, it has an antagonistic effect relative to CCK, enhancing inwardly rectifying potassium channels.71 Patch-clamp studies on isolated taste cells showed that one-third of the cells became hyperpolarized in response to NPY administration. Hyperpolarization occurred because of enhanced activity for the inward-rectifying potassium+ ion channel.70 Although direct modulation of taste perception by NPY has not been confirmed, its expression pattern suggests a possible role in bitter taste transduction.
Oxytocin
As demonstrated by reverse-transcription polymerase chain reaction, oxytocin is not expressed in taste cells but is thought to enter into the taste bud via capillary diffusion. The oxytocin receptor, on the other hand, was found on type I taste cells.72 Oxytocin is presumed to act as an endocrine hormone on taste buds by influencing type I cells and consequently the structure of the taste bud. However, mice deficient in the oxytocin receptor did not show any morphological alterations.72 Still, some studies suggest a role in the consumption of sweet and salty foods.73,74 While these studies postulate an impact on food consumption, oxytocin has not been directly linked to modulation of a specific taste quality.
Insulin
Insulin, which is a peptide hormone produced by beta cells of the pancreas, is a putative modulator of salt taste sensitivity. As previously stated, the sodium channel ENaC is expressed in taste cells and channels the influx of sodium+ into taste cells. One effect of insulin signaling in the gut is the upregulation of ENaC receptors and the increase of ENaC’s permeability.96 Reverse-transcription polymerase chain reaction data have revealed that taste cells express the insulin receptor.75 Furthermore, patch-clamp studies revealed that sodium+ influx increased significantly upon insulin administration in ENaC-responsive taste cells.75 This response was inhibited in the presence of amiloride. The ability of insulin to regulate ENaC function was dependent on phosphoinositide 3-kinase function since treatment with the inhibitor LY294002 abolished insulin-induced changes in ENaC.
Ghrelin
Ghrelin is produced primarily by endocrine cells in the stomach.97,98 It is the only potent orexigenic peptide found in circulation. Ghrelin cognate receptor is the G protein–coupled growth hormone secretagogue receptor. In taste buds, ghrelin expression is found in type I, II, and III cells; G protein–coupled growth hormone secretagogue receptor expression is largely coincident with ghrelin. G protein–coupled growth hormone secretagogue receptor–null mice are less responsive to sodium chloride and citric acid (but not sweet or bitter stimuli) relative to wild-type controls.76
Galanin
Galanin is expressed predominantly in the central nervous system and gastrointestinal tract.99 The physiological roles of galanin as a hormone remain poorly characterized, but the peptide has been linked to such diverse functions as the modulation of food intake, gut secretion, and gut motility.100,101 There are three G protein–coupled galanin receptors: GALR1, GALR2, and GALR3102,103; the expression of the GALR2 receptor has been shown in the taste bud.77 Galanin is expressed in both type II and III taste cells.77 No studies have assessed the role of galanin in the functioning of the peripheral gustatory system.
GENETIC CHEMOSENSORY VARIATION
In addition to hormonal regulation, individual variation in chemosensation alters food choice, and this variation likely has a genetic basis. A myriad of single nucleotide polymorphisms (SNPs) have been identified in the genes encoding taste receptors. SNPs in these genes result in variable sensitivity to different tastants and, therefore, personal food preference.
