SYNOPSIS
Evidence from basic, preclinical, and clinical research points to an important role of estradiol (E2) in the regulation of body composition and bioenergetics. There is consistent evidence from basic and preclinical research that the disruption of E2 signaling, through either genetic manipulation (e.g., estrogen receptor deletion) or surgical intervention (e.g., ovariectomy), accelerates fat accumulation, with a disproportionate increase in abdominal fat. Clinical evidence for the regulation of body composition and bioenergetics by E2 is less consistent. Evidence exists both for and against menopause as the mediator of changes in body composition. This is likely related to the prolonged nature of the menopause transition in women and the associated complexities of distinguishing effects of the loss of gonadal function from other phenomena of aging. However, a need remains to better understand the metabolic actions of estrogens in women because of the potential impact on health after the menopause.
Keywords: menopause, adiposity, body composition, estradiol, estrogen receptor, energy expenditure, energy intake, ovariectomy
INTRODUCTION
There is growing evidence that estradiol (E2) is an important regulator of body composition and bioenergetics. The wide distribution of estrogen receptors (ERs) and their involvement in genomic and non-genomic signaling pathways1 suggests that the loss of E2 at menopause is likely have pronounced effects on numerous factors other than reproduction2. ER expression in the brain, adipose tissue, and skeletal muscle demonstrates the potential role of E2 in body weight regulation and other metabolic processes. Further, the presence of mitochondrial ERs3 suggests a role of E2 in the regulation of cellular bioenergetics. This review will discuss findings from basic, preclinical, and clinical studies that provide insight on the role of E2 and ER signaling in the regulation of energy storage (i.e., fat accrual), regional fat distribution, and energy balance (i.e., energy expenditure and intake).
BASIC RESEARCH
Estrogens have many physiological effects that were long thought to be due to a single receptor, ERα4. However, the discovery of a second receptor, ERβ5, and the recognition that ERs are present not only in the nucleus but also in the plasma membrane6, have advanced the understanding of the metabolic actions of estrogens.
The systemic actions of estrogens are mediated through ER signaling. This can occur through nuclear ERs and the consequent transcription of multiple genes7, or through membrane-bound ERs that mediate rapid, non-genomic effects of estrogens8. E2 binds to ERα and ERβ with equal affinity9. However, ERα and ERβ have distinct and sometimes opposing actions, indicating that the ratio of ERα to ERβ may be an important determinant of tissue-specific responses to E210–12 Both ER subtypes appear to be present in most, if not all, body tissues, but in varying proportions13–15. Knowledge regarding the effects of ER signaling has been advanced through the use of transgenic mice that have deletions of ERα and/or ERβ throughout the body16, 17, in specific cells or tissues18–20, or at the molecular level (nuclear vs membrane)21–23.
Regulation of adiposity by E2
The importance of ER signaling in the regulation of adiposity was highlighted by the discovery of Heine and colleagues that a whole-body knockout of ERα (αERKO) resulted in increased fat accrual in both females and males when compared with wild type (WT) mice24. By 90 days of age, the parametrial and inguinal fat pads were 2-fold larger in female αERKO mice than controls as a result of increased adipocyte size and number. The αERKO mice were also more insulin resistant and glucose intolerant than WT mice, consistent with the excess adiposity. Subsequent studies confirmed that the deletion of ERα increases adiposity in female mice21–23.
The excess fat mass in αERKO mice suggests that ERα plays a protective role against fat accumulation. However, another possibility is that removal of ERα promotes fat accumulation through increased ERβ signaling. One strategy that has been used to test this possibility is to ovariectomize (OVX) αERKO mice to reduce circulating E2, thereby diminishing ERβ signaling25. Indeed, the increase in fat mass that occurred in αERKO-sham mice was attenuated in αERKO-OVX mice. Further, when αERKO-OVX mice were treated with E2, thereby increasing ERβ signaling, fat mass increased to the level of αERKO-sham mice25. The deletion of ERβ in mice (i.e., βERKO) does not result in excess fat mass26 or body mass27 when compared with WT mice, providing additional evidence that the increased fat accumulation in αERKO mice is mediated, at least in part, through increased ERβ signaling. However, ERα also plays a protective role against fat accumulation. When ERα signaling was reduced in βERKO mice through OVX, there was an excess gain in body mass and adiposity27. Finally, when both ERα and ERβ are absent (i.e., double knockout; DERKO), the αERKO phenotype of increased adiposity dominates26.
These studies of the genetic manipulation of ERs in mice demonstrate the complex regulation of body fat accrual by E2. In general, ERα protects against fat accumulation whereas ERβ promotes fat gain. The actions of ERα appear to dominate among inbred mice, but this may depend on the relative density and distribution of ERs under conditions of genetic heterogeneity (e.g., outbred animals, humans). Additional evidence that the net effect of E2 is to prevent excess fat accumulation comes from studies that reduce serum E2 through the deletion of the enzyme that converts androgens to estrogens (i.e., aromatase). Regional and total body adiposity is roughly 2-fold higher in aromatase knockout (ArKO) mice than controls by 12 weeks of age and this difference persists as mice age28. The regulation of adiposity by E2 is further complicated by the discovery of G protein-coupled receptors that associate with E2, known as G protein-coupled ER 1 (GPER1 or GPR30)29, 30. There is some evidence that deletion of this receptor increases fat gain31, but this has not been a consistent observation32.
