Abstract
Aim:
Chronic periodontal disease (CPD) and type 2 diabetes mellitus (T2DM) share common pathogenic pathways. This study aimed to estimate levels of serum interleukin (IL-10), an anti-inflammatory cytokine also associated with T2DM and evaluate its association with hyperglycemia.
Materials and Methods:
This investigation involved sixty participants divided into four groups comprising 15 participants each: Group 1 (healthy controls), Group 2 (CPD patients), Group 3 (T2DM patients with CPD) and Group 4 (T2DM patients). Plaque index, gingival index, probing pocket depths (PPD), clinical attachment loss, bleeding on probing, random blood sugar, glycosylated hemoglobin (HbA1c), and serum IL-10 was measured.
Results:
Interleukin-10 was detected in all four groups. Statistically significant (P < 0.05) differences were observed in most of the variables in all groups. IL-10 correlated significantly with PPD in Group 1 and with HbA1c in Group 4. IL-10 regressed with PPD in Group 1 and with HbA1c in Group 4. IL-10 levels were lower in Group 3 when compared with Group 4 and was lowest in Group 2.
Conclusion:
Low IL-10 levels associated with high HbA1c. Pathogenic mechanisms of CPD seem to regulate IL-10. Serum IL-10 levels may be one of the predictors of glycemia.
Keywords: Chronic periodontitis, diabetes mellitus, glycosylated, hemoglobin A, interleukin-10, type 2
INTRODUCTION
Chronic periodontal disease (CPD) is an infectious inflammatory disease, and type 2 diabetes mellitus (T2DM) is a metabolic disease due to disrupted insulin action. Diabetes and periodontitis are complex chronic diseases with an established bidirectional relationship.[1] Both have similar pathobiologic mechanisms and factors such as obesity and insulin resistance are important predecessors with inflammation being crucial in this association.[2] Nonresolving chronic inflammation has an impact on diabetes control, beta cell function, insulin resistance and the development of T2DM and its complications.[1] CPD may serve as an initiator or propagator of insulin resistance in a way similar to obesity,[2] induce or perpetuate an elevated systemic chronic inflammatory state[3] resulting in increased insulin resistance and poor glycemic control.[1,4] In addition, periodontitis may have a negative impact on T2DM control while the periodontal therapy may lead to improvements in glycemia.[5] Although the bidirectional association between the two has been established with dysregulated immune response as a pathogenic mechanism common to both, the complexity of both these diseases has an impact on our understanding of the various mechanistic links. There is less clarity about the impact of periodontal diseases on glycemic control of diabetes and the mechanisms through which this occurs.[1,2] The biologic association of diabetes mellitus and periodontal disease, though well documented in the literature, has potential in research to better understand the commonalities governing the pathobiology of these two interrelated diseases.
Effective immune responses against pathogenic microbes depend on the balance between pro-and anti-inflammatory reactions. Interleukin-10 (IL-10) is essential in regulating this balance and has gained interest recently as a modulator of the response to infection at the Janus Kinase-Signal Transducers and Activators
of Transcription (JAK-STAT) signaling axis of host responses.[6] IL-10 was first identified by its ability to stop the immune response by inhibiting the production of a number of cytokines.[7] IL-10 has been recognized to have potent and broad-spectrum anti-inflammatory activity, which has been unequivocally established in various models of infection, inflammation, and even in cancer.[8]
Molecular pathways of IL-10 signaling through JAK-STAT are not only associated with antimicrobial defense against potential pathogens, but these pathways are also involved in tolerance to commensal flora. Lactobacillus rhamnosus, for example, as a normal microbe inhabitant of the placental mucosa, triggers signaling pathways involving IL-10 and JAK-STAT to control tumor necrosis factor (TNF)-α production, which prevents preterm birth.[6,9]
Interleukin-10 is known to control or prevent production of TNF-α, IL-6 and other mediators.[6] A meta-analysis has provided strong evidence that IL-10 is associated with risk of T2DM.[10] With regard to CPD, IL-10 levels were higher in healthy controls as compared with chronic periodontitis patients[11] and lower levels of IL-10 in aggressive periodontitis has been reported.[12]
There is evidence for change in systemic cytokine levels in nondiabetics with chronic periodontitis, and whether this occurs in diabetic patients remains to be clarified.[1] Therefore, the present study had the objective of estimating the serum levels of IL-10 and its association with glycemic status in CPD and T2DM and to evaluate the impact of an inflammatory state on glycemic status.
