Abstract
Vismodegib hedgehog signaling inhibition treatment has potential for reducing the burden of multiple skin basal cell carcinomas and jaw keratocystic odontogenic tumors. They are major criteria for the diagnosis of Gorlin syndrome, also called nevoid basal cell carcinoma syndrome. Clinical features of Gorlin syndrome are reported, and the relevance of hedgehog signaling pathway inhibition by oral vismodegib for maxillofacial surgeons is highlighted. In summary, progressed basal cell carcinoma lesions are virtually inoperable. Keratocystic odontogenic tumors have an aggressive behavior including rapid growth and extension into adjacent tissues. Interestingly, nearly complete regression of multiple Gorlin syndrome-associated keratocystic odontogenic tumors following treatment with vismodegib. Due to radio-hypersensitivity in Gorlin syndrome, avoidance of treatment by radiotherapy is strongly recommended for all affected individuals. Vismodegib can help in those instances where radiation is contra-indicated, or the lesions are inoperable. The effect of vismodegib on basal cell carcinomas was associated with a significant decrease in hedgehog-signaling and tumor proliferation. Vismodegib, a new and approved drug for the treatment of advanced basal cell carcinoma, is a specific oncogene inhibitor. It also seems to be effective for treatment of keratocystic odontogenic tumors and basal cell carcinomas in Gorlin syndrome, rendering the surgical resections less challenging.
Keywords: Basal cell nevus syndrome, Gorlin syndrome, hedgehog-signaling inhibition, jaw keratocystic odontogenic tumors, multiple skin basal cell carcinomas, vismodegib
INTRODUCTION
The Gorlin syndrome (GS) (Online Mendelian Inheritance in Man Number 109400), also known as nevoid basal cell carcinoma (BCC) syndrome, is a multi-system genetic disorder, and the cardinal features are multiple skin BCCs and jaw keratocystic odontogenic tumors (KOTs). In 1960, Gorlin and Goltz first defined this syndrome.[1]
So far, treatment is mainly by technically challenging surgical procedures. Other local procedures, like laser ablation, photodynamic therapy and topical chemotherapy, can be used to manage small and superficial BCCs for GS. Lesions progressed to a locally advanced state, and metastatic BCCs are virtually inoperable. These KOTs have an aggressive behavior.
Hedgehog (HH)-signaling plays an important role in guiding developmental processes. In an unaffected adult individual, the HH-signaling pathway is quiescent. The GS is a genetic disorder, which is mainly caused by loss-of-function mutations in the tumor suppressor patched-1 homolog gene. This causes the release of inhibition of the smoothened (SMO) oncogene and subsequent re-activation of the HH-signaling pathway and potentially, GS.
Interestingly, nearly complete regression of multiple GS-associated KOTs following treatment with vismodegib, a specific oncogene-inhibitor and an approved drug for the treatment of advanced BCC (aBCC), was reported in 2011.[2] This study refers to a case report with a single patient and thus should be taken with care.
In this mini-review of the literature, using the search engine PubMed with the following keywords alone or in combination was conducted. Key words: GS, basal cell nevus syndrome, multiple skin BCCs, jaw KOTs, vismodegib, and HH-signaling inhibition. The potential of vismodegib HH-signaling inhibition treatment for reducing the burden of KOTs associated with GS is further discussed.
CLINICAL FEATURES
The diagnosis of GS is made in the presence of two major criteria and one minor criterion or one major criterion and three minor criteria.[3,4] Major criteria are multiple BCCs of the skin, jaw KOTs, pits of the palm and sole, progressive intracranial calcification, or a first-degree relative with GS [Table 1]. Minor criteria are macrocephaly, congenital malformations, other skeletal abnormalities, radiological abnormalities, (ovarian) fibromas, or medulloblastomas [Table 1].[3]
Table 1.
Diagnostic criteria for Gorlin syndrome

The large number of BCCs represents a substantial physical and psychological burden for patients with GS. The quality of life for patients with GS is further severely diminished by the need for frequent, repetitive, and scarring surgical procedures.[5] Although most BCCs are surgically treated with complete resection, some of these lesions might progress to a locally advanced state (i.e., the tumor has infiltrated the connective tissues, bone and/or cartilage), and then might spread to distant sites (i.e., metastatic).