The mechanistic signaling pathways by which E2 regulates fat accumulation remain unclear. Studies that utilized tissue-specific silencing of ERs are beginning to provide some insight for the locus of regulation. The deletion of ERα in the central nervous system (CNS) (e.g., ventromedial nucleus or specific neurons in the hypothalamus) appears to18, 20 play a pivotal role, but systemic deletions (e.g., whole bone marrow or cells of myeloid lineage) also result in excess body weight gain and fat accumulation19.
Collectively, basic research has established a solid foundation of evidence that E2 plays an important role in the regulation of adiposity. Genetic manipulations that disrupt ERα signaling by deleting the receptor (i.e., αERKO and DERKO models) or reducing the ligand (i.e., ArKO model) cause excess fat gain, whereas disrupting only ERβ signaling (i.e., βERKO model) does not (Figure 1). Thus, the dominant action of E2 on the regulation of body composition is to protect against fat accumulation and this is mediated primarily through ERα.
Figure 1.
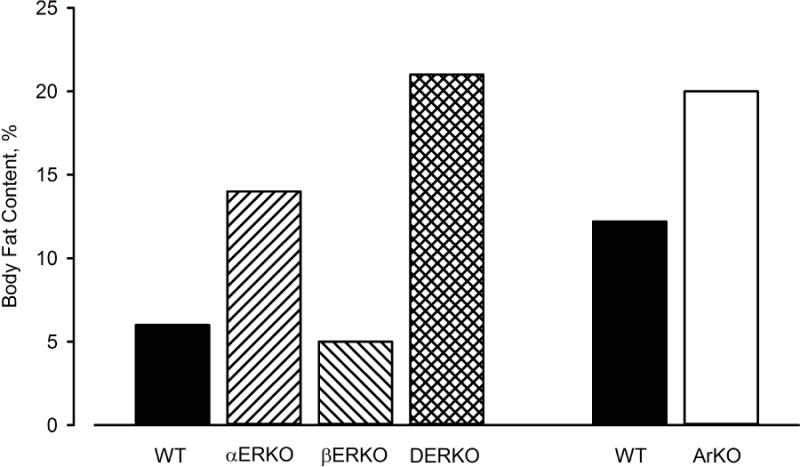
Relative body fat content of wild type (WT) mice and mice with whole-body knockout of estrogen receptor α (αERKO), ERβ (βERKO), both ERα and ERβ (DERKO), or aromatase enzyme (ArKO). Data from Lindberg MK, Weihua Z, Andersson N, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 2002;174(2) and Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol 2001;79(1–5):3–9.
Regulation of bioenergetics by E2
The system-level mechanisms that underlie the excess adiposity triggered by disruptions in E2 signaling include increased energy intake and/or decreased energy expenditure. There is consistent evidence that αERKO mice have little change in energy intake, but have decreased energy expenditure that is attributable to reductions in both locomotion and basal metabolic rate when compared with WT mice21, 24, 33, 34. In contrast, energy expenditure and intake are not altered in βERKO mice33, 35, consistent with no change in adiposity in this model. Running wheel activity was not different among WT-OVX, αERKO-OVX, and βERKO-OVX mice, and was increased in response to E2 treatment in WT-OVX and βERKO-OVX but not αERKO-OVX mice. This indicates that E2 regulation of locomotor activity is mediated through ERα33.
Selective suppression of ERα signaling provides further insight into how E2 mediates energy expenditure and intake. Silencing of ERα in either the CNS20 or in the ventromedial hypothalamic nucleus18, 20 of female rodents resulted in increased energy intake, reduced basal metabolic rate, and reduced locomotor activity. These changes occurred in mice lacking ERα in the CNS despite the fact that circulating E2 was elevated, suggesting that ERα signaling in peripheral tissues did not compensate for the suppression of ERα signaling in the CNS. Silencing of ERα in the medial preoptic area also resulted in a decrease in locomotor activity36. In contrast, deleting ERα in pro-opiomelanocortin neurons resulted in increased energy intake and increased, rather than decreased, energy expenditure20.
Collectively, both whole body and tissue-specific genetic manipulations of ER provide strong evidence that ERα-mediated signaling, in particular, plays a critical role in body weight regulation. The finding that the specific silencing of ERα in various regions of the brain recapitulates disruptions in energy expenditure and/or increasing energy intake that occur with whole body ERα deletion may guide the development of targeted therapies to improve weight regulation after menopause.
PRE-CLINICAL RESEARCH
There is a wealth of pre-clinical evidence that ovarian hormones play an important role in body fat accrual through the regulation of energy balance (food intake and spontaneous physical activity). Because ovaries do not fail in rodents until 11 to 18 months of age37, 38, studies commonly use OVX to remove ovarian hormone production in young female animals as an approach to study the effects of the loss of gonadal function. After removal of ovarian sex hormone production, animals can be treated with exogenous E2 to isolate the action of this hormone. Using these approaches, the effects of ovarian hormones on adiposity and bioenergetics have been well documented. However, a limitation of this approach is that the removal of the ovaries at a young age may not mimic the natural loss of ovarian function at an older age.
Regulation of adiposity by E2
When compared with sham-operated mice, OVX mice gain 25% more weight and up to 5-fold more fat mass in the parametrial, retroperitoneal, and inguinal regions39, 40. In a study that compared the effects of OVX in mice and rats40, the latter gain more weight than sham-operated animals, but primarily through increases in the parametrial and mesenteric (i.e., intra-abdominal) fat pads, suggesting the possibility of species differences in regional fat expansion. However, this species difference has not been a consistent finding, as the expansion of subcutaneous, but not intra-abdominal, fat has been observed in OVX rats41, and the expansion of intra-abdominal fat has been observed in OVX mice42, 43. Most evidence indicates that OVX results in excess fat accumulation in laboratory animals, with a disproportionate amount stored in abdominal regions.