The ability to taste bitterness can be attributed to 1 of approximately 25 bitter taste genes; in humans, this is known as the TAS2R gene family. For example, sensitivity to 6-n-propylthiouracil (PROP) is encoded largely by TAS2R38, which has been localized to chromosome 7. Variation in one’s threshold to detect the bitter taste has been linked to several SNPs including P49A, A262V, and V269I. Haplotype analysis in one study revealed two predominant haplotypes at the three SNPs in this gene, which were named in the order of the three SNPs (A49P, V262A, and I296V) as nontaster haplotype AVI and taster haplotype PAV.104 Additional gene polymorphisms that affect sensitivity to bitterness have also been identified. W35S in the TAS2R43 gene confers sensitivity to the bitterness of the natural plant compounds aloin and aristolochic acid. W35R in the TAS2R31 (formally TAS2R44) gene increases sensitivity to the bitterness of the artificial sweetener saccharin.105 Allowing for detection of harmful cyanogenic glycosides, polymorphisms in the human TAS2R16 gene seem to serve an evolutionary purpose, as the less sensitive K172 ancestral allele is more commonly seen in those of African descent, and the more sensitive N172 variant is found in individuals of European or Asian origin.106 Individuals with the Arg299 allele for TAS2R19 find grapefruit juice to be less bitter and, therefore, more palatable.107
Understanding of genetic variations in TAS2R38 has allowed individuals to be divided by their perception of the taste of PROP. Those who perceived PROP as tasting bitter were termed super tasters or medium tasters, depending on intensity of taste sensation.108,109 Similarly, approximately 30% of individuals are considered nontasters of PROP. While the exact mechanism of phenotypic variation is unknown, characterization of bitter tasting sensation has been linked to several adverse health affects. Nontasters have shown an increased risk of alcoholism,110 higher body mass index in women,111 and dental caries in children,112 while super taster males may have an increased risk of colon cancer.113 Furthermore, the PROP-insensitive T allele (T785C) of TAS2R38 has been associated with eating behavior. Amish women with the T allele (T785C) reported higher disinhibition when eating behavior was assessed using the three-factor eating questionnaire.114
TAS1R2 and TAS1R3 are genes that encode the heterodimer of G protein–coupled receptors that are responsible for detection of natural and artificial sweeteners. The five receptor sites within the heterodimer may exist for various structural classes of sweeteners.115 Fushan et al.116 located two SNPs, C1266T and C1572T, in the promoter region of the TAS1R3 gene that were associated with variable sweetness perception, though influence on intake of sweet food was not confirmed. SNPs in a gene encoding a subunit of gustducin, a protein involved in the signaling of sweet as well as bitter and umami taste, have also been linked to perceived sweetness of sucrose.117 Eny et al.118 linked genetic variation in TAS1R with habitual consumption of sugar, suggesting a possible link of this gene with individual response to dietary counseling.
The umami receptor is also encoded by TAS1R1 and TAS1R3.34 Lugaz et al.119 showed that, like bitter taste, there seems to be a spectrum of ability to taste monosodium l-glutamate, with thresholds differing approximately five-fold between tasters and hypotasters. Shigemura et al.120 identified two SNPs that conferred variation in taste recognition threshold: TASR1-372 and TAS1R3-757 haplotypes. The TASR1 A372T SNP confers increased sensitivity to umami, while the TAS1R3 R757C SNP results in a higher threshold for detection. Two additional SNPs, C329T in TAS1R1 and C2269T in TAS1R3, have been identified in nontasters, and G1114A in TAS1R1 has been identified in tasters.121 Chen et al.122 demonstrated that variations in perception of umami taste correlate with R757C, A5T, and R247H in the human TAS1R3 gene. While many different genotypes have been elucidated, genetic variation alone cannot necessarily explain alterations in umami food intake.
MACRONUTRIENT SELECTION
The human diet consists of three macronutrients: protein, carbohydrate, and fat. Regulation of macronutrient selection is a complicated interplay of learned behaviors, hormonal signaling, and hedonics. As described above, sensitivity to tastants is affected by an individual’s hormonal milieu, which in turn affects food intake. Sensitivity to sweet tastants, influenced by leptin, endocannabinoids, VIP, and GLP-1, directly affects carbohydrate intake, which serves as the greatest source of energy for most people. More recently, much interest has surrounded gustatory regulation of fat intake.
Oral stimulation with fatty meals stimulates a myriad of physiologic responses such as gastric lipase and insulin secretion, elevation of triglycerides, and ghrelin suppression, all of which lead to appetite reduction.123 In addition to the five accepted food qualities, the presence of fatty acids in the oral cavity can be detected in both rodents and humans. More recently, a variety of fatty acid receptors, including CD36, Delayed rectifying potassium (DRKs), and several G protein–coupled receptors, have been identified on taste receptor cells. CD36, in particular, seems to play a key role in fatty acid detection. El-Yassimi et al.124 showed that linoleic acid binds to mouse gustatory cells that express CD36, triggering the release of noradrenaline and 5-hydroxytryptamine via calcium signaling. The large interindividual variation in the ability to detect oral fatty acids likely affects food intake and weight. Individuals with a higher body mass index who also report higher habitual dietary energy and fat intakes have demonstrated a decreased ability to detect fatty acids, thereby affecting body weight regulation.125
Interestingly, sensitivity to bitter taste also affects individual macronutrient selection. The majority of white individuals are sensitive to a low concentration of PROP, whereas approximately 30% are unable to taste the compound.126 In addition to the ability to taste other bitter compounds, sensitivity to PROP has also been associated with food preference. Kamphuis et al.126 demonstrated a difference in macronutrient selection among healthy adults depending on PROP tasting status. When offered a mixed meal, PROP tasters ate relatively more fat and less carbohydrate than nontasters, without a difference in energy intake, hedonics, or appetite. When compared with super tasters, nontasters enjoyed a wider variety of foods, including bitter- and strong-tasting items, and were shown to prefer high-fat dairy products and salad dressings.127 When exposed to three consecutive days of buffet consumption, young, healthy-weight women who were nontasters consumed more energy and a greater percentage of energy from fat compared with super tasters.128 Additionally, nontasters consumed more energy from between-meal snacks and sweets, while super tasters consumed more fruits and vegetables. While this was a population of lean, moderately active women, eating patterns that include high fat content, minimal fruits and vegetables, and frequent snacking are often associated with weight gain. Therefore, in addition to affecting food preference, PROP tasting status places individuals at risk for excess fat and caloric intake, potentially promoting obesity.
