The actin-polymerizing protein complex Arp2/3 was crystallized in two new space groups. The new electron-density maps from these new crystal forms reveal some details that may help to elucidate how nucleation-promoting factors bind to the complex.
Keywords: Arp2/3, Wiskott–Aldrich syndrome, actin
Abstract
Co-crystals of the bovine Arp2/3 complex with the CA motif from N-WASP in two new space groups were analyzed by X-ray diffraction. The crystals in the orthorhombic space group P212121 contained one complex per asymmetric unit, with unit-cell parameters a = 105.48, b = 156.71, c = 177.84 Å, and diffracted to 3.9 Å resolution. The crystals in the tetragonal space group P41 contained two complexes per asymmetric unit, with unit-cell parameters a = b = 149.93, c = 265.91 Å, and diffracted to 5.0 Å resolution. The electron-density maps of both new crystal forms had densities for small segments of subdomains 1 and 2 of Arp2. Both maps had density at the binding site on Arp3 for the C-terminal EWE tripeptide from N-WASP and a binding site proposed for the C motif of N-WASP in the barbed-end groove of Arp2. The map from the tetragonal crystal form had density near the barbed end of Arp3 that may correspond to the C helix of N-WASP. The noise levels and the low resolution of the maps made the assignment of specific molecular structures for any of these CA peptides impossible.
1. Introduction
The Arp2/3 complex initiates branched actin filaments for cell motility (Pollard, 2007 ▸; Goley & Welch, 2006 ▸; Yang & Svitkina, 2011 ▸) and the spread of some intracellular pathogenic bacteria (Welch & Way, 2013 ▸) as well as metastasizing tumor cells (Nürnberg et al., 2011 ▸; Gross, 2013 ▸). The complex consists of actin-related proteins (Arps) 2 and 3 as well as five other subunits. Numerous crystal structures with resolutions extending to 2.0 Å (Robinson et al., 2001 ▸) have been obtained from crystals of the bovine Arp2/3 complex in space group P212121 with and without adenine nucleotides (Nolen et al., 2004 ▸) or small-molecule inhibitors bound to the Arps (Nolen et al., 2009 ▸; Luan & Nolen, 2013 ▸). The bovine Arp2/3 complex with the regulatory protein glial maturation factor bound to Arp2 crystallized in space group P65 (Luan & Nolen, 2013 ▸). A structure of the fission yeast Arp2/3 complex from crystals of space group P4222 is also available (Nolen & Pollard, 2008 ▸).
When activated by a nucleation-promoting factor such as N-WASP or WAVE (Stradal & Scita, 2006 ▸; Higgs & Pollard, 2001 ▸; Rotty et al., 2012 ▸) and an actin filament, the Arp2/3 complex forms a new actin filament that grows as a branch on the side of the pre-existing filament (Mullins et al., 1998 ▸; Machesky et al., 1999 ▸). Nucleation-promoting factors characteristically feature a VCA domain containing a V motif (also called a WH2 motif) that binds an actin monomer and connecting (C) and acidic (A) motifs that bind to the Arp2/3 complex. Most structures of the Arp2/3 complex had little density for subdomains 1 and 2 of Arp2, presumably owing to flexibility, except after chemical cross-linking with glutaraldehyde (Nolen & Pollard, 2007 ▸) or with bound glial maturation factor (GMF; Luan & Nolen, 2013 ▸).
WASP-family CA motifs bind to at least two, and possibly three, sites on the Arp2/3 complex (Padrick et al., 2011 ▸; Boczkowska et al., 2014 ▸). VCA binding to sites on Arp2 and Arp3 (Padrick et al., 2011 ▸) promotes branch formation in two ways: by increasing the affinity of the Arp2/3 complex for the side of the actin filament (Ti et al., 2011 ▸) and by delivering actin monomers bound to the V motif to the barbed ends of Arp2 and Arp3 to initiate the daughter filament (Padrick et al., 2011 ▸). The order of addition of the first two actin subunits of the daughter filament has not been settled. They might add in order, with the first associating with Arp3 and the second with Arp2, but an attractive alternative is that the first actin monomer binds to Arp2 followed by a second actin monomer, forming a transitional complex with two NPF-bound actin molecules associated with the Arp2/3 complex (Boczkowska et al., 2014 ▸). In either case, the binding of actin at the barbed end of Arp3 depends on a large conformational change that moves Arp2 by ∼30 Å (Rouiller et al., 2008 ▸; Dalhaimer & Pollard, 2010 ▸).
Relatively little is known about how WASP-family CA motifs bind the Arp2/3 complex. One of our crystal structures of the bovine Arp2/3 complex had density for the three C-terminal residues of the A motif (EWE) bound in a pocket in subdomain 4 of Arp3 (Ti et al., 2011 ▸). We proposed that the C motif of VCA forms a two-turn α-helix that binds in the barbed-end groove of Arp3, where a short helix from ARPC1 in an adjacent complex forms a crystal contact in the original 2.0 Å resolution structure. The placement of this helix in Arp3 is similar to the binding sites of short helices of the GAB motif of Ena/VASP (Ferron et al., 2007 ▸) and of the V/WH2 region of VCA (Chereau et al., 2005 ▸) in the barbed-end groove of actin. With the C and A motifs bound to Arp3, the V motif is positioned to deliver an actin subunit to the barbed end of Arp3, the first subunit in the daughter filament. Data from chemical cross-linking (Padrick et al., 2011 ▸), small-angle X-ray scattering (Boczkowska et al., 2008 ▸) and spectroscopy (Boczkowska et al., 2014 ▸) are consistent with a second binding site for the C motif bound near the barbed-end groove of Arp2, with the A motif bound to a basic patch involving ARPC1. Binding to this site would allow the V motif to deliver the second actin subunit to the barbed end of Arp2. In addition, CA binding to a high-affinity site inhibits interaction of the Arp2/3 complex with the side of the actin filament (Ti et al., 2011 ▸). The location of this site has not been firmly established, but one candidate is the density at the pointed end of Arp3 in reconstructions of electron micrographs of the Arp2/3 complex with three different nucleation-promoting factors (Xu et al., 2012 ▸).