MATERIALS AND METHODS
Sixty participants (36 males and 24 females) including healthy volunteers and patients visiting the Department of Periodontics, and the Department of General Medicine of the associated hospital were recruited in this study. An ethical clearance (Ref: 2013/S/PERIO/14) was obtained from the institutional ethical committee, and an informed written consent was obtained from all the patients before their participation in the study.
The inclusion criteria were patients in the age range of 30–55 years, presence of at least 20 natural teeth, minimum of 4 teeth with a probing pocket depth (PPD) ≥5 mm, and clinical attachment loss (AL) ≥2 mm, which were positive for bleeding on probing (BoP) and which showed radiographic evidence of bone loss when evaluated using the long cone technique, defined chronic periodontitis. The glycemic status of patients previously diagnosed with T2DM was confirmed by their glycosylated hemoglobin (HbA1c) ≥7% and random blood sugar (RBS) levels >200 mg/dl, with no major diabetic complications, a body mass index (BMI) <30 and total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglycerides (TG) within normal limits. All the groups were age and gender matched. The T2DM patients in this study were diagnosed with this disease for at least 1-year. They were on oral hypoglycemic agents/insulin as per the prescribed treatment regimen of their respective physician/endocrinologist, with no major alteration in the diabetes treatment in the past 1-year.
The exclusion criteria were smokers or those who chewed any form of tobacco, pregnancy or lactating females, immunosuppressed individuals, any condition requiring prophylactic antibiotics prior to dental treatment, prolonged bleeding as a result of any medication, any other systemic disorder that could compromise safe participation in the study and any participant who had undergone nonsurgical therapy for periodontal disease in the last 3 months or were on an antibiotic or/and anti-inflammatory drug regimen prior to the study.
A dental and medical history was recorded for the selected participants, and a single examiner conducted the intraoral examination. On the basis of their plaque index (PlI),[13] gingival index (GI),[14] PPDs, clinical AL, BoP, BMI, radiographic evidence of bone loss, total cholesterol, TG, LDL, HDL, glycated hemoglobin (HbA1c) levels (Axis-Shield, Oslo, Norway) and RBS levels (Accurex Biomedicals Pvt. Ltd., New Delhi, India), the selected participants were then divided into the following four groups of 15 each: Group 1 (healthy controls) included individuals who were nondiabetic, systemically and periodontally healthy; Group 2 (CPD only) were nondiabetic and systemically healthy and diagnosed with periodontal disease; Group 3 (T2DM with CPD) were diagnosed as type 2 diabetes with periodontal disease and Group 4 (T2DM without CPD) were diagnosed as type 2 diabetes with no evidence of CPD. Blood samples were drawn from all participants and serum IL-10 was measured using a commercially available ELISA kit (Krishgen BioSystems, Mumbai, India).
Statistical analysis
A sample of 15 individuals for each group was estimated to achieve a 95% power with an error of 5% in each of the groups. The Kolmogorov–Smirnov and Shapiro–Wilk tests were first performed to assess for normality of distribution of all the variables in all the groups. Having found a normal distribution, parametric tests were applied for the comparison of the variables, which included one-way ANOVA for comparing all the variables between the four groups and Tukey's multiple post-hoc procedures for multiple comparisons and the pairwise comparison of the variables for each group using the t-test. The Karl Pearson's correlation coefficient was used to analyze for correlations. A multiple stepwise regression analysis was done to establish a relationship between IL-10, HbA1c and clinical variables.
The statistical significance was set at P < 0.05. Data were analyzed using computer software, Statistical Package for Social Sciences (SPSS) version 20 (SPSS Inc., Chicago, IL, USA).
RESULTS
Participants (36 males, 24 females) in the four groups were aged between 30 and 55 years with the average age being 42.7 years. The mean values of all the variables of the four groups were expressed as means ± standard deviations [Table 1].
Table 1.