OVX appears to expand adipose tissue by increasing adipocyte size and preadipocyte differentiation39, 44. Controlling food intake in OVX animals through paired feeding with sham animals attenuated, but did not prevent, the fat gain observed in ad libitum fed OVX rats40. Treatment of OVX animals with E2 effectively prevented the gains in fat mass when compared with placebo-treated animals37, 41, 45, 46. The increase in total and abdominal adiposity following OVX has been found to occur under various dietary conditions, including caloric restriction, low-fat feeding, and high-fat feeding and, in all cases, is markedly diminished by E2 treatment (Figure 2)47. Other preclinical models of ovarian failure, including treatment with 4-vinylcyclohexene diepoxide48 and gonadotropin releasing hormone (GnRH) analogs49, also accelerate weight gain. Thus, there is consistent evidence in laboratory animals that the removal of ovarian hormones results in a positive energy balance and that this is prevented with E2 treatment.
Figure 2.
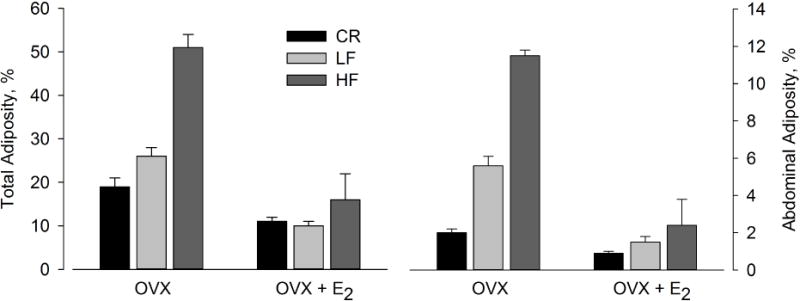
Effects of ovariectomy (OVX) and treatment with estradiol (E2) on total body adiposity and abdominal adiposity in mice on caloric restriction (CR), low-fat diet (LF), or high-fat diet (HF). Data from Stubbins RE, Holcomb VB, Hong J, et al. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 2012;51(7):861–870.
Regulation of bioenergetics by E2
The regulation of energy intake by E2 appears to differ in mice and rats. When compared with sham-operated mice with intact ovaries, OVX mice have either no change in energy intake39, 40, or a small decrease that is reversed with E2 treatment50. In contrast, rats that undergo OVX increase energy intake by ~20% for at least several weeks after surgery40, 46, 51. However, when Ferreira and colleagues46 monitored food intake for 20 weeks after OVX, it returned to the level of sham-operated controls after 10 weeks. Introducing E2 treatment 20 weeks after OVX decreased food intake to below that of sham controls. A unique aspect of the Ferreira study was that the OVX was introduced in mature animals (i.e., aged 10 to 12 months). It is not known whether younger animals would also demonstrate a waning of the hyperphagic effects of OVX over time.
In contrast to the discordant effects of OVX on energy intake in mice versus rats, OVX causes a marked decline in spontaneous physical activity in both mice39, 40, 45, 50 and rats40, 46, 52, particularly in the dark phase when activity level is typically high. The magnitude of decrease in physical activity in these studies ranged from 30% to 80% (Figure 3). Importantly, Ferreira et al. did not observe a waning of the effects of OVX to reduce physical activity over 20 weeks, as they did with energy intake46. Rather, there was an acute decrease in daily activity of more than 50% after OVX that persisted for 20 weeks. In most studies that treated OVX animals with E2 within 2 to 20 weeks after surgery, there was a full rescue of physical activity by E245, 46, 52. One exception was a study of mice treated with E2 at the time of OVX, in which E2 did not prevent the OVX-related decline in physical activity50. Paradoxically, many of the unfavorable effects of OVX, including increased adiposity, reduced energy expenditure, and increased insulin resistance, were prevented by E2 treatment. The fact that energy expenditure was increased in OVX+E2 mice when compared with OVX controls (i.e., to the level of sham-operated animals), despite similar or lower activity levels, suggests that E2 increases basal metabolic rate. Others have also found that energy expenditure is lower in OVX mice than OVX+E2 mice when activity is similar39.
Figure 3.
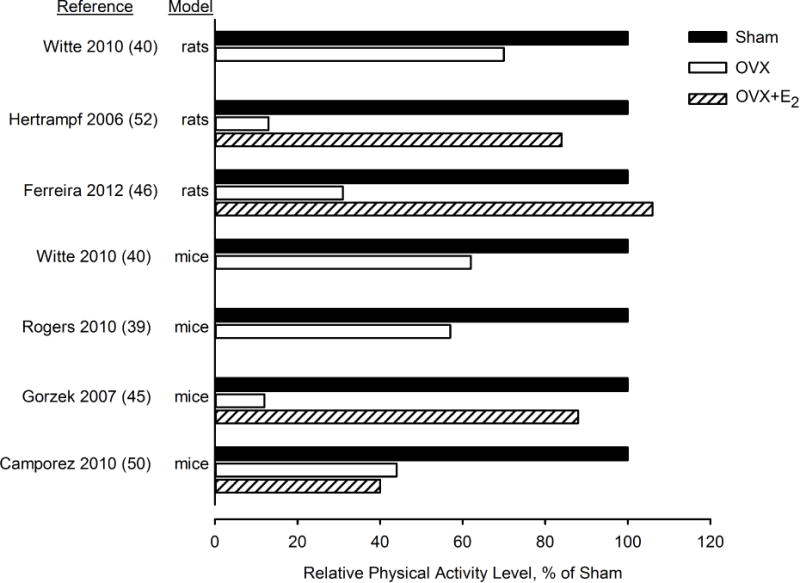
Physical activity level of mice and rats in response to ovariectomy (OVX) or OVX with estradiol (E2) treatment relative to sham-operated controls. Data from references 39, 40, 45, 46, 50 and 52.