CONCLUSION
Taste is a key factor that impacts food intake. Although much research has been devoted to the study of the peripheral gustatory system and taste quality, current understanding of the specific interplay of receptor activation, signaling, and hormonal modulation remains complex. Genotypic variation results in various phenotypes of food preference and nutrient intake. Additionally, the hormonal milieu impacts food hedonics and macronutrient intake. Increased knowledge of chemosensory variation will allow insight into individual ingestive behavior and potentially identify therapeutic targets for chronic health problems such as obesity.
Acknowledgments
Funding. N.S. received support from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (P30 DK072488).
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Small DM. Flavor is in the brain. Physiol Behav. 2012;107:540–552. [DOI] [PubMed] [Google Scholar]
- 2.Rolls ET. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc Nutr Soc. 2012;7:488–501. [DOI] [PubMed] [Google Scholar]
- 3.Veldhuizen MG, Shepard TG, Wang MF, Marks LE. Coactivation of gustatory and olfactory signals in flavor perception. Chem Senses. 2010;35:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dotson CD, Colbert CL, Garcea M, et al. The consequences of gustatory deafferentation on body mass and feeding patterns in the rat. Am J Physiol Regul Integr Comp Physiol. 2012;303:R611–R623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartoshuk LM. Taste. In: Stevens SS, Atkinson RC, eds. Stevens’ Handbook of Experimental Psychology, 2nd ed. New York: Wiley; 1988:461–499. [Google Scholar]
- 6.Breslin PA, Spector AC. Mammalian taste perception. Curr Biol. 2008;18:R148–R155. [DOI] [PubMed] [Google Scholar]
- 7.Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. [DOI] [PubMed] [Google Scholar]
- 8.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartoni C, Yasumatsu K, Ohkuri T, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura S, Mizushige T, Yoneda T, et al. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed Res. 2007;28:49–55. [DOI] [PubMed] [Google Scholar]
- 11.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. [DOI] [PubMed] [Google Scholar]
- 12.Smith AA, Farbman A, Dancis J. Tongue in familial dysautonomia, a diagnostic sign. Am J Dis Child. 1965;110:152–154. [DOI] [PubMed] [Google Scholar]
- 13.Smith A, Farbman A, Dancis J. Absence of taste-bud papillae in familial dysautonomia. Science. 1965;147:1040–1041. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doty RL. Gustation. WIRES Cogn Sci. 2012;3:29–46. [DOI] [PubMed] [Google Scholar]
- 16.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. [DOI] [PubMed] [Google Scholar]
- 17.Kinnamon JC, Taylor BJ, Delay RJ, et al. Ultrastructure of mouse vallate taste buds. I. Taste cells and their associated synapses. J Comp Neurol. 1985;235:48–60. [DOI] [PubMed] [Google Scholar]
- 18.Roper SD. Taste buds as peripheral chemosensory processors. Sem Cell Dev Biol. 2013;24:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DL, Sullivan SL, Lavoie EG, et al. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandenbeuch A, Anderson CB, Parnes J, et al. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A. 2013;110:14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, et al. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009;517:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandrashekar J, Kuhn C, Oka Y, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida R, Horio N, Murata Y, et al. NaCl responsive taste cells in the mouse fungiform taste buds. Neuroscience. 2009;159:795–803. [DOI] [PubMed] [Google Scholar]
- 26.Yang R, Tabata S, Crowley HH, et al. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol. 2000;425:139–151. [DOI] [PubMed] [Google Scholar]
- 27.Kusuhara Y, Yoshida R, Ohkuri T, et al. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol. 2013;591:1967–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmanov AA, Li X, Reed DR, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitagawa M, Kusakabe Y, Miura H, et al. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–242. [DOI] [PubMed] [Google Scholar]
- 30.Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. [DOI] [PubMed] [Google Scholar]
- 31.Montmayeur JP, Liberles SD, Matsunami H, et al. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. [DOI] [PubMed] [Google Scholar]
- 32.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. [DOI] [PubMed] [Google Scholar]
- 33.Sainz E, Korley JN, Battey JF, et al. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem. 2001;77:896–903. [DOI] [PubMed] [Google Scholar]