Mean and SD values of the PlI, GI, BoP, PPD, AL, HbA1c, RBS and IL-10 in the four groups
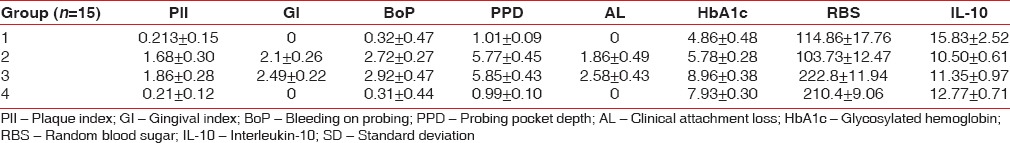
Interleukin-10 was detected (pg/ml) in all the four groups, highest (15.83 ± 2.52) in Group 1 and lowest (10.50 ± 0.61) in Group 2. Group 3 and Group 4 had 11.35 ± 0.97 and 12.77 ± 0.71, respectively.
Intergroup comparisons of the clinical and biochemical parameters are summarized in Table 2. Statistically significant differences (P < 0.05) were observed in most of the comparisons. The periodontal parameters for the groups, which were periodontally healthy (Groups 1 and 4) revealed no statistically significant differences (P < 0.05), as also in PlI, BoP, PPD, IL-10 for the two groups, which had CPD (Groups 2 and 3), and RBS for Groups 1 compared with 2 (both were systemically healthy), and Groups 3 compared with 4 (both were T2DM). Statistically significant difference (P < 0.05) was observed in HbA1c levels between Groups 1 and 2.
Table 2.
Consolidated pairwise comparison (P value) between the four groups for PlI, GI, BoP, PPD, AL, HbA1c, RBS and IL-10 (P<0.05)
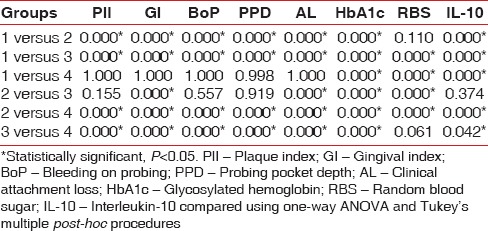
Karl Pearson's correlation coefficient was calculated to analyze for any correlations between IL-10 levels and the clinical and biochemical parameters assessed in all the four groups. IL-10 correlated significantly (P < 0.05) with PPD in Group 1 (P = 0.05) and with HbA1c in Group 4 (P = 0.05).
Stepwise multiple linear regression analysis was performed for all groups and individual groups keeping IL-10 as the dependent variable on the clinical and biochemical parameters evaluated. IL-10 related only with PPD in Group 1 and with HbA1c in Group 4.
DISCUSSION
This study was conducted to estimate and compare the concentration of IL-10 from the serum of healthy individuals and T2DM patients with and without CPD, to evaluate the relationship between an inflammatory state and glycemic status.
Gingival index[14] was selected for assessing the severity and quantity of gingival inflammation. A GI score of >1 indicated the presence of active periodontal disease.[15] Periodontal pockets and subgingival inflammation give evidence of actual periodontal infection, whereas AL and bone loss are indicators of former periodontitis that has caused tissue destruction in the past.[16] Thus, PPD ≥5 mm, AL ≥2 mm and radiographic evidence of bone loss were considered as defining CPD.[17]
Plaque index, GI and BoP were higher in the T2DM with CPD group (Group 3), consistent with other reports.[18,19] This group had mean HbA1c level of 8.96 ± 0.38 and mean values in mm of PPD 5.85 ± 0.43 and AL 2.58 ± 0.43, which is comparable to the study by Duarte et al.,[20] who reported two groups of periodontitis patients with HbA1c levels of ≤8 and ≥8, with mean PPD of 3.4 ± 0.6 and 3.7 ± 0.7 and mean AL 4.3 ± 0.8 and 4.3 ± 1, respectively.
Periodontitis is associated with increased HbA1c in individuals with and without T2DM. In people without diabetes, progression of periodontitis over 5–10 years was associated with increasing HbA1c and impaired glucose tolerance.[1] The comparison of HbA1c levels was statistically significant in all groups in this study.