Contacts or solvent conditions in the original P212121 crystal form of the Arp2/3 complex may be unfavorable for CA binding, so we conducted crystallization trials seeking new crystal forms that would allow CA to bind to the proposed binding sites on Arp2 and Arp3. Sparse-matrix screening of purified bovine Arp2/3 complex with the CA fragment of N-WASP yielded crystals in the tetragonal space group P41. Further screening around this condition gave crystals in the orthorhombic space group P212121. The original crystal structure (Robinson et al., 2001 ▸) was also in space group P212121, but the unit-cell parameters were different. Electron-density maps of both crystal forms had some density in the first two subdomains of Arp2, confirming that its topology is similar to that of actin. Novel densities in the map near the barbed ends of Arp2 and Arp3 may correspond to bound CA, but the resolution and map quality were too low to fit a model.
2. Materials and methods
2.1. Protein purification
We purified native Arp2/3 complex (Nolen et al., 2004 ▸) from calf thymus and concentrated it to 35 µM before flash-freezing it in liquid nitrogen for storage. The concentration was determined using an extinction coefficient of 139 000 M −1 cm−1 measured at 290 nm (Kiselar et al., 2007 ▸). We purified recombinant CA domain (residues Gly466–Asp505) of bovine N-WASP (Ti et al., 2011 ▸). The N-WASP CA construct with a GST tag in a pG67 vector was transformed into Escherichia coli Rosetta cells. The protein was purified by glutathione affinity chromatography, cleavage of the peptide from GST with TEV protease, ion exchange on a Mono Q column and gel filtration. N-WASP CA was concentrated to 5 mM and flash-frozen in liquid nitrogen for storage.
2.2. Protein crystallization and cooling
2.2.1. Tetragonal space group
The Arp2/3 complex was crystallized by the hanging-drop vapor-diffusion method in the tetragonal space group P41 with 0.1 M Tris–HCl pH 7.0, 15% ethanol in the presence of 0.5 mM CA peptide. The drops were prepared using 1 µl 10 mg ml−1 Arp2/3 in 100 mM NaCl, 20 mM Tris–HCl pH 8.0 mixed with 1 µl well solution. The crystals grew with octahedral geometry to approximately 150 and 75 µm along the longest and shortest axes, respectively, after 2–4 weeks at 277 K. Crystals did not grow with ATP in the buffer. The crystals were very fragile and difficult to handle. Within 1–2 min after removing the cover slip from the hanging-drop chamber, the crystals started to crack and dissolve, likely owing to evaporation of ethanol from the drop. Immediately covering the hanging drop with mineral oil protected the crystals from evaporation and kept them intact for long enough to remove them from the mother liquor for cooling in liquid nitrogen. No further cryoprotection was performed, since the crystals shattered immediately when moved to soaking solutions containing higher concentrations of cryoprotectants.
2.2.2. Orthorhombic space group
Attempts to optimize the crystals in the tetragonal space group gave crystals that belonged to the orthorhombic space group P212121. The drops were prepared using 1 µl 10 mg ml−1 Arp2/3 with 0.5 mM CA peptide in 100 mM NaCl, 20 mM Tris–HCl pH 8.0 mixed with 1 µl well solution. Crystals grew in hanging drops after 2–4 weeks at 277 K in 0.1 M Tris–HCl pH 7.4, 10% ethanol, 15% 1,4-butanediol. The rectangular crystals of approximate dimensions 150 × 40 × 10 µm were more robust than those belonging to the tetragonal space group. Crystals did not grow with ATP in the buffer. The high concentration of 1,4-butanediol in the mother liquor was a suitable cryoprotectant for cooling in liquid nitrogen.