KOTs mostly occur in the jaws [Figure 1], with potential for substantial (facial) disfigurement and speech impediment. The current treatment option is complete surgical resection, however, the procedures are often technically challenging, because of the location of the cyst to inaccessible sites – due to complex anatomical structures of the head and neck region. Therefore, complete resection is not always achievable, and subsequently, there are high recurrence rates. More than 65% of patients with GS have KOTs. The KOTs that are related to GS grow to sizes larger than any other.[6] These KOTs have an aggressive behavior including rapid growth and extension into adjacent tissues.[7] Obviously, this is of importance for the maxillofacial surgeons.
Figure 1.
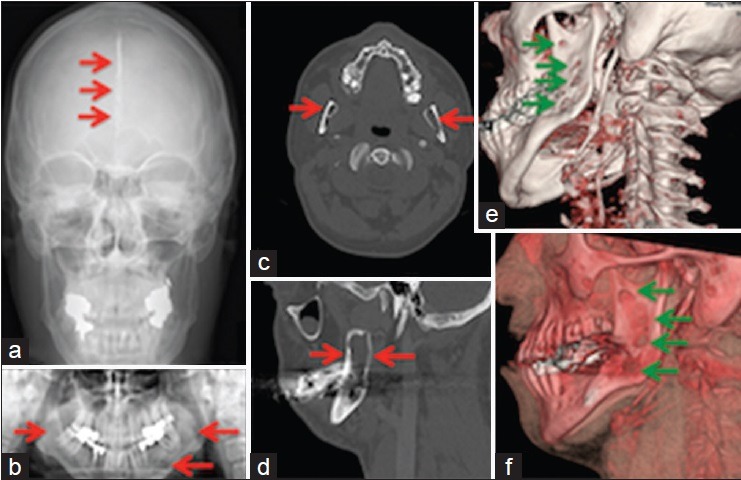
Images of a patient with Gorlin-Goltz syndrome: (a) Calcification of the falx cerebri ( ). (b) Lesions (
). (b) Lesions ( ) of the mandible. (c) Mandibular cystic lucencies (
) of the mandible. (c) Mandibular cystic lucencies ( ). (d) Considerable loss of bone (
). (d) Considerable loss of bone ( ) in the mandible. (e and f) Mandibular odontogenic keratocysts (
) in the mandible. (e and f) Mandibular odontogenic keratocysts ( )
)
EPIDEMIOLOGY
GS is an autosomal-dominant inherited disorder, characterized by a predisposition to multiple neoplasms and a wide range of developmental abnormalities.[8] The syndrome has an estimated prevalence of 1 in 31,000–1/256,000.[9,10,11,12,13] All affected individuals have certain key features [Table 1], but there is significant phenotypic variability within and among kindreds with respect to malformations.[14]
ETIOLOGY
Mutations in the tumor suppressor patched homolog (PTCH) genes[15] and the suppressor of fused gene are shown in Table 2. These genes are components of the HH-signaling pathway. Some of the associated developmental defects may also arise through a two-hit mechanism.[16] Modifying genes and germline variants may play an important role in determining the phenotype.[17]
Table 2.
Mutations identified in Gorlin syndrome patients
In an unaffected adult individual, the HH-signaling pathway is quiescent and under inhibition. The pathway gets activated upon binding of HH ligands to the transmembrane receptor PTCH1, thus allowing the transmembrane protein SMO to transfer signals through various proteins like glioma-associated oncogene (GLI) transcription factors. Vismodegib is a small molecule inhibitor of SMO. PTCH1 normally inhibits the signaling activity of SMO. HH ligands release PTCH1 protein's suppression on SMO-mediated signal transduction. In GS, the “functional-loss” mutated PTCH1 protein cannot inhibit SMO, allowing unregulated signal transduction and constitutive activation of target genes and their products. Vismodegib specifically binds and inactivates SMO and suppresses BCC growth.[8]
MOLECULAR PATHOGENESIS
Unlike in digestive tract tumors, where pathway activity and cell growth are driven by endogenous expression of HH ligands, GS-associated tumors are mostly due to mutations in the PTCH1 gene on chromosome 9q22.3. Patients with GS inherit one defective allele of the PTCH1 gene and through a “second hit” (from external factors like ultraviolet (UV) radiation exposure) functional loss of the second allele effectively eliminates PTCH1 as the “gatekeeper” that upon HH binding, normally suppresses the activation of SMO. An activated SMO would promote the transcription of HH target genes (e.g., transcription factor GLI1 gene). It also induces PTCH1 transcription, which creates a negative feedback loop. This PTCH1 is a primary component of the HH-signaling pathway [Figure 2]. It is located in the cell membrane and constitutes an HH (secreted protein/ligand) receptor; A pivotal genetic and molecular contributor in the oncogenesis of BCCs and KOTs.
Figure 2.