- 34.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Staszewski L, Xu H, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci U S A. 2002;99:4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adler E, Hoon MA, Mueller KL, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. [DOI] [PubMed] [Google Scholar]
- 37.Chandrashekar J, Mueller KL, Hoon MA, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. [DOI] [PubMed] [Google Scholar]
- 38.Zhao FL, Herness S. Resynthesis of phosphatidylinositol 4,5-bisphosphate mediates adaptation of the caffeine response in rat taste receptor cells. J Physiol. 2009;587:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Majerus PW. Phosphatidylinositol signalling reactions. Sem Cell Dev Biol 1998;9:153–160. [DOI] [PubMed] [Google Scholar]
- 41.Taruno A, Vingtdeux V, Ohmoto M, et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang YA, Roper SD. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata Y, Yasuo T, Yoshida R, et al. Action potential-enhanced ATP release from taste cells through hemichannels. J Neurophysiol. 2010;104:896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finger TE, Danilova V, Barrows J, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. [DOI] [PubMed] [Google Scholar]
- 45.Bo X, Alavi A, Xiang Z, et al. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. [DOI] [PubMed] [Google Scholar]
- 46.Dando R, Dvoryanchikov G, Pereira E, et al. Adenosine enhances sweet taste through A2B receptors in the taste bud. J Neurosci. 2012;32:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci. 2009;29:13909–13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kataoka S, Baquero A, Yang D, et al. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS One. 2012;7:e30032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang YA, Stone LM, Pereira E, et al. Knocking out P2X receptors reduces transmitter secretion in taste buds. J Neurosci. 2011;3:13654–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baryshnikov SG, Rogachevskaja OA, Kolesnikov SS. Calcium signaling mediated by P2Y receptors in mouse taste cells. J Neurophysiol. 2003;90:3283–3294. [DOI] [PubMed] [Google Scholar]
- 51.Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. J Neurosci. 2008;28:13088–13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obata H, Shimada K, Sakai N, et al. GABAergic neurotransmission in rat taste buds: immunocytochemical study for GABA and GABA transporter subtypes. Brain Res Mol Brain Res. 1997;49:29–36. [DOI] [PubMed] [Google Scholar]
- 53.Dvoryanchikov G, Huang YA, Barro-Soria R, et al. GABA, its receptors, and GABAergic inhibition in mouse taste buds. J Neurosci. 2011;31:5782–5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y, Zhao FL, Kolli T, et al. GABA expression in the mammalian taste bud functions as a route of inhibitory cell-to-cell communication. Proc Natl Acad Sci U S A. 2009;106:4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS One. 2011;6:e25471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kataoka S, Yang R, Ishimaru Y, et al. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chandrashekar J, Yarmolinsky D, von Buchholtz L, et al. The taste of carbonation. Science. 2009;326:443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang A, Chen X, Hoon M, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Salle F, Cantone E, Savarese MF, et al. Effect of carbonation on brain processing of sweet stimuli in humans. Gastroenterology. 2013;145:537–539. [DOI] [PubMed] [Google Scholar]
- 60.Nakamura Y, Sanematsu K, Ohta R, et al. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes. 2008;57:2661–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida R, Ohkuri T, Jyotaki M, et al. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci U S A. 2010;107:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin Y, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin C, Passilly-Degrace P, Chevrot M, et al. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012;53:2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herness S, Zhao FL, Lu SG, et al. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002;22:10018–10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu SG, Zhao FL, Herness S. Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett. 2003;351:157–160. [DOI] [PubMed] [Google Scholar]