The regulation of physical activity by E2 appears to be mediated by ERα, which is consistent with results of studies that genetically manipulated ERs. Evidence for this comes from a study in which rats were treated after OVX with E2, an ERα agonist, an ERβ agonist, or genistein, which is a phytoestrogen that is thought to bind primarily to ERβ53. E2 and ERα agonist treatments increased wheel running activity 4-fold over the level in OVX controls, whereas ERβ agonist and genistein treatments had no effect; the differences among treatments in physical activity mirrored differences in body weight.
In summary, OVX appears to increase energy intake in rats but not mice. OVX causes a marked decrease in spontaneous physical activity in both mice and rats. Some studies have also reported a decrease in energy expenditure in OVX animals beyond that explained by decreased physical activity, suggesting a decrease in basal metabolic rate. Thus, pre-clinical studies provide convincing evidence that the loss of gonadal function via OVX in young or mature rodents disrupts energy balance in a manner that accelerates fat accumulation.
CLINICAL RESEARCH
Investigations of the potential effects of the menopause on adiposity, regional fat distribution, and bioenergetics have included cross-sectional comparisons of pre- and postmenopausal women, prospective cohort studies of women through the menopause, randomized trials of estrogen-based hormone therapy (HT) in postmenopausal women, and the pharmacologic suppression of ovarian function in premenopausal women. Each of these approaches has limitations. A well-recognized disadvantage of cross-sectional comparisons is that many factors other than menopausal status contribute to differences between pre- and postmenopausal women. Prospective cohort studies are valuable because they capture individual changes over time. However, one limitation is that, because the menopause transition is a process that can last several years, age is inextricably linked with the menopause. Thus, it is very challenging (if not impossible) to distinguish effects of the menopause from those of aging. Randomized trials of menopausal HT might be considered the gold standard for evaluating the effects of the menopause, but numerous factors regarding the type of HT regimen (e.g., type and dose of estrogens and progestins, oral versus transdermal delivery) may influence results. Finally, the pharmacologic suppression of ovarian function in premenopausal women with GnRH analogs is another approach for experimentally controlling ovarian hormones, but changes in hormones occur relatively abruptly and, thus, may not mimic the effects of the natural menopause. The discussion of clinical research will focus on studies that fall into the latter two categories.
Regulation of adiposity by estrogens
The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial was the first large randomized controlled trial of HT to demonstrate protective effects of HT against weight gain54. Women randomized to take conjugated equine estrogens (CEE; 0.625 mg/d) with or without a progestin gained 50% as much weight over 3 years as women randomized to placebo treatment. Body composition was not measured in this study, but HT attenuated the increase in waist girth when compared with placebo treatment. Also, although differences between CEE alone and CEE+progestin were not significantly different, the smallest increases in body weight and waist size occurred in the CEE alone group, suggesting a regulatory role of estrogens. On average, when compared with the placebo group, the increases in body weight and waist girth in the CEE group were attenuated by 67% and 61%, respectively. The Women’s Health, Osteoporosis, Progestin, Estrogen (HOPE) trial also found that treatment with CEE 0.625 mg/d attenuated weight gain over 1 year by 49% when compared with placebo; smaller doses of CEE were less effective55. The attenuation of weight gain in postmenopausal women by HT is not a consistent observation. Most notably, in a subgroup of women who participated in the CEE+progestin and placebo arms of the Women’s Health Initiative trial, there was no attenuation of weight gain by HT56.
Most of the large trials of HT in postmenopausal women did not assess body composition. One exception to this was the Danish Osteoporosis Prevention study, which evaluated changes in total and regional adiposity over 5 years in early postmenopausal women randomized to HT (E2 +progestin or E2 alone in women with hysterectomy) or no HT57. A limitation of this trial was that it was not placebo-controlled. In the HT group, the increases in body weight, total fat mass, and trunk fat mass after 5 years of intervention were 25% smaller than in the No-HT group. These differences increased to 35% to 40% in on-treatment analyses. Body composition was also assessed in a subset of women who participated in the CEE+progestin and placebo arms of the Women’s Health Initiative trial56. Although there were no differences between the groups in the change in body weight or fat mass after 3 years of intervention, the trunk-to-leg fat ratio decreased in the HT group, suggesting a protective effect of HT against abdominal fat distribution. Another interesting finding in this study was an attenuation of the loss of lean mass by HT. A 2006 meta-analysis of HT intervention trials found that HT reduced waist circumference by −0.8% and abdominal adiposity by −6.8%58. HT was also associated with reductions in insulin resistance, new-onset diabetes, and dyslipidemia. The extent to which the effects of HT to improve metabolic function are mediated indirectly by beneficial effects on body composition or fat distribution remains unclear. However, at least some of the benefit of HT to reduce insulin resistance appears to be through direct effects of estrogens59, 60.