Schematic of the hedgehog-signaling pathway: Hedgehog ligands bind to patched homolog 1, causing release of the suppression of smoothened by patched homolog 1. Smoothened interacts with suppressor of fused, which promotes glioma-associated oncogene transcription factors. Constitutive activation of smoothened plays a role in carcinogenesis. Vismodegib inhibits smoothened
There are different manifestations of the BCCs depending on whether they are associated to the GS or not.[18] Essentially, all BCCs have enhanced HH-signaling.[19] BCC is the most common cancer in the Western world. Sporadic (i.e., not associated to the GS) BCCs are usually caused by UV radiation exposure leading to depression of the local immune system and DNA damage. These sporadic BCCs most commonly develop in the head- and neck-region. An additional risk factor for the sporadic BCC is fair skin. The number of BCCs, when not associated with GS, is only one. These sporadic BCCs are more frequent in men. In GS, the number of BCCs is 50–100. These GS-BCCs have an equal frequency in both sexes, independent of skin color. The GS-BCCs are not mainly located on the head and neck, but also on the back, thorax, abdomen, and upper part of the body.[18] A high rate (about 40%) of GS cases represents a de novo mutation.[20] They are distributed throughout the entire PTCH1 gene and it was predicted that most would cause protein truncation, resenting a null allele, and subsequent functional inactivation of the PTCH1 protein, which allows the uninhibited up-regulation of the HH-signaling - unfortunately, the mutation type cannot be used to predict disease burden.[4]
GENOTYPE/PHENOTYPE CORRELATION
Phenotypic variability in GS is a complex genetic event. No genotype-phenotype correlation between the position of the (truncation; high rate of about 86% is predicted to cause protein truncation) mutations and major clinical features is evident. The developmental defects associated with GS are likely due to haploinsufficiency.[21] Reduction in the expression of the PTCH1 gene can cause the GS.[15] Modifying genes and germline variants may play an important role in further determining the phenotype.[17]
TREATMENT
Until recently, there was no approved therapy for aBCC. Vismodegib (trade name Erivedge®) is a first-in-class drug for the treatment of aBCC. US Food and Drug Administration (FDA)-approval for vismodegib was obtained in January 2012. Meanwhile, approval for use in the European Union has been obtained.
In 2011, Goldberg et al. reported the nearly complete regression of multiple GS-associated KOTs following nonsurgical 270 mg/d (phase I study) treatment with vismodegib.[2] A pivotal phase II trial earned vismodegib (2-chloro-N-[4-chloro-3-(2-pyridininyl) phenyl]-4-[methylsulfonyl] benzamide) US FDA-approval for the treatment of locally advanced and metastatic BCCs.[22] The current literature seems to indicate that inhibition of the HH pathway is relatively safe in adults; appropriate toxicology studies have been performed.[22] Vismodegib is a pregnancy category D drug teratogenic.[23] Vismodegib is embryonic-lethal in rats.[24] The toxic effects appear to be related to inhibition of the drug target, as LDE 225 – same class; SMO inhibition did also cause taste loss and muscle cramps.[25,26] The most common side effects of vismodegib are muscle cramps/spasms, hair loss (alopecia), “distortion of the sense of taste” (dysgeusia), weight loss, and fatigue. The side effects of vismodegib have resulted in occasional discontinuation of the drug in trials.
Vismodegib, also known as GDC-0449, inhibits HH-signaling through binding and inhibiting SMO receptor and subsequent downstream HH target genes/proteins (e.g., transforming growth factor-beta [TGF-β], BCL2, BMP2, and MMP2) responsible for cell proliferation, differentiation, and tissue maintenance, via the GLI family of transcription factors.
Although radiotherapy is used for sporadic BCCs, patients with GS should avoid radiation therapy for BCCs. Due to radio-hypersensitivity in GS, avoidance of treatment by radiotherapy is strongly recommended for all affected individuals.[27] Treatment of medulloblastomas in patients with GS, radiation therapy can induce invasive BCCs in the fields of therapy.[28] Exposure to X-ray and UV radiation in GS patients induces expression of multiple BCCs. Increased skin pigmentation may be protective against UV, but not ionizing radiation (e.g., radiotherapy).[27] Patched (Ptch) heterozygous knockout mice, models of GS, demonstrated that Ptch inactivation causes radiation hypersensitivity and is essential for tumorigenesis and developmental defects associated with the GS.[29,30] Vismodegib can help in those instances where radiation therapy is contra-indicated, or the lesions are inoperable (mainly due to the topology; the head-and neck region being extremely complex and does not allow for “R0-resections”) for example, in the case of GS-BCCs and KOTs.