- 67.Martin B, Shin YK, White CM, et al. Vasoactive intestinal peptide-null mice demonstrate enhanced sweet taste preference, dysglycemia, and reduced taste bud leptin receptor expression. Diabetes. 2010;59:1143–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.La Sala MS, Hurtado MD, Brown AR, et al. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J. 2013; 27:5022–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurtado MD, Acosta A, Riveros PP, et al. Distribution of y-receptors in murine lingual epithelia. PLoS One. 2012;7:e46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009;97:581–591. [DOI] [PubMed] [Google Scholar]
- 71.Zhao FL, Shen T, Kaya N, et al. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005;102:11100–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinclair MS, Perea-Martinez I, Dvoryanchikov G, et al. Oxytocin signaling in mouse taste buds. PLoS One. 2010;5:e11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sclafani A, Rinaman L, Vollmer RR, et al. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puryear R, Rigatto KV, Amico JA, et al. Enhanced salt intake in oxytocin deficient mice . Exp Neurol. 2001;171:323–328. [DOI] [PubMed] [Google Scholar]
- 75.Baquero AF, Gilbertson TA. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol-Cell Physiol. 2011;300:C860–C871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shin YK, Martin B, Kim W, et al. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One. 2010;5:e12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seta Y, Kataoka S, Toyono T, et al. Expression of galanin and the galanin receptor in rat taste buds. Arch Histol Cytol. 2006;69:273–280. [DOI] [PubMed] [Google Scholar]
- 78.Seeley RJ, Yagaloff KA, Fisher SL, et al. Melanocortin receptors in leptin effects. Nature. 1997;390:349. [DOI] [PubMed] [Google Scholar]
- 79.Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377:530–532. [DOI] [PubMed] [Google Scholar]
- 80.Kawai K, Sugimoto K, Nakashima K, et al. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 2000;97:11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shigemura N, Ohta R, Kusakabe Y, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–847. [DOI] [PubMed] [Google Scholar]
- 82.Holst JJ. The physiology of glucagon-like peptide 1 . Physiol Rev. 2007;87:1409–1439. [DOI] [PubMed] [Google Scholar]
- 83.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev. 2005;6:297–306. [DOI] [PubMed] [Google Scholar]
- 85.Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. [DOI] [PubMed] [Google Scholar]
- 86.Barden N, Mérand Y, Rouleau D, et al. Regional distributions of somatostatin and cholecystokinin-like immunoreactivities in rat and bovine brain. Peptides. 1981;2:299–302. [DOI] [PubMed] [Google Scholar]
- 87.Baldwin BA, Parrott RF, Ebenezer IS. Food for thought: a critique on the hypothesis that endogenous cholecystokinin acts as a physiological satiety factor. Prog Neurobiol. 1998;55:477–507. [DOI] [PubMed] [Google Scholar]
- 88.Cheng CA, Geoghegan JG, Lawson DC, et al. Central and peripheral effects of CCK receptor antagonists on satiety in dogs. Am J Physiol. 1993;265:G219–G223. [DOI] [PubMed] [Google Scholar]
- 89.Shiraishi T. CCK as a central satiety factor: behavioral and electrophysiological evidence. Physiol Behav. 1990;48:879–885. [DOI] [PubMed] [Google Scholar]
- 90.Winzell MS, Ahren B. Role of VIP and PACAP in islet function. Peptides. 2007;28:1805–1813. [DOI] [PubMed] [Google Scholar]
- 91.Shen T, Kaya N, Zhao FL, et al. Co-expression patterns of the neuropeptides vasoactive intestinal peptide and cholecystokinin with the transduction molecules alpha-gustducin and T1R2 in rat taste receptor cells. Neuroscience. 2005;130:229–238. [DOI] [PubMed] [Google Scholar]
- 92.Gehlert DR. Multiple receptors for the pancreatic polypeptide (PP-fold) family: physiological implications. Proc Soc Exp Biol Med. 1998;218:7–22. [DOI] [PubMed] [Google Scholar]
- 93.Michel MC, Beck-Sickinger A, Cox H, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- 94.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. [DOI] [PubMed] [Google Scholar]
- 95.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–654. [DOI] [PubMed] [Google Scholar]
- 96.Blazer-Yost BL, Liu X, Helman SI. Hormonal regulation of ENaCs: insulin and aldosterone. Am J Physiol. 1998;274:C1373–C1379. [DOI] [PubMed] [Google Scholar]
- 97.Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–456. [DOI] [PubMed] [Google Scholar]
- 98.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. [DOI] [PubMed] [Google Scholar]
- 99.Tatemoto K, Rokaeus A, Jornvall H, et al. Galanin – a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. [DOI] [PubMed] [Google Scholar]
- 100.Koegler FH, Ritter S. Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol Behav. 1998;63:521–527. [DOI] [PubMed] [Google Scholar]
