Abstract
Background:
Many paraclinical methods are available today for an accurate assessment of the periodontal status prior and during the periodontal treatment. The microbial-enzymatic N-benzoyl-DL-arginine-2-napthylamide (BANA) test is one of the modern alternatives to bacterial cultures. It detects the presence of three periodontal pathogens in the subgingival plaque (Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia).
Aims and Objective:
The aim and objective of this study was to detect the presence of BANA micro-organisms and also to determine the effect of scaling and root planning in adult periodontitis patients.
Materials and Methods:
A total number of 20 patients (80 sites) all having periodontitis were selected. Four test sites (permanent molar from each quadrant) were selected from each patient and assessed for plaque index, bleeding index and pocket depth before and after scaling and root planning. BANA test was used for the detection and prevalence of the “red complex” bacteria in plaque samples.
Results:
Showed that the BANA tests are statistically correlated with the severity of periodontal destruction. There was a significant correlation between the BANA test results and the quantity of bacterial plaque, the test being influenced by the composition of bacterial plaque.
Conclusion:
This study encourages the use of such chair-side tests for a proper diagnosis of periodontal disease and for a good evaluation of the treatment results.
Keywords: N-benzoyl-DL-arginine-2-napthylamide test, periodontitis, red complex
INTRODUCTION
Periodontal disease is among the infectious disease caused by micro-organisms that colonize the tooth surfaces at or below the gingival margins. These micro-organisms lead to the destruction of the periodontal ligament and alveolar bone that surrounds the teeth thus causing loss of attachment to the tooth. Moreover, when these micro-organisms are attached on the tooth surface in microbial communities, they form a layer, known as dental plaque.
The overall pattern observed in dental plaque development is a very characteristic shift from the early predominance of Gram-positive, facultative micro-organisms to the later predominance of Gram-negative anaerobic micro-organisms, as the plaque mass accumulates and matures.[1]
Analysis of these periodontal pathogens is becoming an important aspect of diagnosis and treatment of periodontal diseases. However, bacterial culturing is expensive technique, sensitive and requires substantial time for completion,[2] and some organisms will not grow reliably on available culture media. Darkfield and phase contrast microscopic analysis can detect number of micro-organisms and their morphotypes, but have limited application due to their inability to fully specify these micro-organisms. Microscopic evaluation can also be helpful in detecting mobile organisms but is not effective in identifying periodontal pathogens, which are nonmotile.[3]
Until date, the diagnostic tools are based upon enzymes diagnostic markers and DNA probes in order to identify specific periodontopathic bacteria, so as to enforce preventive and therapeutic measures toward disease control.
BANA-Enzymatic test™ kit (Ora Tec Corporation, Manassas, USA) [Figure 1] is a rapid and reliable chair side diagnostic test, which can be performed in about 15 min time, that can give information about the presence of three of the putative pathogens in subgingival plaque samples, that is, Porphyromonas gingivalis, Treponema denticola and Tannerella forsythia that share unique ability of hydrolyzing the trypsin substrate, BANA.
Figure 1.
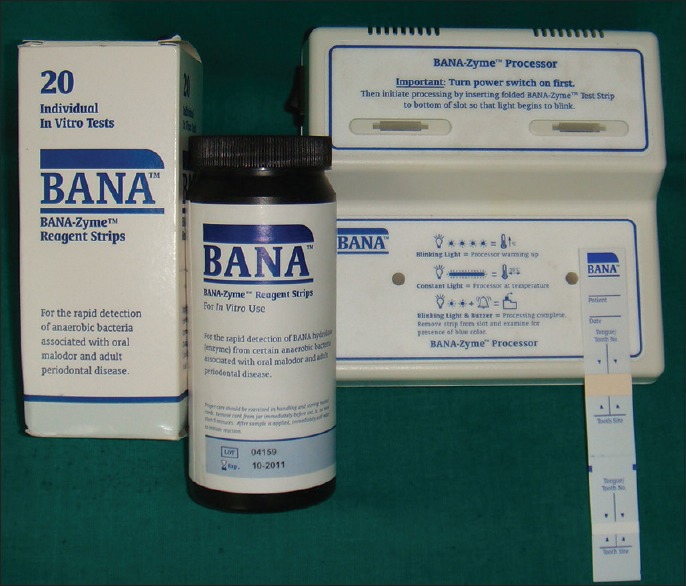
BANA-Enzymatic™ test kit
Loesche et al. studied a strong relationship between a BANA positive reaction and high levels of plaque spirochetes.[4] Vandana showed the variation of BANA positive micro-organisms in adult periodontitis before and after initial periodontal therapy.[2]
In this clinical study, attempt had been made to detect the BANA micro-organisms before and after scaling and root planning in individual suffering from adult periodontitis, using the BANA-Enzymatic™ test kit.