Interleukin-10 is a prototypic anti-inflammatory cytokine that is produced in response to a multitude of pathogens[21] that plays a central role in limiting host immune response to pathogens, thereby preventing damage to the host and maintaining normal tissue homeostasis, dysregulation of IL-10 is associated with enhanced immunopathology in response to infection as well as increased risk for development of many diseases.[22] IL-10-mediated anti-inflammatory response (AIR) represents an essential homeostatic mechanism that controls the degree and duration of inflammation.[23] It also inhibits the secretion of inflammatory cytokines, including interferon, TNF-α, IL-1, IL-2, and granulocyte-monocyte colony-stimulating factor (GM-CSF), as well as several chemokines.[24,25,26,27]
van Exel et al.,[28] proposed that high IL-10 levels prevent the development of T2DM and metabolic syndrome by limiting the effects of the inflammatory response that is, by counter regulating the effects of pro-inflammatory cytokines such as TNF-α and IL-6. They[28] further proposed that IL-10 at least partly represents the effect of an AIR on T2DM and metabolic syndrome, based on another study that suggests IL-10 to be a key regulator and powerful suppressor of the immune response.[7] This hypothesis is partly derived from van Exel et al.,[28] findings, which suggest that when the production capacity of IL-10 is taken into account, the production capacity of TNF-α adds little to markers of T2DM, such as TG and HbA1c. van Exel et al., further reported[28] that low IL-10 production capacity is associated with T2DM, wherein the IL-10 levels decreased with an increase in HbA1c levels. While van Exel et al., conducted their study in individuals of old age, others[29,30,31,32] have corroborated the association of low levels of IL-10 with T2DM, insulin resistance and metabolic syndrome in age groups other than old age, such as middle-aged individuals, and in obese women.
In theory, higher levels of IL-10 should cause an upregulation of tyrosine kinase activity of the insulin receptor causing a decrease in lipolysis, by counter regulating the effects of TNF-α and IL-6.[33,34,35,36] Therefore, high IL-10 levels could provide protection against T2DM, whereas low IL-10 levels may predispose an individual to T2DM. However, in our study, the serum IL-10 levels exhibited a similar reported behavior in Group 3 and 4; it was significantly lower in the former as compared with the latter, implying the added influence of both the diseases on IL-10 levels.
Interleukin-10 levels were higher in healthy controls as compared with chronic periodontitis patients with a negative association between the serum level of IL-10 and the extent of BoP, PPD and AL according to Passoja et al.,[11] They also proposed that the level of IL-10 was indicative of a dose-dependent association with chronic periodontitis. Górska et al.,[37] examined the relationship between clinical periodontal variables and cytokine profiles in patients with chronic periodontitis. Their results indicate that IL-10 from inflamed gingival tissue samples and serum obtained from the periodontitis patients and healthy controls were generally low or even undetectable, and remained, on average, on the same level. However, the frequency of IL-10 (72%) was much higher in healthy gingival tissues.
In our study, IL-10 was the highest in the healthy group. IL-10 correlated and regressed significantly with PPD in Group 1 and with HbA1c in Group 4, which implies that IL-10 levels are predictable with PPD and HbA1c. The predictability of IL-10 levels changing with PPD was 46% and 35.9% for HbA1c, as per the regression analysis.
Schmidt et al.,[38] and Duncan et al.,[39] reported that a variety of inflammatory markers, including white blood cell count, low serum albumin, α1-acid glycoprotein, fibrinogen, and sialic acid, predict the development of T2DM in a middle-aged population. An ongoing cytokine-induced acute-phase response or low-grade inflammation is closely involved in the pathogenesis of T2DM,[40] and CPD is associated with changes in serum components consistent with an acute-phase response.[41] Almost 90% of all patients with T2DM show insulin resistance, which also precedes the first symptoms of diabetes. Subclinical chronic inflammation is implicated as an important pathogenic factor in the development of insulin resistance and T2DM. Surrogate markers for this low-grade chronic inflammation include CRP, IL-6 and TNF-α.[42]
In the present study, IL-10 levels were lower in patients with T2DM and CPD when compared with patients with only T2DM. The levels were lowest in the patients with only CPD, which is interesting because the anticipated IL-10 levels in Group 3 having CPD plus T2DM should have been lowest considering the influence of both the diseases. But this does not seem to have an impact on the IL-10 levels, contradicting anticipated lower levels, with no statistically significant difference between Groups 2 and 3.