2.3. Data collection and processing
2.3.1. Tetragonal space group
Diffraction data were collected on beamline X25 at the National Synchrotron Light Source (NSLS) using a Q315 CCD detector from Area Detector Systems. Data indexing and reduction were performed using the HKL-2000 suite of programs (Otwinowski & Minor, 1997 ▸). Molecular replacement was carried out using the CNS suite of programs (Brünger et al., 1998 ▸) using a 2.0 Å resolution crystal structure of the bovine Arp2/3 complex (PDB entry 1k8k; Robinson et al., 2001 ▸). Cross-rotation function calculations were made between 5 and 15 Å resolution in CNS (Brünger et al., 1998 ▸). The first two cross-rotation peak heights were significantly higher (0.0715 and 0.0684) than the remaining peaks (ranging from 0.0325 to 0.0248). We took this to indicate the presence of two separate Arp2/3 complexes in the asymmetric unit, so two copies of the Arp2/3 complex were modeled in the asymmetric unit. We used CNS for rigid-body, simulated-annealing and B-factor refinement, followed by calculation of 2F o − F c and simulated-annealing OMIT maps. These maps showed additional density for Arp2 that was not found in the reference structure. Model building was performed in Coot (Emsley & Cowtan, 2004 ▸) using the secondary-structure method (SSM) superpose function to overlay the structure of actin with bound WASP (Chereau et al., 2005 ▸) to fit parts of the Arp2 polypeptide into the fragmentary densities for subdomains 1 and 2 of Arp2. Additional structure built into Arp2 included residues 16–45 and 55–145. Further rounds of refinement were performed in REFMAC (Murshudov et al., 2011 ▸) from the CCP4 suite (Winn et al., 2011 ▸) and PHENIX (Adams et al., 2010 ▸), and focused on Arp2 with the remaining structure of the complex unchanged. Twofold noncrystallographic symmetry parameters were used throughout the refinement process. The solvent content was calculated to be 63%. Final refinement was carried out in PHENIX using xyz coordinate, rigid-body and individual B-factor refinement strategies. Automatic X-ray/stereochemistry weighting was performed in PHENIX. Reference model restraints were also used by incorporating the 2.0 Å resolution structure of the Arp2/3 complex (PDB entry 1k8k) into the refinement process. Two copies were made of the 2.0 Å resolution structure by overlaying it on each copy of the Arp2/3 complex in the asymmetric unit of the tetragonal structure. Backbone atoms were built using the overlaid structure of actin as a guide (PDB entry 2a3z; Chereau et al., 2005 ▸). Manual adjustments of side chains were made with geometric outliers, but most of them were left unchanged owing the low resolution making it difficult to accurately determine whether a different rotomeric conformation should be modeled. Real-space refinement was not used in order to avoid overrefinement.
2.3.2. Orthorhombic space group
Diffraction data extending to 3.9 Å resolution were collected on beamline ID-24-E at the Advanced Photon Source using a Q315 CCD detector from Area Detector Systems. Cryoannealing did not improve the diffraction. The data were processed using the HKL-2000 suite of programs (Otwinowski & Minor, 1997 ▸). The solvent content was calculated to be 60%. Molecular-replacement structure solution was performed using the crystal structure of the Arp2/3 complex (PDB entry 1k8k). Cross-rotation function calculations were made using CNS (Brünger et al., 1998 ▸) and showed one complex per asymmetric unit. This was followed by rigid-body, simulated-annealing and B-factor refinement. Further rounds of refinement used REFMAC (Murshudov et al., 2011 ▸) from the CCP4 suite (Winn et al., 2011 ▸). Maps were calculated using the CNS and PHENIX (Adams et al., 2010 ▸) suites of programs. Model building was performed in Coot (Emsley & Cowtan, 2004 ▸). As for the tetragonal space group, the maps were used in conjunction with the structure of WASP-bound actin overlaid onto Arp2 as a guide for building into fragmentary density. Additional structure was built including residues 82–94 and the C-terminal residues 344–358. Final refinement was carried out in PHENIX (Adams et al., 2010 ▸) using the same refinement parameters as for the tetragonal space group owing to the low resolution of the data. As with the tetragonal space group, reference model restraints were used by incorporating a single 2.0 Å resolution structure of Arp2/3 (PDB entry 1k8k) into the refinement process. All figures were generated using PyMOL (DeLano, 2002 ▸). Table 1 ▸ gives the data-collection and refinement statistics.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Space group | Tetragonal | Orthorhombic |
|---|---|---|
| PDB code | 4xf2 | 4xei |
| Data collection | ||
| Source | NSLS | APS |
| Beamline | X25 | ID-24-E |
| Wavelength () | 1 | 1 |
| Resolution () | 505.0 (5.205.00) | 503.9 (4.043.90) |
| Space group | P41 | P212121 |
| Unit-cell parameters | ||
| a () | 149.93 | 105.48 |
| b () | 149.93 | 156.71 |
| c () | 265.91 | 177.84 |
| = = () | 90 | 90 |
| R merge (%) | 9.0 (46.9) | 19.4 (66.1) |
| Mean I/(I) | 20.3 (2.3) | 6.8 (1.8) |
| Multiplicity | 6.4 (3.4) | 3.3 (3.3) |
| Completeness (%) | 96.8 (84.6) | 97.6 (99.4) |
| Refinement | ||
| Modeled protein atoms | 27846 | 13081 |
| Water molecules | 0 | 0 |
| No. of reflections | 156605 | 87034 |
| No. of unique reflections | 24560 | 26649 |
| Bond-length r.m.s.d. () | 0.007 | 0.007 |
| Bond-angle r.m.s.d. () | 1.443 | 1.623 |
| R work (%) | 30.0 (38.3) | 29.5 (35.8) |
| R free (%) | 32.3 (46.5) | 33.3 (40.3) |
3. Results and discussion
3.1. Description of diffraction data and overall maps
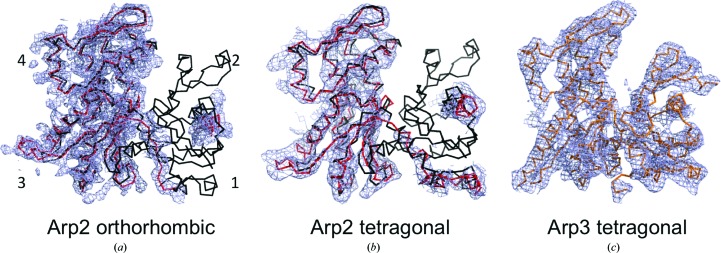
The electron-density maps of the Arp2/3 complex in both new space groups include all of the subunits seen in previous structures. Like previous structures of the Arp2/3 complex without bound nucleotides, the nucleotide-binding cleft of Arp3 was partially open (Fig. 1 ▸). The new maps have more density for subdomains 1 and 2 of Arp2 than the original P212121 crystals, where this part of Arp2 was in a large cavity (Robinson et al., 2001 ▸), allowing high flexibility. The following description focuses on Arp2 and on densities that may correspond to bound CA.