Several studies have evaluated the effects of 4 to 6 months of GnRH agonist (GnRHAG) therapy on body composition (Figure 4)61–66. In general, these studies suggest that the magnitude of fat accrual increases with duration of ovarian hormone suppression. The only study that did not follow this pattern65 was also the only study that enrolled healthy volunteers rather than women who had a clinical indication for GnRHAG therapy. Because the consenting process for this study disclosed weight gain as a risk of the intervention, it is possible that participants were sensitized to the possibility of gaining weight and made behavioral changes to minimize this. However, even though total adiposity did not increase after 5 months of GnRHAG therapy, there was a 12% increase in intra-abdominal fat area measured by computed tomography, which was prevented in women treated with GnRHAG plus E2 add-back therapy65. Fat-free mass was consistently decreased in response to ovarian hormone suppression across all studies, but the magnitude of decrease was not aligned with the duration of treatment (Figure 4). This raises the question of whether the decreases reflected the loss of the water component of fat-free mass. However, thigh skeletal muscle area measured via computed tomography decreased in response to 5 months of GnRHAG therapy, and was prevented by GnRHAG+E265, supporting an effect of E2 on muscle mass and not just water.
Figure 4.
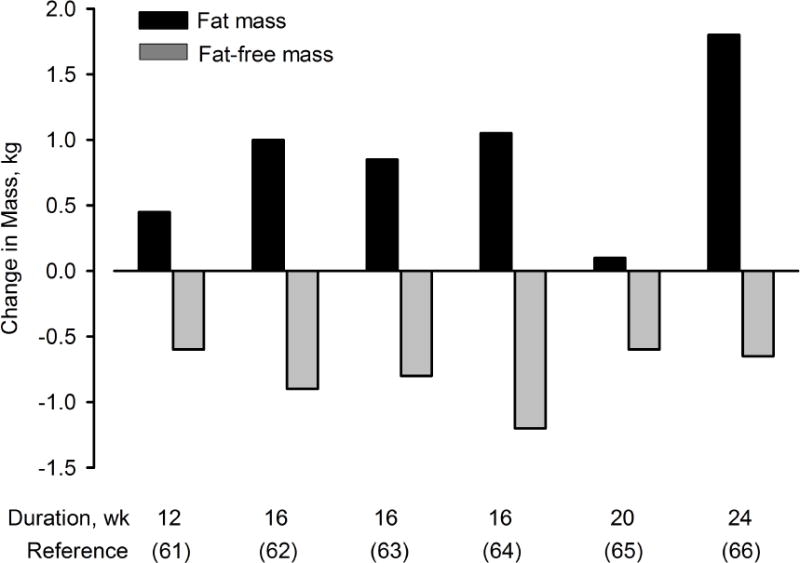
Changes in fat mass and fat-free mass in premenopausal women in response to 12 to 24 weeks of gonadotropin releasing hormone agonist (GnRHAG) therapy. Data from references 61–66.
Regulation of bioenergetics by estrogens
The regulation of energy intake and expenditure by estrogens in women has not been well studied. The scant evidence available suggests that the loss of estrogens disrupts energy balance through decreases in resting energy expenditure (REE) and physical activity, which is consistent with basic and preclinical research.
Both acute (6 days) and more chronic (5 months) ovarian hormone suppression have been found to reduce REE. In a small group of premenopausal women, REE was measured during the mid-luteal phase of the menstrual cycle, when E2 was elevated, during the early follicular phase, when E2 was low, and after 6 days of GnRH antagonist treatment, which further reduced E267. The changes in REE across these 3 conditions paralleled the changes in E2. REE was highest in the mid-luteal phase, lower (−29 kcal/d) in the early follicular phase, and reduced further (−42 kcal/d) after GnRH antagonist treatment. There were no significant differences across the conditions in energy intake, as assessed by 3-day food records. In a larger trial, 45 premenopausal women underwent 5 months of GnRHAG therapy with randomization to concurrent transdermal E2 or placebo treatment to isolate the effects of the loss of estrogens on bioenergetics (Melanson et al.; under review). REE and total energy expenditure (TEE) were measured in the early follicular phase of the menstrual cycle at baseline and again at the end of the intervention. REE decreased (−54 kcal/d) in response to ovarian hormone suppression, and this was prevented by E2 treatment (+6 kcal/d). The decrease in TEE in response to GnRHAG (−128 kcal/d) was not prevented by E2 (−96 kcal/d). Although the measurement of TEE by whole-room calorimetry in this study is state of the science, it provides only a snapshot (i.e., 24 hours) of TEE and does not reflect free-living conditions. It will be important to use another approach, such as the doubly-labeled water technique, which can measure free-living TEE over many days, to determine how estrogens influence bioenergetics in women.