We hypothesize with caution that the inflammatory mechanisms of CPD alone may have a likely role in the regulation of IL-10 considering that serum IL-10 levels were lowest in the group with CPD alone. IL-10 is independently associated with both T2DM and CPD. Demmer et al.,[43] reported a positive nonlinear association between baseline periodontal disease and incident type 2 diabetes in the National Health and Nutrition Examination Survey–I and its epidemiologic follow-up study. This association persisted regardless of the periodontal disease definition. When compared with healthy participants, individuals with intermediate levels of periodontal disease had a two-fold increased odds of incident diabetes, and the odds remained elevated among participants with the highest levels of periodontal disease. This may justify in part, our hypothesis that the lowest levels of IL-10 in CPD to have a potential role as one of the predictors for the changes in glycemic status.
CONCLUSION
It is tempting to hypothesize a link between IL-10 levels, glycemic changes, CPD, and T2DM. Since no other cytokines were assessed in this study, it is a limitation that cannot definitively conclude a relationship between serum IL-10 levels and the glycemic status in association with other cytokines.
However, we detected serum IL-10 in health, CPD, T2DM and in patients with both these diseases. Also, IL-10 levels have an inverse relationship with HbA1c in health and these diseases. Pathogenic mechanisms of CPD seem to have a potential role in the regulation of IL-10. Hence, serum IL-10 levels can be one of the predictors for glycemic alteration that may worsen glycemic control in T2DM. Longitudinal investigations involving larger samples are necessary.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chapple IL, Genco R. Working group of joint EFP/AAP workshop. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S106–12. doi: 10.1111/jcpe.12077. [DOI] [PubMed] [Google Scholar]
- 2.Mealey BL, Oates TW. American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 3.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–15. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 4.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 Suppl):2075–84. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 5.Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M. Diabetes and periodontal diseases: Interplay and links. Curr Diabetes Rev. 2011;7:433–9. doi: 10.2174/157339911797579205. [DOI] [PubMed] [Google Scholar]
- 6.Carey AJ, Tan CK, Ulett GC. Infection-induced IL-10 and JAK-STAT: A review of the molecular circuitry controlling immune hyperactivity in response to pathogenic microbes. JAKSTAT. 2012;1:159–67. doi: 10.4161/jkst.19918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 8.Mosser DM, Zhang X. Interleukin-10: New perspectives on an old cytokine. Immunol Rev. 2008;226:205–18. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeganegi M, Leung CG, Martins A, Kim SO, Reid G, Challis JR, et al. Lactobacillus rhamnosus GR-1-induced IL-10 production in human placental trophoblast cells involves activation of JAK/STAT and MAPK pathways. Reprod Sci. 2010;17:1043–51. doi: 10.1177/1933719110377237. [DOI] [PubMed] [Google Scholar]
- 10.Hua Y, Shen J, Song Y, Xing Y, Ye X. Interleukin-10 -592C/A, -819C/T and -1082A/G Polymorphisms with Risk of Type 2 Diabetes Mellitus: A HuGE Review and Meta-analysis. PLoS One. 2013;8:e66568. doi: 10.1371/journal.pone.0066568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passoja A, Puijola I, Knuuttila M, Niemelä O, Karttunen R, Raunio T, et al. Serum levels of interleukin-10 and tumour necrosis factor-a in chronic periodontitis. J Clin Periodontol. 2010;37:881–7. doi: 10.1111/j.1600-051X.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- 12.Mattuella LG, Campagnaro MB, Vargas AE, Xavier LL, Oppermann RV, Chies JA, et al. Plasma cytokines levels in aggressive and chronic periodontitis. Acta Odontol Scand. 2013;71:683–8. doi: 10.3109/00016357.2012.715191. [DOI] [PubMed] [Google Scholar]
- 13.Silness J, Loe H. Periodontal disease in pregnancy. II. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol. 1986;13:590–6. doi: 10.1111/j.1600-051x.1986.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 16.Eickholz P. Clinical periodontal diagnosis: Probing pocket depth, vertical attachment level and bleeding on probing. Periodontology. 2004;1:75–80. [Google Scholar]