Figure 1.
2F o − F c electron-density maps (blue) contoured at 1σ of Arp2 and Arp3 from the new crystals. (a) Arp2 from the P212121 crystals at 3.9 Å resolution fitted with a backbone model of Arp2 (red) and compared with a backbone model of actin (black). Subdomains are labeled by the numbers 1–4. (b) Arp2 from the P41 crystals at 5.0 Å resolution fitted with a backbone model of Arp2 (red) and compared with a backbone model of actin (black). (c) Arp3 from the P41 crystals at 5.0 Å resolution fitted with an orange backbone model refined against the new data.
3.2. Crystal contacts
Both new crystal forms have more intermolecular contacts in the crystal lattice than the original orthorhombic crystals (Supplementary Fig. S1). The numbers of residues making crystal contacts are 17 in the new orthorhombic crystals, 22 in the tetragonal crystals and 15 in the old orthorhombic space group (Table 2 ▸). Crystal contacts were determined by identifying interactions between symmetry-related complexes that were close enough to participate in noncovalent interactions. This was performed using the cell and symmetry function in Coot, which calculated the positions of symmetry-related complexes close to our model. Once regions were identified that were close between symmetry-related complexes, we zoomed in to identify and measure the distances between atoms in residues that may interact. Hydrogen bonds were determined by a length of 2.7–3.3 Å between hydrogen-bond donor and accepter heteroatoms. Salt bridges were determined by a distance of approximately 4.5 Å between charged side chains. Cation–π interactions were determined by a length of approximately 3.2 Å between the cationic and aromatic side-chain moieties (for example Arg328 of Arp2 and Phe302 of ARPC1 in the P41 space group). π-Stacking interactions were determined by two aromatic residues less than 4 Å apart (e.g. Trp153 of Arp3 and Phe302 of ARPC1 in the P212121 structure of Robinson et al., 2001 ▸). Hydrophobic interactions were determined by hydrophobic side chains that were close enough to disallow a water molecule from coming in between them (for example Met383 of Arp3 and Phe302 of ARPC1 in the P212121 structure of Robinson et al., 2001 ▸). In many cases multiple residues contribute to a single contact, as indicated by the ‘Contact’ column in Table 2 ▸. In the original orthorhombic space group Arp2 made a single crystal contact in subdomain 4 (Table 2 ▸). In both new space groups the barbed-end groove of Arp2 is located in solvent channels without crystal contacts that would sterically hinder the binding of CA. The additional density seen in subdomains 1 and 2 in both new space groups may also be owing to stability induced by the density bound at the barbed end of Arp2.
Table 2. List of crystal contacts in the three crystal forms of the Arp2/3 complex.
The columns labeled ‘Contact’ group the residues that together form one extended contact.
| Contact | Chain | Residue | Contact chain | Contact residue |
|---|---|---|---|---|
| Old orthorhombic | ||||
| 1 | Arp3 | Arg4 | ARPC3 | Ser38 |
| 2 | Arp3 | Thr25 | ARPC1 | Ala298 |
| 2 | Arp3 | Trp153 | ARPC1 | Phe302 |
| 2 | Arp3 | Thr154 | ARPC1 | Arg301 |
| 2 | Arp3 | Glu160 | ARPC1 | Lys307 |
| 2 | Arp3 | Arg161 | ARPC1 | Leu305 |
| 3 | Arp3 | Glu218 | Arp2 | Lys331 |
| 4 | Arp3 | Glu266 | ARPC3 | Lys109 |
| 5 | Arp3 | Met383 | ARPC3 | Phe302 |
| 6 | ARPC1 | Gly39 | ARPC2 | Gly90 |
| 7 | ARPC1 | Lys139 | ARPC2 | Asn101 |
| 7 | ARPC1 | Glu193 | ARPC2 | Lys106 |
| 8 | ARPC1 | Arg179 | ARPC2 | Glu20 |
| 9 | ARPC1 | Arg360 | ARPC2 | Asn49 |
| 10 | ARPC3 | Thr101 | ARPC5 | Arg74 |
| New orthorhombic | ||||
| 1 | Arp3 | Thr247 | ARPC2 | Arg190 |
| 2 | Arp3 | Lys265 | ARPC5 | Asn120 |
| 3 | Arp3 | Lys340 | Arp2 | Glu335 |