- 101.Wang S, Ghibaudi L, Hashemi T, et al. The GalR2 galanin receptor mediates galanin-induced jejunal contraction, but not feeding behavior, in the rat: differentiation of central and peripheral effects of receptor subtype activation. FEBS Lett. 1998;434:277–282. [DOI] [PubMed] [Google Scholar]
- 102.Branchek T, Smith KE, Walker MW. Molecular biology and pharmacology of galanin receptors. Ann N Y Acad Sci. 1998;863:94–107. [DOI] [PubMed] [Google Scholar]
- 103.Branchek TA, Smith KE, Gerald C, et al. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. [DOI] [PubMed] [Google Scholar]
- 104.Kim UK, Jorgenson E, Coon H, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. [DOI] [PubMed] [Google Scholar]
- 105.Pronin A, Xu H, Tang H, et al. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Curr Biol. 2007;17:1403–1408. [DOI] [PubMed] [Google Scholar]
- 106.Soranzo N, Bufe B, Sabeti P, et al. Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16. Curr Biol. 2005;15:1257–1265. [DOI] [PubMed] [Google Scholar]
- 107.Hayes JE, Wallace MR, Knopik VS, et al. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem Senses. 2011;36:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bartoshuk LM. Sweetness – History, preference, and genetic variability. Food Technol-Chicago. 1991;45:108–113. [Google Scholar]
- 109.Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994;56:1165–1171. [DOI] [PubMed] [Google Scholar]
- 110.Duffy VB, Davidson AC, Kidd JR, et al. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldstein GL, Daun H, Tepper BJ. Adiposity in middle-aged women is associated with genetic taste blindness to 6-n-propylthiouracil. Obes Res. 2005;13:1017–1023. [DOI] [PubMed] [Google Scholar]
- 112.Rupesh S, Nayak UA. Genetic sensitivity to the bitter taste of 6-n propylthiouracil: a new risk determinant for dental caries in children. J Indian Soc Pedod Prev Dent. 2006;24:63–68. [DOI] [PubMed] [Google Scholar]
- 113.Basson M, Bartoshuk L, Dichello S, et al. Association between 6-n-propylthiouracil (PROP) bitterness and colonic neoplasms. Dig Dis Sci. 2005;50:483–489. [DOI] [PubMed] [Google Scholar]
- 114.Dotson C, Shaw H, Mitchell B, et al. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite. 2010;54:98–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morini G, Bassoli A, Temussi PA. From small sweeteners to sweet proteins: anatomy of the binding sites of the human T1R2_T1R3 receptor. J Med Chem. 2005;48:5520–5529. [DOI] [PubMed] [Google Scholar]
- 116.Fushan A, Simons C, Slack J, et al. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fushan AA, Simons CT, Slack JP, et al. Association between common variation in genes encoding sweet taste signaling components and human sucrose perception. Chem Senses. 2010;35:579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eny KM, Wolever TM, Corey PN, et al. Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am J Clin Nutr. 2010;92:1501–1510. [DOI] [PubMed] [Google Scholar]
- 119.Lugaz O, Pillias AM, Faurion A. A new specific ageusia: some humans cannot taste L-glutamate. Chem Senses. 2002;27:105–115. [DOI] [PubMed] [Google Scholar]
- 120.Shigemura N, Shirosaki S, Sanematsu K, et al. Genetic and molecular basis of individual differences in human umami taste perception. PLoS One. 2009;4:e6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Raliou M, Boucher Y, Wiencis A, et al. Tas1R1-Tas1R3 taste receptor variants in human fungiform papillae. Neurosci Lett. 2009;45:217–221. [DOI] [PubMed] [Google Scholar]
- 122.Chen QY, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr. 2009;90:770S–779S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity—oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. [DOI] [PubMed] [Google Scholar]
- 124.El-Yassimi A, Hichami A, Besnard P, et al. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. [DOI] [PubMed] [Google Scholar]
- 125.Stewart JE, Feinle-Bisset C, Golding M, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. [DOI] [PubMed] [Google Scholar]
- 126.Kamphuis MM, Westerterp-Plantenga MS. PROP sensitivity affects macronutrient selection. Physiol Behav. 2003;79:167–172. [DOI] [PubMed] [Google Scholar]
- 127.Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Ann N Y Acad Sci. 1998;855:802–804. [DOI] [PubMed] [Google Scholar]
- 128.Shafaie Y, Koelliker Y, Hoffman DJ, et al. Energy intake and diet selection during buffet consumption in women classified by the 6-n-propylthiouracil bitter taste phenotype. Am J Clin Nutr. 2013;98:1583–1591. [DOI] [PubMed] [Google Scholar]