- 17.Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458–67. doi: 10.1111/j.1600-051X.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed HG, Idris SB, Ahmed MF, Bøe OE, Mustafa K, Ibrahim SO, et al. Association between oral health status and type 2 diabetes mellitus among Sudanese adults: A matched case-control study. PLoS One. 2013;8:e82158. doi: 10.1371/journal.pone.0082158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J Clin Periodontol. 2013;40(Suppl 14):S135–52. doi: 10.1111/jcpe.12080. [DOI] [PubMed] [Google Scholar]
- 20.Duarte PM, Miranda TS, Lima JA, Dias Gonçalves TE, Santos VR, Bastos MF, et al. Expression of immune-inflammatory markers in sites of chronic periodontitis in patients with type 2 diabetes. J Periodontol. 2012;83:426–34. doi: 10.1902/jop.2011.110324. [DOI] [PubMed] [Google Scholar]
- 21.Duell BL, Tan CK, Carey AJ, Wu F, Cripps AW, Ulett GC. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol Med Microbiol. 2012;64:295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 22.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: Recent developments and future challenges. Brief Funct Genomics. 2013;12:489–98. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG. de Vries JE Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guichelaar T, ten Brink CB, van Kooten PJ, Berlo SE, Broeren CP, van Eden W, et al. Autoantigen-specific IL-10-transduced T cells suppress chronic arthritis by promoting the endogenous regulatory IL-10 response. J Immunol. 2008;180:1373–81. doi: 10.4049/jimmunol.180.3.1373. [DOI] [PubMed] [Google Scholar]
- 26.Joyce DA, Steer JH. IL-4, IL-10 and IFN-gamma have distinct, but interacting, effects on differentiation-induced changes in TNF-alpha and TNF receptor release by cultured human monocytes. Cytokine. 1996;8:49–57. doi: 10.1006/cyto.1996.0007. [DOI] [PubMed] [Google Scholar]
- 27.Macatonia SE, Doherty TM, Knight SC, O’Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993;150:3755–65. [PubMed] [Google Scholar]
- 28.van Exel E, Gussekloo J, de Craen AJ, Frölich M, Bootsma-Van Der Wiel A, Westendorp RG Leiden Plus Study. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: The Leiden 85-Plus Study. Diabetes. 2002;51:1088–92. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- 29.Nishida M, Moriyama T, Sugita Y, Yamauchi-Takihara K. Interleukin-10 associates with adiponectin predominantly in subjects with metabolic syndrome. Circ J. 2007;71:1234–8. doi: 10.1253/circj.71.1234. [DOI] [PubMed] [Google Scholar]
- 30.Chang JS, Chang CC, Chien E, Lin SS, Cheng-Shiuan T, Bai CH, et al. Association between interleukin 1ß and interleukin 10 concentrations: A cross-sectional study in young adolescents in Taiwan. BMC Pediatr. 2013;13:123. doi: 10.1186/1471-2431-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Straczkowski M1, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005;28:2036–7. doi: 10.2337/diacare.28.8.2036. [DOI] [PubMed] [Google Scholar]
- 32.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, et al. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–8. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins PN, Hunt SC, Wu LL, Williams GH, Williams RR. Hypertension, dyslipidemia, and insulin resistance: Links in a chain or spokes on a wheel? Curr Opin Lipidol. 1996;7:241–53. doi: 10.1097/00041433-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, et al. Association between C-reactive protein and features of the metabolic syndrome: A population-based study. Diabetes Care. 2000;23:1835–9. doi: 10.2337/diacare.23.12.1835. [DOI] [PubMed] [Google Scholar]
- 35.Feingold KR, Grunfeld C. Role of cytokines in inducing hyperlipidemia. Diabetes. 1992;41(Suppl 2):97–101. doi: 10.2337/diab.41.2.s97. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994;94:1543–9. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30:1046–52. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): A cohort study. Lancet. 1999;353:1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 39.Duncan BB, Schmidt MI, Offenbacher S, Wu KK, Savage PJ, Heiss G. Factor VIII and other hemostasis variables are related to incident diabetes in adults. The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care. 1999;22:767–72. doi: 10.2337/diacare.22.5.767. [DOI] [PubMed] [Google Scholar]
- 40.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 41.Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007–16. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 42.Sjöholm A, Nyström T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev. 2006;22:4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 43.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: Results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–9. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]