| 4 | Arp2 | Trp86 | ARPC5 | Arg74 |
| 5 | Arp2 | Phe97 | ARPC2 | Lys136 |
| 6 | Arp2 | Lys336 | Arp3 | Arg333 |
| 7 | ARPC1 | Gly83 | ARPC2 | Asn86 |
| 7 | ARPC1 | Arg84 | ARPC2 | Tyr64 |
| 8 | ARPC2 | Glu20 | ARPC5 | Lys131 |
| 8 | ARPC2 | Ala24 | ARPC4 | Arg6 |
| 9 | ARPC2 | Lys106 | ARPC5 | Lys131 |
| 9 | ARPC2 | Asp107 | ARPC5 | Asn89, Lys93 |
| 10 | ARPC2 | Lys158 | ARPC3 | Gln27 |
| 11 | ARPC2 | Gln183 | ARPC3 | Glu150 |
| 11 | ARPC2 | Lys186 | ARPC3 | Glu150 |
| 11 | ARPC2 | Leu199 | ARPC3 | Glu146 |
| New tetragonal | ||||
| 1 | Arp3 | Pro194 | Arp2 | Asn370 |
| 2 | Arp3 | Thr247 | ARPC2 | Arg190 |
| 2 | Arp3 | Asp248 | ARPC4 | Arg158 |
| 3 | Arp3 | Ser263 | ARPC5 | Asn120 |
| 3 | Arp3 | Lys265 | ARPC4 | Asn56 |
| 3 | Arp3 | Glu266 | ARPC4 | Thr4 |
| 4 | Arp3 | Glu352 | ARPC5 | Ser44 |
| 5 | Arp2 | Glu274 | ARPC1 | Lys307 |
| 5 | Arp2 | Arg328 | ARPC1 | Phe302 |
| 6 | Arp2 | Lys366 | ARPC4 | Pro290 |
| 7 | ARPC2 | Ala23 | ARPC4 | Arg6 |
| 8 | ARPC2 | Asp159 | ARPC3 | Arg25 |
| 8 | ARPC2 | Arg160 | ARPC3 | Gln27 |
| 9 | ARPC2 | Gln183 | ARPC3 | Pro151 |
| 9 | ARPC2 | Glu187 | ARPC3 | Lys147 |
| 9 | ARPC2 | His193 | ARPC3 | Asp38 |
| 9 | ARPC2 | Ser201 | ARPC3 | Lys88 |
| 9 | ARPC2 | Glu204 | ARPC3 | Ser89 |
| 9 | ARPC2 | Leu207 | ARPC3 | Glu92 |
| 9 | ARPC2 | Asp211 | ARPC3 | Lys93 |
| 10 | ARPC3 | Lys14 | ARPC5 | Asn65 |
| 11 | ARPC3 | Glu125 | ARPC5 | Gly30 |
Although the map of Arp2 in the new P212121 space group is noisy (owing to the low resolution and quality of the diffraction data reflected by the high R merge values), it includes density for an α-helix in subdomain 2 corresponding to residues 82–94 (Fig. 1 ▸ a). This 2F o − F c map generated at 1.0σ shows the helix located in subdomain 2 viewed down its axis. The superimposed model of actin shows the missing regions of Arp2 (Fig. 1 ▸ a). A crystal contact (Table 2 ▸) appears to stabilize the C-terminal residues of Arp2, resulting in density for the backbone of residues 344–358.
The resolution of the maps from the P41 crystals was lower, but densities are clear for the helix of subdomain 2 of Arp2 consisting of residues 80–94 (Fig. 1 ▸ b). Additional density was seen at the C-terminal end spanning residues 344–379. Owing to the low resolution, these residues were modeled as polyalanine.
Although the new maps have more density for subdomains 1 and 2 of Arp2 than the maps of crystals in the original P212121 space group, even more density was present for these subdomains when the original P212121 crystals were cross-linked with glutaraldehyde (Nolen & Pollard, 2007 ▸) or when the Arp2/3 complex was cocrystallized with bound GMF (glial maturation factor; Luan & Nolen, 2013 ▸). Neither new map has density corresponding to residues 95–114 as seen in the maps for the cross-linked Arp2/3 complex (Nolen & Pollard, 2007 ▸).
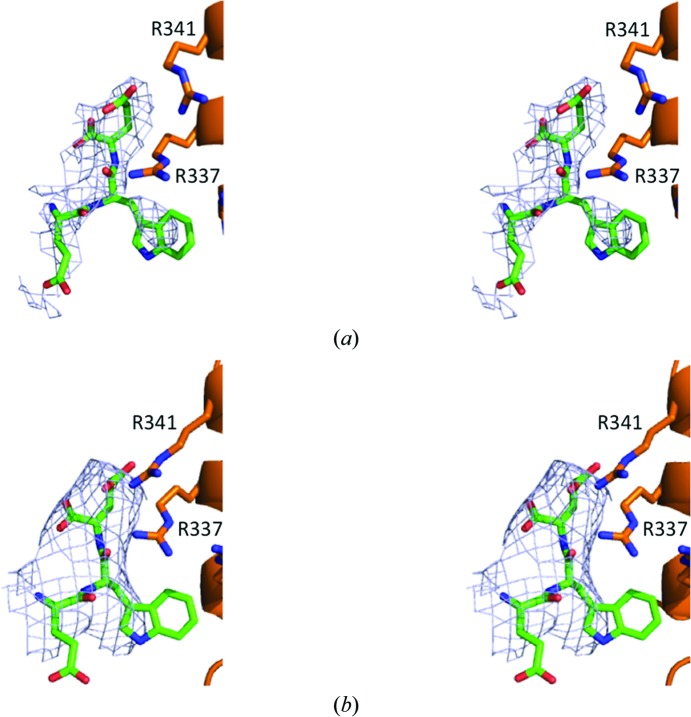
3.3. A-motif binding sites
We explored the electron-density maps of the Arp2/3 complex in both new space groups for bound CA. The 2F o − F c maps from both space groups contoured at 1σ have density next to subdomain 4 of Arp3 corresponding to our model of the bound C-terminal EWE tripeptide of the A motif (Figs. 2 ▸ a and 2 ▸ b). The density for the peptide is clearer in the higher resolution orthorhombic map (Fig. 2 ▸ a) than in the tetragonal map (Fig. 2 ▸ b). A crystal contact in the orthorhombic space group is close to the binding site for the A motif on Arp3, but leaves sufficient space for the model of the EWE tripeptide of the A motif (Fig. 2 ▸ a). It is not possible to predict whether crystal contacts sterically hinder the binding of other residues of the A and C motifs. In the tetragonal space group the region of Arp3 that interacts with EWE is fully exposed to solvent and is not close to any crystal contact.