MATERIALS AND METHODS
A prospective, microbiological study was planned on 20 patients (80 sites), having periodontitis and were selected on the basis of clinical examination. The criteria for the selection of the patient were:
Age group with 35–55 years
Having no systemic diseases
Subjects are not having any local or systemic antimicrobial and anti-inflammatory therapy for last 6 months
No history of smoking or tobacco chewing
Pregnant women or lactating women were not selected for study
No periodontal therapy is other than standard prophylaxis during the previous 6 months.
Each patient had a minimum of 4 sites (permanent molars from each quadrant) of periodontal pockets measuring 5–7 mm in depth. The following clinical parameters, that is, plaque index (Silness and Loe),[5] bleeding index (Ainamo and Bay)[6] and periodontal pocket depth was analyzed on 0 day, that is, before treatment and 1 month after treatment.
Data analyzed employed Chi-Square test for comparison of BANA-Enzymatic™ test score at baseline and after 1 month. The accuracy of test was expressed using following formula: Sensitivity, true positive (TP)/(TP + false negative [FN]); specificity, true negative (TN)/(false positive [FP] + TN); Overall accuracy, prevalence × sensitivity + (1 − prevalence) × specificity where prevalence is TP + FN/(TP + FP + TN + FN).
Principle of N-benzoyl-DL-arginine-2-napthylamide-Enzymatic™ test
Anaerobic bacteria contain enzymes that can hydrolyze peptides, but only P. gingivalis, T. denticola, and T. forsythia possess significant amounts of a unique enzyme. Taking advantage of these unique characteristic, researchers at the University of Michigan developed a synthetic peptide, BANA to detect the presence of the shared enzyme.
The BANA-Zyme™ reagent strips are plastic cards to which separate reagent containing matrices are affixed. The lower matrix is impregnated with BANA. Subgingival plaque samples were applied to the lower matrices. The upper reagent matrix contains a chromogenic diazo reagent (fast black K). Peptidase in certain anaerobic micro-organisms associated with periodontal diseases can hydrolyze the peptide analog BANA. The upper reagent matrix, which reacts from BANA by bacterial enzyme reacts with fast black K forming a permanent blue color. The blue color of a positive or weak positive reaction appears in the upper matrix and is permanent.[7]
Blood, and saliva do not hydrolyze BANA and do not interfere with the test, but blood in the sample may obscure the visualization of the blue color.
Specimen collection and preparation
The information of the desired tooth and sites were recorded
Supragingival plaque was removed and discarded prior to sampling. First series of subgingival plaque samples were obtained by the means of a curette from the most apical portion of the periodontal pocket. Each sample was applied on the reagent matrix affixed to the lower portion of the strip in a location corresponding to the number of the tooth where the specimen was taken [Figure 2]
Before taking another specimen, wipe the curette on a clean cotton gauze pad to prevent carry-over of plaque
After sampling all desired sites, moisten the upper pad of the test strip (salmon colored pad) with distilled water using an autoclaved cotton swab
The reagent strip was folded at the crease mark so that the upper and lower matrices meet
The reagent strip was then placed into one of the two top slots of the BANA-Zyme™ processor and heated for 15 min at 55°C ± 5°C. The processor cycle begins when the indicator light comes on. Incubation is complete when the bell sounds [Figure 3]
The lower portion of the test strips was separated from the upper strip and discarded
The upper strip showed a blue color reaction and it was permanent. This was then compared with the sample chart on the BANA-Enzymatic™ test bottle label [Figure 4].