In a phase I trial, including 68 patients with aBCC, it was shown that vismodegib has an acceptable safety profile and encouraging anti-tumor activity.[31] In another phase I clinical trial, the safety and pharmacokinetics of vismodegib were assessed in 33 patients with locally advanced or metastatic BCC. Vismodegib appeared to have antitumor activity in these carcinomas.[32]
Vismodegib reduces BCC tumor burden and blocks the growth of new BCC in patients with GS.[19] The low incidence of serious adverse effects, along with the efficacy of the drug against BCC, suggested that vismodegib might be suitable for patients with GS. However, the common terminology criteria grade 1–2 for adverse events (e.g., muscle cramps, hair loss, weight loss, and fatigue) associated with treatment led to discontinuation for over half of treated patients with GS. An individual was described with GS, who demonstrated “rapid BCCs rebound” with the cessation of vismodegib.[33] However, Ally et al. commented on this that they feel like these conclusions are unsubstantiated.[34] Recurrence of some tumors is a known phenomenon with vismodegib cessation, rather than a “rapid BCCs rebound.”[19,22] There have been no reports of treatment resistance in BCCs, unlike medulloblastomas.[35]
The effect of vismodegib on BCC was associated with a significant decrease in HH-signaling, as shown by a 90% decrease (P < 0.001) in GLI1 messenger RNA in biopsy specimens from BCCs in patients treated for 1-month. One month of vismodegib treatment also significantly reduced tumor proliferation, as assessed by the Ki-67 expression. Vismodegib significantly reduced the rate of appearance of new surgically eligible BCCs among patients with GS.[8]
CLINICAL TRIALS
Fifty-three clinical studies were found for vismodegib using the ClinicalTrial. gov search engine. The completed ones with results studied vismodegib in BCC, metastatic colorectal cancer, or ovarian cancer.
Two studies of vismodegib in GS are not recruiting and active.
In the first trial (phase II, estimated enrollment 24-focus on adverse events and efficacy), the effects of intermittent vismodegib versus photodynamic therapy in patients with multiple BCCs are compared.
In the second trial (phase II, estimated enrollment 41), the purpose is to determine the efficacy and safety of a systemic HH pathway antagonist (vismodegib) in patients with GS.
FUTURE PERSPECTIVE
Vismodegib, useful for the treatment of BCC, also appears to be effective for treatment of KOTs in GS.[2,36] It is a sort of “killing two birds with one stone,” as it not only reduces the burden of the BCCs but also of the KOTs and renders the surgical resections less cumbersome through neo-adjuvant features in GS; the administration of oral vismodegib (150 mg/d FDA approved dose) seems to shrink KOTs in patients with GS[36] before surgery.
This new oral drug, vismodegib, is a low-molecular-weight systemic inhibitor of the HH-signaling pathway. Thereby decreasing the production of proliferation factors (e.g., TGF-β) and ultimately suppressing KOTs in GS.
Further studies to address the potential of vismodegib as “neo-adjuvant” treatment of KOTs before their surgical treatment should be carried out. Possibly, the use of vismodegib can reduce the KOTs burden in these patients with GS.[42]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960;262:908–12. doi: 10.1056/NEJM196005052621803. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg LH, Landau JM, Moody MN, Kazakevich N, Holzer AM, Myers A. Resolution of odontogenic keratocysts of the jaw in basal cell nevus syndrome with GDC-0449. Arch Dermatol. 2011;147:839–41. doi: 10.1001/archdermatol.2011.50. [DOI] [PubMed] [Google Scholar]
- 3.Kimonis VE, Goldstein AM, Pastakia B, Yang ML, Kase R, DiGiovanna JJ, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299–308. [PubMed] [Google Scholar]
- 4.Jones EA, Sajid MI, Shenton A, Evans DG. Basal cell carcinomas in Gorlin syndrome: A review of 202 patients. J Skin Cancer 2011. 2011:217378. doi: 10.1155/2011/217378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah M, Mavers M, Bree A, Fosko S, Lents NH. Quality of life and depression assessment in nevoid basal cell carcinoma syndrome. Int J Dermatol. 2011;50:268–76. doi: 10.1111/j.1365-4632.2010.04658.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohki K, Kumamoto H, Ichinohasama R, Sato T, Takahashi N, Ooya K. PTC gene mutations and expression of SHH, PTC, SMO, and GLI-1 in odontogenic keratocysts. Int J Oral Maxillofac Surg. 2004;33:584–92. doi: 10.1016/j.ijom.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Eryilmaz T, Ozmen S, Findikcioglu K, Kandal S, Aral M. Odontogenic keratocyst: An unusual location and review of the literature. Ann Plast Surg. 2009;62:210–2. doi: 10.1097/SAP.0b013e31817dad9c. [DOI] [PubMed] [Google Scholar]