Figure 2.
Stereoviews of the electron density associated with the A-motif binding site on Arp3 in the two new crystal forms. (a) Map from the P212121 crystals. (b) Map from the P41 crystals. Details of 2F o − F c electron-density maps contoured at 1σ from the region of Arp3 known to bind the C-terminal EWE sequence of WASP are shown in blue. The orange models are ribbon diagrams of the backbone of Arp3 with stick figures of several key side chains based on refined data from the new crystals. The green stick figure of the C-terminal EWE sequence of WASP is positioned as in our previous work (PDB entry 3rse; Ti et al., 2011 ▸).
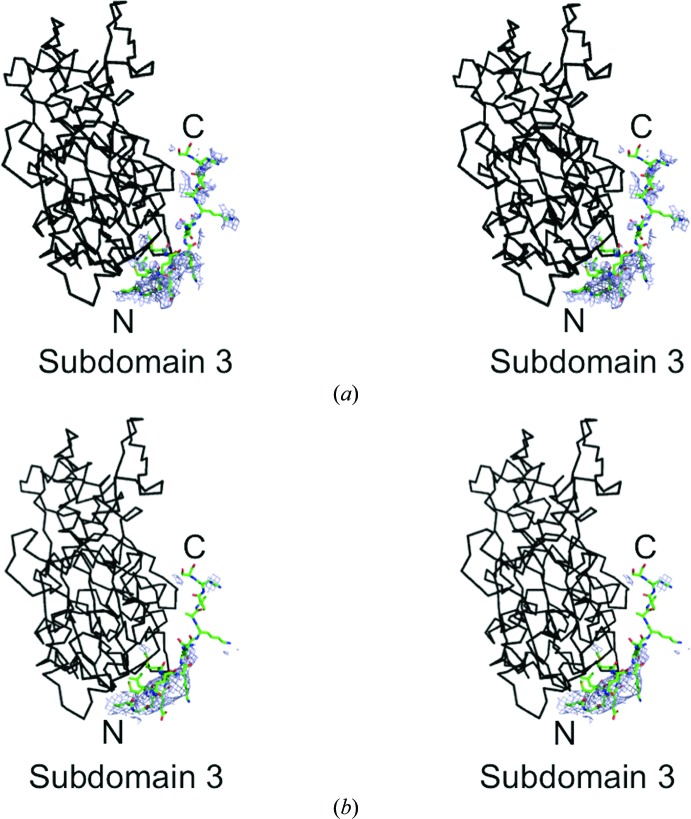
3.4. Possible C-motif binding sites
Maps from both space groups have an extended density that follows the surface of Arp2 out of the barbed-end groove (Fig. 3 ▸). The details are clearer in the map of the orthorhombic space group (Fig. 3 ▸ a) than the lower resolution map of the tetragonal space group (Fig. 3 ▸ b). These densities are located where the C motif of the VCA domain is proposed to form a helix bound to Arp2 (Boczkowska et al., 2008 ▸) in a site similar to that of the short helices of WASP WH2 (Chereau et al., 2005 ▸) and the G-actin binding (GAB) motif of Ena/VASP that bound near the barbed-end groove of actin (Ferron et al., 2007 ▸). We tested this hypothesis by superimposing the model of actin with a bound WASP WH2 motif (Chereau et al., 2005 ▸) on the maps of Arp2 from both new crystal forms. With the secondary-structural elements of actin aligned with the densities of Arp2 subdomains 1, 3 and 4, a model of N-WASP residues 432–440 built onto the backbone of the WH2 helix roughly fits into the densities in the barbed-end groove of Arp2 in both of the new maps.
Figure 3.
Stereoviews of small parts of composite OMIT maps contoured at 1σ showing density at the barbed end of Arp2 from crystals in the (a) orthorhombic and (b) tetragonal space groups in blue. Docked onto the model of Arp2 is a black backbone model of actin with a green stick figure of bound human WASP WH2 (PDB entry 2a3z; Chereau et al., 2005 ▸). The side chains in the WH2 model are from the C motif of Bos taurus N-WASP with N-terminal residue Arg431 and C-terminal residue Thr447.