Figure 2.
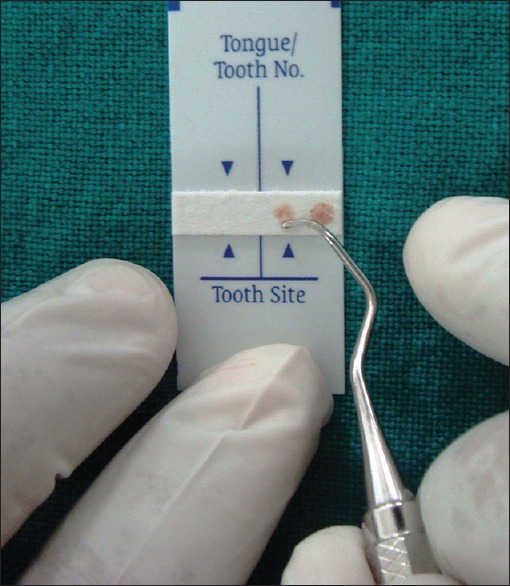
BANA -Zyme™ reagent strip with subgingival plaque sample
Figure 3.

Incubation of N-benzoyl-DL-arginine-2-napthylamide-Zyme™ reagent strip for 15 min at 55°C ± 5°C
Figure 4.

Interpretation chart
Weak Positive: Small faint trace of blue coloration on a pale red-brown background.
Positive: Patches of blue coloration somewhat larger and darker than weak positive on a pale red-brown background.
Negative: No blue color observable on a pale red-brown background.
If the BANA Test is negative in the presence of clinical disease, two possibilities exist:
It might be a technical error due to poor sampling technique or handling
It's possible that the infection involves non-BANA bacterial species.
In these cases, the BANA Test should be repeated making sure that an adequate plaque sample has been taken and carefully tested.
The upper strips were sealed with nonporous transparent tape and stored in plastic albums individually
After taking plaque samples from the selected sites and first series of plaque sampling for the BANA-Enzymatic™ test, a conventional nonsurgical periodontal treatment was done to manage periodontal disease, that is, full mouth scaling and root planning without any prescription of antibiotics and mouthwashes
Second series of subgingival plaque samples were obtained from the same selected sites after 1 month and were subjected for BANA-Enzymatic™ test.
The results obtained from both the series of samples were subjected for statistical analysis.
RESULTS
The data were tabulated and analyzed using the SPSS version 16.0 SPSS Inc. Chicago.
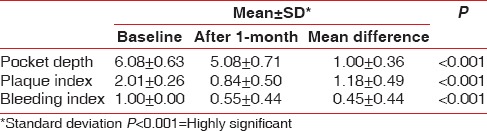
The plaque index, bleeding index and pocket depths were analyzed before and after 1 month of initial periodontal treatment and reduction in their mean values were observed with P < 0.001 that is, statistically highly significant [Table 1].
Table 1.
Comparison of mean values of pocket depth, plaque index and bleeding index at baseline and after 1-month
At baseline, that is, before treatment, 0 site showed “negative” (0.0%), 15 sites showed “weak positive” (18.8%) and 65 sites showed “positive” (81.3%). After treatment, that is, after 1 month, 27 sites showed “negative” (33.8%), 22 sites showed “weak positive” (27.5%) and 31 sites showed “positive” (38.8%). These analyzes were made using Chi-square test that showed marked reduction in bacterial count after treatment [Table 2].
Table 2.
Comparison of BANA-Enzymatic™ test scores at baseline and after 1-month

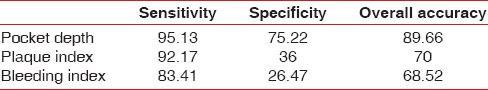
Assessment of validity of BANA test reflected a sensitivity of 92–95%, specificity of 36–75% and overall accuracy of 70–89%, using validity test [Table 3].