The effects of estrogens on REE in postmenopausal women have been examined primarily through cross-sectional comparisons of premenopausal women with postmenopausal women on or not on HT, with varied results. These studies are not reviewed here because of the multiple factors other than hormone status that can influence bioenergetics. However, one intervention trial measured changes in REE in 18 younger (45 to 55 y) and 15 older (70 to 80 y) postmenopausal women after 2 months of newly initiated HT68. REE was lower in the older group than the younger group, even when adjusted for fat-free mass, but was not influenced by HT in either group. Physical activity level, as assessed by questionnaire, was also unchanged in response to HT. Another short-term intervention study also found that 2 weeks of E2 did not increase REE in postmenopausal women69. Physical activity, as assessed by questionnaire, was unchanged in both studies. Because of the consistent finding in preclinical research that spontaneous physical activity is regulated by E2, it will be important to determine whether this occurs in humans using objective measures of activity. To date, we are not aware of any intervention trials of either hormone suppression in premenopausal women or HT in postmenopausal women that have done so. However, one prospective study of women going through the menopause transition measured physical activity annually by accelerometry and found that it decreased by more than 50% over the 4 years leading up to the onset of menopause70. Energy intake, assessed by 4-day food records, also decreased over this time interval. Thus, the limited data on the effects of estrogens on bioenergetics suggest that energy expenditure may decline as a result of the loss of ovarian function, resulting in increased risk for fat gain.
SUMMARY
There is consistent evidence from basic and preclinical research that the disruption of E2 signaling, through either genetic manipulation (e.g., ER deletion) or surgical intervention (e.g., OVX), accelerates fat accumulation. The excess fat appears to accumulate disproportionately in the abdominal region and leads to insulin resistance and dyslipidemia. Treatment of OVX animals with E2 prevents these phenotypic changes, thereby isolating E2 as the regulatory ovarian factor, and transgenic studies indicate that the effects are mediated, in large part, through ERα. The primary system-level mechanism for the increased fat accumulation is a decrease in energy expenditure, although energy intake also increases in some species. The lower energy expenditure is the result of a marked decline in spontaneous physical activity and a decrease in resting metabolic rate.
Clinical evidence for the regulation of body composition and bioenergetics by E2 is less consistent. Cross-sectional comparisons of pre- and postmenopausal women and prospective cohort studies of women through the menopause transition have yielded evidence both for and against menopause as the mediator of changes in body composition. This is likely related to the prolonged nature of the menopause transition in women and the associated complexities of distinguishing effects of the loss of gonadal function from other phenomena of aging. However, even controlled interventions that evaluate changes in body composition and bioenergetics in response to HT in postmenopausal women or suppression of ovarian function in premenopausal women do not always reveal a clear role of estrogens. A myriad of factors, such as the type, dose, and duration of treatment, could contribute to the variable results. It is also possible that inter-individual differences in the distribution of ERα and ERβ influence the changes women experience in response to the withdrawal of ovarian estrogens and in response to exogenous E2 treatment.
The totality of evidence from basic, preclinical, and clinical research points to an important physiologic role of E2 in the regulation of bioenergetics and body composition. Further advances in basic and preclinical research will help to elucidate the mechanistic targets of estrogens. However, it is important to recognize the limitations of translating basic and preclinical discoveries to humans. For example, dehydroepiandrosterone (DHEA) is secreted primarily by the adrenal gland in humans and it is the major progenitor for androgens and estrogens in postmenopausal women. In contrast, DHEA is secreted primarily by the gonads in rodents, which means that OVX leads to the loss of both biologically active sex hormones and prohormones. This suggests that the phenotypic consequences of OVX in animals may be more severe than those that occur with the loss of ovarian function in women.
There is a need to better understand the mechanisms for the metabolic actions of estrogens in women because of the potential adverse impact on health after the menopause. It is well known that the menopausal decline in estrogens accelerates the loss of bone mineral, thereby increasing risk for osteoporosis. If the loss of ovarian estrogens also triggers such changes as a decline in physical activity and an increase in abdominal adiposity in women, as it does in laboratory animals, this could contribute to increased risk for other age-related chronic diseases, such as diabetes and cardiovascular disease. Thus, it is important to continue to advance studies in women that experimentally control the sex hormone environment to better understand the metabolic and bioenergetics consequences of the menopausal loss of estrogens.
KEY POINTS.
Consistent evidence from basic and preclinical research indicates that the disruption of estradiol (E2) signaling accelerates abdominal fat accumulation.
Treatment of ovariectomized animals with E2 prevents fat accumulation, thereby isolating E2 as the regulatory ovarian factor, and transgenic studies indicate that these effects are mediated primarily through estrogen receptor alpha (ERα).
The major system-level mechanism for excess fat accumulation in response to the loss of E2 in animals is a decrease in energy expenditure, which occurs as a result of reductions in spontaneous physical activity and resting metabolic rate.
Clinical evidence for the regulation of body composition by E2 is less consistent, but the suppression of ovarian function does promote fat gain.
If the loss of ovarian estrogens triggers a decline in physical activity and increase in abdominal adiposity in women, as it does in laboratory animals, this could increase risk for diabetes and cardiovascular disease in postmenopausal women.
Acknowledgments
The expertise of the authors on this topic was generated, in part, by research supported by the following awards: P50 HD073063, R01 DK088105, T32 AG000279, F32 AG046957, UL1 TR001082, P30 DK048520.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have nothing to disclose.