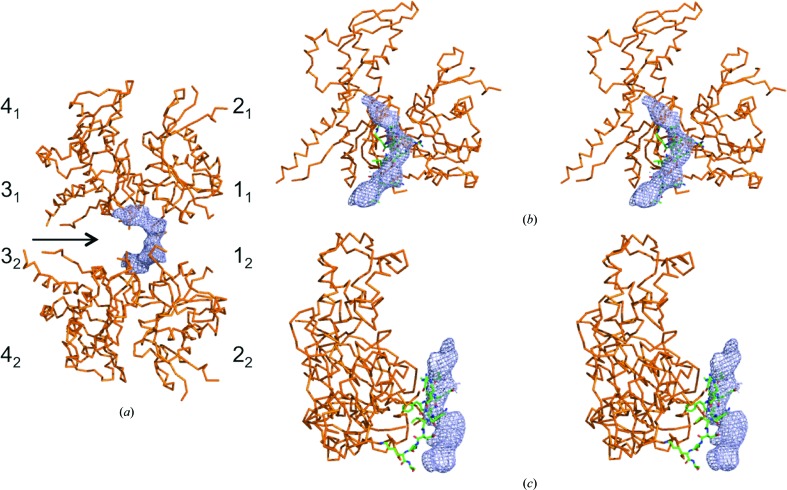
The simulated-annealing composite OMIT maps from the P41 crystals had a similar extended density at the barbed end of Arp3 (Fig. 4 ▸). The continuous lobe of density in these maps is located near the twofold noncrystallographic axis and must be contributed to by both molecules. The crystal contact in the P41 crystals may stabilize this density next to Arp3, since the barbed-end groove of Arp3 is exposed to solvent but is empty in the new P212121 crystals. The position of this density is similar but not identical to that of a crystal contact in the original P212121 crystals that placed a two-turn helix of ARPC1 in the barbed-end groove of Arp3 (Robinson et al., 2001 ▸). This crystal contact with ARPC1 precluded binding of the C motif to Arp3, but the ARPC1 helix was too distant from the barbed end of Arp3 to interact in the new crystals. The position of this density differs from the binding sites for WH2 on actin and the Ena/VASP G-actin binding domain on actin. If this is the CA helix, it is expected to have the opposite orientation from the ARPC1 helix in the original P212121 crystals.
Figure 4.
Analysis of density near the barbed-end groove of Arp3 from crystals in the tetragonal space group. The density shown in blue is from a simulated-annealing composite OMIT map contoured at 1σ. Arp3 is shown as an orange backbone model. (a) View of two Arp3 molecules located on the noncrystallographic twofold rotational symmetry axis at the interface between the Arp2/3 complexes in the asymmetric unit. The four subdomains of Arp3 are numbered with subscripts 1 and 2 to indicate different Arp3 molecules. (b, c) Stereo pairs of models of Arp3 with a green backbone model of the ARPC1 insert helix from the structure of the Arp2/3 complex (PDB entry 3rse). The orientation of Arp3 in (b) is similar to that of the top Arp3 in (a) but is rotated about 20° around the x axis. The view in (c) is rotated 90° about the y axis relative to (b).
To determine whether the C-motif bound to this site on Arp3 would interfere with the first actin subunit in the daughter filament, we overlaid the new structure of Arp3 and the associated density in the P41 space group onto the EM structure of the branch junction of the Arp2/3 complex with an F-actin filament (Pfaendtner et al., 2012 ▸; Supplementary Fig. S2). The density next to Arp3 in these crystals does not clash with actin subunit 1, indicating that VCA bound to this site could deliver the first actin to the daughter filament rather than interfering with this interaction like a WH2 motif bound to actin.
In the original orthorhombic crystals (Robinson et al., 2001 ▸) a crystal contact with a helix from ARPC1 blocked the pointed end of Arp3 where the inhibitory CA may bind (Ti et al., 2011 ▸; Xu et al., 2012 ▸). Neither new crystal has this interaction with ARPC1, but no new density is seen in either crystal form.
3.5. Prospects for further studies
Both new crystal forms have several densities with clues about the interactions of CA with the Arp2/3 complex. Both have density in the barbed-end groove of Arp2 where the C motif is predicted to bind (Boczkowska et al., 2008 ▸) but had not appeared in previous crystals. Both new crystal forms have density corresponding to the A-motif tripeptide that binds to Arp3. Furthermore, the P41 crystals have density in the barbed-end groove of Arp3 that cannot be the ARPC1 helix observed in the original P212121 crystals and may be bound C motif. As noted previously (Ti et al., 2011 ▸), the A motif is long enough to link the C-terminal tripeptide bound to subdomain 4 of Arp3 to the C-motif helix in the barbed-end groove of Arp3, while the V motif is long enough to dock actin 1 of the daughter filament. Our new evidence strengthens the case that a pair of VCAs dock the first two subunits of the daughter filament (Padrick et al., 2011 ▸).
These crystals are a promising step towards characterizing the interactions between nucleation-promoting factors and the Arp2/3 complex. Hopefully, improvements in crystallization and data collection will yield better maps of Arp2 as well as definitive crystallographic evidence regarding the second and third N-WASP binding sites.
Supplementary Material
PDB reference: Arp2/3 complex, 4xei
PDB reference: 4xf2
Supporting Information.. DOI: 10.1107/S2053230X15013515/hv5302sup1.pdf
Acknowledgments
The research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award No. PO1GM066311. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the editor and anonymous reviewers for their constructive comments that strengthened the paper. We thank staff at the Richards Center at Yale University for supplying both software and computational support for all modeling and refinement applications. We thank Dr Gregor Blaha for collecting the data used for the new orthorhombic Arp2/3 complex crystals. We thank the National Synchrotron Light Source, Brookhaven National Laboratory, which is supported by the US Department of Energy, Office of Science and Office of Basic Energy Sciences under Contract No. DE-AC02-98CH10886. We thank the NSLS beamline X25 staff supported by the Offices of Biological and Environmental Research and of Basic Energy Sciences of the US Department of Energy and the National Center for Research Resources (P41RR012408) and the National Institute of General Medical Sciences (P41GM103473) of the National Institutes of Health. We thank the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357, and the NE-CAT staff supported by a grant from the National Institute of General Medical Sciences (P41 GM103403) from the National Institutes of Health for the use of beamline ID-24-E.
References
- Adams, P. D. et al. (2010). Acta Cryst. D66, 213–221.