Table 3.
Comparison of validity test of BANA-Enzymatic™ test results
DISCUSSION
Periodontal infection is a Gram-negative, anaerobic oral infection. The bacteria responsible for this condition are capable of producing a variety of biochemical inflammatory markers that directly affect the host. Bacterial plaque contributes to the periodontal breakdown by direct injury to tissue and by stimulating host mediated responses that results in tissue destruction; direct injury is caused by both endotoxins and exotoxins produced by the bacterial mass and enzymes, secreted by bacteria to facilitate its penetration by breaking down the structure barrier.[8]
Considerable interest has been developed in methods of detecting periodontopathogens in plaque samples with the development of the diagnostic systems.[9] A diagnostic test should be useful, ideally leading to a choice of treatment that would confer benefits upon the patient. It should be simple and aimed at identifying certain forms of periodontal disease associated with some predominant periodontopathogens, which possess few common characteristics. Instead of identifying individual bacterial species on the basis of these common characteristics, a group of micro-organisms can be detected for diagnosis of periodontal disease. Here, BANA-Enzymatic test™ screen subgingival plaque samples for trypsin-like proteolytic activity that is common to only few known periodontopathogens such as P. gingivalis, T. denticola, and T. forsythia.
Most of the clinical studies showed a strong relationship between a BANA positive reaction and high levels of plaque spirochetes.[4] However, there are possibilities that other plaque species and host enzyme could be contributing to this reaction. Screening of 255 strains from 51 species does not hydrolyze BANA, that is, demonstrated a uniform BANA negative reaction, and several Capnocytophaga species were variable in giving a weak BANA reaction. Though BANA hydrolyze activity was found in Capnocytophaga species, it could be associated with the BANA reaction because Capnocytophaga species were found in very low proportions in both the BANA positive and BANA negative results.[7]
It had been seen that BANA hydrolysis by plaque samples has the potential to be the marker of periodontal morbidity as assessed by probing depth measurements and by plaque proportions of spirochetes.[10]
The plaque sample is often contaminated with ground clutter filter (GCF) and/or blood, but neither blood, saliva nor GCF was found to be able to hydrolyze BANA.[2]
A positive BANA test indicates more spirochetal load than bacterial load, as indicated by the ability of the BANA test to identify subgingival plaque with elevated spirochetes, but not elevated bacteria in the treated patients. Bretz and Loesche agreed with the previous data, which showed that BANA positive test in subgingival plaque occurred only when appreciable levels of spirochetes were present.[11]
Limitation of the BANA test is, firstly, it does not identify which of the three BANA positive species is present in plaque, but since they all are anaerobic species, it should enable the clinician to diagnose an anaerobic infection, and only such a diagnose could be useful for the treatment and management of periodontal disease of the patient. Secondly, to preserve the shelf life of the test strips, ensure that top to the dispensing bottle is tightly closed on the bottle after removing a test strip, and the desiccant is contained within the dispensing bottle. Thirdly, the unavailability of this kit in India.
The presence of a BANA positive plaque around a tooth site at the conclusion of the initial periodontal treatment indicates still presence of higher proportions of anaerobic bacteria in the periodontal pocket as a residual infection. This is also indicative of the future attachment loss, which is having potentially greater clinical significance.[2]
N-benzoyl-DL-arginine-2-napthylamide test kit result is showing weak positive reaction in four sites before treatment exhibited a positive response after initial periodontal therapy. It had been shown that conversion of BANA positive plaque to BANA negative plaque, leading to the reduction in the need of surgical intervention was significantly modified by certain host factors. These host factors are host immune responses or patient's ability to maintain the oral hygiene at an optimum level. All these intrinsic and extrinsic host factors determine the tooth site-specific response to a certain extent.[12] These possible factors might have allowed sudden growth of micro-organisms in the periodontal pocket and resulted in positive BANA reaction.