References
- 1.Gruber CJ, Tschugguel W, Schneeberger C, et al. Production and actions of estrogens. N Engl J Med. 2002;346(5):340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 2.Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35(1):8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SH, Liu R, Perez EJ, et al. Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci U S A. 2004;101(12):4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen EV. On the mechanism of estrogen action. Perspect Biol Med. 1962;6:47–59. doi: 10.1353/pbm.1963.0005. [DOI] [PubMed] [Google Scholar]
- 5.Kuiper GG, Enmark E, Pelto-Huikko M, et al. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9(5):404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 7.O’Malley BW, Tsai MJ. Molecular pathways of steroid receptor action. Biol Reprod. 1992;46(2):163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- 8.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67(2):77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 10.Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277(5331):1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 11.Dieudonne MN, Leneveu MC, Guidicelli Y, et al. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:655–661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- 12.Barros RP, Gabbi C, Morani A, et al. Participation of ERalpha and ERbeta in glucose homeostasis in skeletal muscle and white adipose tissue. Am J Physiol Endocrinol Metab. 2009;297(1):E124–133. doi: 10.1152/ajpendo.00189.2009. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi H, Fujimoto J, Aoki I, et al. Expression of estrogen receptor alpha and beta in myometrium of premenopausal and postmenopausal women. Steroids. 2003;68(1):11–19. doi: 10.1016/s0039-128x(02)00111-3. [DOI] [PubMed] [Google Scholar]
- 14.Gavin KM, Cooper EE, Hickner RC. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism. 2013;62(8):1180–1188. doi: 10.1016/j.metabol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Wend K, Wend P, Krum SA. Tissue-specific effects of loss of estrogen during menopause and aging. Front Endocrinol (Lausanne) 2012;3:19. doi: 10.3389/fendo.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubahn DB, Moyer JS, Golding TS, et al. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993;90(23):11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95(26):15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musatov S, Chen W, Pfaff DW, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104(7):2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas V, Drew BG, Le JA, et al. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci U S A. 2011;108(39):16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Nedungadi TP, Zhu L, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CJ, Zhao Z, Glidewell-Kenney C, et al. Genetic rescue of nonclassical ERalpha signaling normalizes energy balance in obese ERalpha-null mutant mice. J Clin Invest. 2011;121(2):604–612. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedram A, Razandi M, Kim JK, et al. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284(6):3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Syed FA, Fraser DG, Monroe DG, et al. Distinct effects of loss of classical estrogen receptor signaling versus complete deletion of estrogen receptor alpha on bone. Bone. 2011;49(2):208–216. doi: 10.1016/j.bone.2011.03.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine PA, Taylor JA, Iwamoto GA, et al. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naaz A, Zakroczymski M, Heine P, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm Metab Res. 2002;34(11–12):758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 26.Lindberg MK, Weihua Z, Andersson N, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174(2) doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 27.Seidlova-Wuttke D, Nguyen BT, Wuttke W. Long-term effects of ovariectomy on osteoporosis and obesity in estrogen-receptor-beta-deleted mice. Comp Med. 2012;62(1):8–13. [PMC free article] [PubMed] [Google Scholar]
- 28.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79(1–5):3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 29.Revankar CM, Cimino DF, Sklar LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307(5715):1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 30.Thomas P, Pang Y, Filardo EJ, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 31.Davis KE, Carstens EJ, Irani BG, et al. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav. 2014;66(1):196–207. doi: 10.1016/j.yhbeh.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isensee J, Meoli L, Zazzu V, et al. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1730. doi: 10.1210/en.2008-1488. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa S, Chan J, Gustafsson JA, et al. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144(1):230–239. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 34.Geary N, Asarian L, Korach KS, et al. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142(11):4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 35.Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet. 2008;4(6):e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiteri T, Ogawa S, Musatov S, et al. The role of the estrogen receptor alpha in the medial preoptic area in sexual incentive motivation, proceptivity and receptivity, anxiety, and wheel running in female rats. Behav Brain Res. 2012;230(1):11–20. doi: 10.1016/j.bbr.2012.01.048. [DOI] [PubMed] [Google Scholar]
- 37.Felicio LS, Nelson JF, Finch CE. Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod. 1984;31(3):446–453. doi: 10.1095/biolreprod31.3.446. [DOI] [PubMed] [Google Scholar]
- 38.Durbin PW, Williams MH, Jeung N, et al. Development of spontaneous mammary tumors over the life-span of the female Charles River (Sprague-Dawley) rat: the influence of ovariectomy, thyroidectomy, and adrenalectomy-ovariectomy. Cancer Res. 1966;26(3):400–411. [PubMed] [Google Scholar]