- Boczkowska, M., Rebowski, G., Kast, D. J. & Dominguez, R. (2014). Nature Commun. 5, 3308. [DOI] [PMC free article] [PubMed]
- Boczkowska, M., Rebowski, G., Petoukhov, M. V., Hayes, D. B., Svergun, D. I. & Dominguez, R. (2008). Structure, 16, 695–704. [DOI] [PMC free article] [PubMed]
- Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst. D54, 905–921. [DOI] [PubMed]
- Chereau, D., Kerff, F., Graceffa, P., Grabarek, Z., Langsetmo, K. & Dominguez, R. (2005). Proc. Natl Acad. Sci. USA, 102, 16644–16649. [DOI] [PMC free article] [PubMed]
- Dalhaimer, P. & Pollard, T. D. (2010). Biophys. J. 99, 2568–2576. [DOI] [PMC free article] [PubMed]
- DeLano, W. L. (2002). PyMOL. http://www.pymol.org. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Ferron, F., Rebowski, G., Lee, S. H. & Dominguez, R. (2007). EMBO J. 26, 4597–4606. [DOI] [PMC free article] [PubMed]
- Goley, E. D. & Welch, M. D. (2006). Nature Rev. Mol. Cell Biol. 7, 713–726. [DOI] [PubMed]
- Gross, S. R. (2013). Cell Adh. Migr. 7, 199–213. [DOI] [PMC free article] [PubMed]
- Higgs, H. N. & Pollard, T. D. (2001). Annu. Rev. Biochem. 70, 649–676. [DOI] [PubMed]
- Kiselar, J. G., Mahaffy, R., Pollard, T. D., Almo, S. C. & Chance, M. R. (2007). Proc. Natl Acad. Sci. USA, 104, 1552–1557. [DOI] [PMC free article] [PubMed]
- Luan, Q. & Nolen, B. J. (2013). Nature Struct. Mol. Biol. 20, 1062–1068. [DOI] [PMC free article] [PubMed]
- Machesky, L. M., Mullins, R. D., Higgs, H. N., Kaiser, D. A., Blanchoin, L., May, R. C., Hall, M. E. & Pollard, T. D. (1999). Proc. Natl Acad. Sci. USA, 96, 3739–3744. [DOI] [PMC free article] [PubMed]
- Mullins, R. D., Heuser, J. A. & Pollard, T. D. (1998). Proc. Natl Acad. Sci. USA, 95, 6181–6186. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Nolen, B. J., Littlefield, R. S. & Pollard, T. D. (2004). Proc. Natl Acad. Sci. USA, 101, 15627–15632. [DOI] [PMC free article] [PubMed]
- Nolen, B. J. & Pollard, T. D. (2007). Mol. Cell, 26, 449–457. [DOI] [PMC free article] [PubMed]
- Nolen, B. J. & Pollard, T. D. (2008). J. Biol. Chem. 283, 26490–26498. [DOI] [PMC free article] [PubMed]
- Nolen, B. J., Tomasevic, N., Russell, A., Pierce, D. W., Jia, Z., McCormick, C. D., Hartman, J., Sakowicz, R. & Pollard, T. D. (2009). Nature (London), 460, 1031–1034. [DOI] [PMC free article] [PubMed]
- Nürnberg, A., Kitzing, T. & Grosse, R. (2011). Nature Rev. Cancer, 11, 177–187. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Padrick, S. B., Doolittle, L. K., Brautigam, C. A., King, D. S. & Rosen, M. K. (2011). Proc. Natl Acad. Sci. USA, 108, E472–E479. [DOI] [PMC free article] [PubMed]
- Pfaendtner, J., Volkmann, N., Hanein, D., Dalhaimer, P., Pollard, T. D. & Voth, G. A. (2012). J. Mol. Biol. 416, 148–161. [DOI] [PMC free article] [PubMed]
- Pollard, T. D. (2007). Annu. Rev. Biophys. Biomol. Struct. 36, 451–477. [DOI] [PubMed]
- Robinson, R. C., Turbedsky, K., Kaiser, D. A., Marchand, J. B., Higgs, H. N., Choe, S. & Pollard, T. D. (2001). Science, 294, 1679–1684. [DOI] [PubMed]
- Rotty, J. D., Wu, C. & Bear, J. E. (2012). Nature Rev. Mol. Cell Biol. 14, 7–12. [DOI] [PubMed]
- Rouiller, I., Xu, X.-P., Amann, K. J., Egile, C., Nickell, S., Nicastro, D., Li, R., Pollard, T. D., Volkmann, N. & Hanein, D. (2008). J. Cell Biol. 180, 887–895. [DOI] [PMC free article] [PubMed]
- Stradal, T. E. & Scita, G. (2006). Curr. Opin. Cell Biol. 18, 4–10. [DOI] [PubMed]
- Ti, S.-C., Jurgenson, C. T., Nolen, B. J. & Pollard, T. D. (2011). Proc. Natl Acad. Sci. USA, 108, E463–E471. [DOI] [PMC free article] [PubMed]
- Welch, M. D. & Way, M. (2013). Cell Host Microbe, 14, 242–255. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Xu, X.-P., Rouiller, I., Slaughter, B. D., Egile, C., Kim, E., Unruh, J. R., Fan, X., Pollard, T. D., Li, R., Hanein, D. & Volkmann, N. (2012). EMBO J. 31, 236–247. [DOI] [PMC free article] [PubMed]
- Yang, C. & Svitkina, T. (2011). Cell. Adh. Migr. 5, 402–408. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: Arp2/3 complex, 4xei
PDB reference: 4xf2
Supporting Information.. DOI: 10.1107/S2053230X15013515/hv5302sup1.pdf