Thus, the ability of BANA to detect a particular threshold of anaerobic periodontopathic bacteria was found to be a valuable diagnostic tool for screening the individual at risk for an anaerobic infection and also as an objective indicator of periodontal disease activity that could be used in combination with the clinical criteria both to initiate therapy and as a means to monitor the efficacy of treatment.
Further research direction regarding the use of this test may include: (1) Studies to monitor the efficacy of selected clinical or antimicrobial procedures. (2) As prospective cohort studies, where risk indicators such as positive BANA test and subsequent development of clinical disease could be followed throughout time. (3) School-based screening programs to identify risk groups for periodontal diseases at an early age.
CONCLUSION
From the observation of the study, following the conclusion was drawn suggesting that BANA-Enzymatic test™ may be a potential diagnostic tool, which could be employed:
As a reliable indicator of BANA positive species in dental plaque
As a simple, chair side test to detect a BANA hydrolyses from P. gingivalis, T. denticola and T. forsythia, anaerobic bacteria associated with adult periodontal disease
As an objective means of determining diseased sites, requiring some form of periodontal treatment
It also helps in the different treatment options: Either the traditional approach of surgery or extraction of hopeless teeth or an approach based on an antimicrobial strategy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Loesche WJ, Syed SA, Schmidt E, Morrison EC. Bacterial profiles of subgingival plaques in periodontitis. J Periodontol. 1985;56:447–56. doi: 10.1902/jop.1985.56.8.447. [DOI] [PubMed] [Google Scholar]
- 2.Aparna B, Vandana KL, Mehta DS. The detection of BANA positive micro-organisms in adult periodontitis before and after the initial periodontal therapy using ‘Perioscan’-A rapid chairside diagnostic test. J Indian Dent Assoc. 1998;69:75–80. [Google Scholar]
- 3.Genco RJ, Goldman H, Cohen DW. Contemporary Periodontics. The CV Mosby Company; 1990. Pathogenesis and host response in periodontal disease; pp. 184–93. [Google Scholar]
- 4.Loesche WJ, Syed SA, Stoll J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease? J Periodontol. 1987;58:266–73. doi: 10.1902/jop.1987.58.4.266. [DOI] [PubMed] [Google Scholar]
- 5.Silness J, Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 6.Newbrun E. Indices to measure gingival bleeding. J Periodontol. 1996;67:555–61. doi: 10.1902/jop.1996.67.6.555. [DOI] [PubMed] [Google Scholar]
- 7.Loesche WJ, Bretz WA, Kerschensteiner D, Stoll J, Socransky SS, Hujoel P, et al. Development of a diagnostic test for anaerobic periodontal infections based on plaque hydrolysis of benzoyl-DL-arginine-naphthylamide. J Clin Microbiol. 1990;28:1551–9. doi: 10.1128/jcm.28.7.1551-1559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed SA, Gusberti FA, Loesche WJ, Lang NP. Diagnostic potential of chromogenic substrates for rapid detection of bacterial enzymatic activity in health and disease associated periodontal plaques. J Periodontal Res. 1984;19:618–21. doi: 10.1111/j.1600-0765.1984.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 9.Tencate JM. Biofilms, a new approach to microbiology of dental plaque. J Periodontol. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 10.Loesche WJ, Giordano J, Hujoel PP. The utility of the BANA test for monitoring anaerobic infections due to spirochetes (Treponema denticola) in periodontal disease. J Dent Res. 1990;69:1696–702. doi: 10.1177/00220345900690101301. [DOI] [PubMed] [Google Scholar]
- 11.Bretz WA, Loesche WJ. Characteristics of trypsin-like activity in subgingival plaque samples. J Dent Res. 1987;66:1668–72. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- 12.Hujoel PP, Moulton LH, Loesche WJ. Estimation of sensitivity and specificity of site-specific diagnostic tests. J Periodontal Res. 1990;25:193–6. doi: 10.1111/j.1600-0765.1990.tb00903.x. [DOI] [PubMed] [Google Scholar]