- 39.Rogers NH, Perfield JW, 2nd, Strissel KJ, et al. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witte MM, Resuehr D, Chandler AR, et al. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen Comp Endocrinol. 2010;166(3):520–528. doi: 10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gloy V, Langhans W, Hillebrand JJ, et al. Ovariectomy and overeating palatable, energy-dense food increase subcutaneous adipose tissue more than intra-abdominal adipose tissue in rats. Biol Sex Differ. 2011;2:6. doi: 10.1186/2042-6410-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson KC, Wohlers LM, Lovering RM, et al. Ectopic lipid deposition and the metabolic profile of skeletal muscle in ovariectomized mice. Am J Physiol Regul Integr Comp Physiol. 2013;304(3):R206–217. doi: 10.1152/ajpregu.00428.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yonezawa R, Wada T, Matsumoto N, et al. Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. Am J Physiol Endocrinol Metab. 2012;303(4):E445–456. doi: 10.1152/ajpendo.00638.2011. [DOI] [PubMed] [Google Scholar]
- 44.Fu Y, Li R, Zhong J, et al. Adipogenic differentiation potential of adipose-derived mesenchymal stem cells from ovariectomized mice. Cell Prolif. 2014;47(6):604–614. doi: 10.1111/cpr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorzek JF, Hendrickson KC, Forstner JP, et al. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc. 2007;39(2):248–256. doi: 10.1249/01.mss.0000241649.15006.b8. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira JA, Foley AM, Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur J Appl Physiol. 2012;112(8):3007–3018. doi: 10.1007/s00421-011-2271-y. [DOI] [PubMed] [Google Scholar]
- 47.Stubbins RE, Holcomb VB, Hong J, et al. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 48.Wright LE, Christian PJ, Rivera Z, et al. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res. 2008;23(8):1296–1303. doi: 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth CL, Neu C, Jarry H, et al. Different effects of agonistic vs. antagonistic gnrh-analogues (triptorelin vs. cetrorelix) on bone modeling and remodeling in peripubertal female rats. Exp Clin Endocrinol Diabetes. 2005;113(8):451–456. doi: 10.1055/s-2005-865710. [DOI] [PubMed] [Google Scholar]
- 50.Camporez JP, Jornayvaz FR, Lee HY, et al. Cellular mechanism by which estradiol protects female ovariectomized mice from high-fat diet-induced hepatic and muscle insulin resistance. Endocrinology. 2013;154(3):1021–1028. doi: 10.1210/en.2012-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42(4):461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 52.Hertrampf T, Degen GH, Kaid AA, et al. Combined effects of physical activity, dietary isoflavones and 17beta-estradiol on movement drive, body weight and bone mineral density in ovariectomized female rats. Planta Med. 2006;72(6):484–487. doi: 10.1055/s-2006-931579. [DOI] [PubMed] [Google Scholar]
- 53.Hertrampf T, Seibel J, Laudenbach U, et al. Analysis of the effects of oestrogen receptor alpha (ERalpha)- and ERbeta-selective ligands given in combination to ovariectomized rats. Br J Pharmacol. 2008;153(7):1432–1437. doi: 10.1038/sj.bjp.0707664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Espeland MA, Stefanick ML, Kritz-Silverstein D, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab. 1997;82(5):1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 55.Utian WH, Gass ML, Pickar JH. Body mass index does not influence response to treatment, nor does body weight change with lower doses of conjugated estrogens and medroxyprogesterone acetate in early postmenopausal women. Menopause. 2004;11(3):306–314. doi: 10.1097/01.gme.0000117062.54779.bd. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition–a substudy of the estrogen plus progestin trial of the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 57.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18(2):333–342. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 58.Salpeter SR, Walsh JM, Ormiston TM, et al. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab. 2006;8(5):538–554. doi: 10.1111/j.1463-1326.2005.00545.x. [DOI] [PubMed] [Google Scholar]
- 59.Van Pelt RE, Gozansky WS, Schwartz RS, et al. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab. 2003;285(2):E311–317. doi: 10.1152/ajpendo.00490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Pelt RE, Schwartz RS, Kohrt WM. Insulin secretion and clearance after subacute estradiol administration in postmenopausal women. J Clin Endocrinol Metab. 2008;93(2):484–490. doi: 10.1210/jc.2007-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nowicki M, Adamkiewicz G, Bryc W, et al. The influence of luteinizing hormone-releasing hormone analog on serum leptin and body composition in women with solitary uterine myoma. Am J Obstet Gynecol. 2002;186(3):340–344. doi: 10.1067/mob.2002.120485. [DOI] [PubMed] [Google Scholar]
- 62.Yamasaki H, Douchi T, Yamamoto S, et al. Body fat distribution and body composition during GnRH agonist therapy. Obstet Gynecol. 2001;97(3):338–342. doi: 10.1016/s0029-7844(00)01181-9. [DOI] [PubMed] [Google Scholar]
- 63.Douchi T, Kuwahata R, Yamasaki H, et al. Inverse relationship between the changes in trunk lean and fat mass during gonadotropin-releasing hormone agonist therapy. Maturitas. 2002;42(1):31–35. doi: 10.1016/s0378-5122(02)00004-x. [DOI] [PubMed] [Google Scholar]
- 64.Douchi T, Kuwahata T, Yoshimitsu N, et al. Changes in serum leptin levels during GnRH agonist therapy. Endocr J. 2003;50(3):355–359. doi: 10.1507/endocrj.50.355. [DOI] [PubMed] [Google Scholar]
- 65.Shea KL, Gavin KM, Melanson EL, et al. Body composition and bone mineral density after ovarian hormone suppression with or without estradiol treatment. Menopause. 2015 doi: 10.1097/GME.0000000000000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Revilla R, Revilla M, Villa LF, et al. Changes in body composition in women treated with gonadotropin-releasing hormone agonists. Maturitas. 1998;31(1):63–68. doi: 10.1016/s0378-5122(98)00080-2. [DOI] [PubMed] [Google Scholar]
- 67.Day DS, Gozansky WS, Van Pelt RE, et al. Sex hormone suppression reduces resting energy expenditure and {beta}-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90(6):3312–3317. doi: 10.1210/jc.2004-1344. [DOI] [PubMed] [Google Scholar]
- 68.Anderson EJ, Lavoie HB, Strauss CC, et al. Body composition and energy balance: lack of effect of short-term hormone replacement in postmenopausal women. Metabolism. 2001;50(3):265–269. doi: 10.1053/meta.2001.21015. [DOI] [PubMed] [Google Scholar]
- 69.Bessesen DH, Cox-York KA, Hernandez TL, et al. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: impact of menopause and estradiol. Obesity (Silver Spring) 2015;23(1):145–153. doi: 10.1002/oby.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lovejoy JC, Champagne CM, de Jonge L, et al. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes. 2008;32(6):949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]