- 8.Lam C, Ou JC, Billingsley EM. PTCH-ing it together: A basal cell nevus syndrome review. Dermatol Surg. 2013;39:1557–72. doi: 10.1111/dsu.12241. [DOI] [PubMed] [Google Scholar]
- 9.Evans DG, Howard E, Giblin C, Clancy T, Spencer H, Huson SM, et al. Birth incidence and prevalence of tumor-prone syndromes: Estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A:327–32. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 10.Farndon PA, Del Mastro RG, Evans DG, Kilpatrick MW. Location of gene for Gorlin syndrome. Lancet. 1992;339:581–2. doi: 10.1016/0140-6736(92)90868-4. [DOI] [PubMed] [Google Scholar]
- 11.Shanley S, Ratcliffe J, Hockey A, Haan E, Oley C, Ravine D, et al. Nevoid basal cell carcinoma syndrome: Review of 118 affected individuals. Am J Med Genet. 1994;50:282–90. doi: 10.1002/ajmg.1320500312. [DOI] [PubMed] [Google Scholar]
- 12.Endo M, Fujii K, Sugita K, Saito K, Kohno Y, Miyashita T. Nationwide survey of nevoid basal cell carcinoma syndrome in Japan revealing the low frequency of basal cell carcinoma. Am J Med Genet A. 2012;158A:351–7. doi: 10.1002/ajmg.a.34421. [DOI] [PubMed] [Google Scholar]
- 13.Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome) Orphanet J Rare Dis. 2008;3:32. doi: 10.1186/1750-1172-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimkets R, Gailani MR, Siu VM, Yang-Feng T, Pressman CL, Levanat S, et al. Molecular analysis of chromosome 9q deletions in two Gorlin syndrome patients. Am J Hum Genet. 1996;59:417–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–51. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 16.Levanat S, Gorlin RJ, Fallet S, Johnson DR, Fantasia JE, Bale AE. A two-hit model for developmental defects in Gorlin syndrome. Nat Genet. 1996;12:85–7. doi: 10.1038/ng0196-85. [DOI] [PubMed] [Google Scholar]
- 17.Bale AE. Variable expressivity of patched mutations in flies and humans. Am J Hum Genet. 1997;60:10–2. [PMC free article] [PubMed] [Google Scholar]
- 18.Ortega García de Amezaga A, García Arregui O, Zepeda Nuño S, Acha Sagredo A, Aguirre Urizar JM. Gorlin-Goltz syndrome: Clinicopathologic aspects. Med Oral Patol Oral Cir Bucal. 2008;13:E338–43. [PubMed] [Google Scholar]
- 19.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–8. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorlin R. J. Personal Communication. Minneapolis, Minn. 1982 [Google Scholar]
- 21.Wicking C, Shanley S, Smyth I, Gillies S, Negus K, Graham S, et al. Most germ-line mutations in the nevoid basal cell carcinoma syndrome lead to a premature termination of the PATCHED protein, and no genotype-phenotype correlations are evident. Am J Hum Genet. 1997;60:21–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Genentech. Erivedge (Vismodegib) Capsule. Full Prescribing Information. 2012. Available on: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203388lbl.pdf .
- 24.Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: A new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039–50. doi: 10.1016/j.clinthera.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 25.Ahnert JR, Baselga J, Tawbi HA, Shou Y, Granvil C, Dey J, et al. A phase I dose-escalation study of LDE225, a smoothened (Smo) antagonists, in patients with advanced solid tumors. J Clin Oncol. 2010;28:15S. [Google Scholar]
- 26.Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218–22. doi: 10.1158/1078-0432.CCR-12-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korczak JF, Brahim JS, DiGiovanna JJ, Kase RG, Wexler LH, Goldstein AM. Nevoid basal cell carcinoma syndrome with medulloblastoma in an African-American boy: A rare case illustrating gene-environment interaction. Am J Med Genet. 1997;69:309–14. doi: 10.1002/(sici)1096-8628(19970331)69:3<309::aid-ajmg17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.O’Malley S, Weitman D, Olding M, Sekhar L. Multiple neoplasms following craniospinal irradiation for medulloblastoma in a patient with nevoid basal cell carcinoma syndrome. Case report. J Neurosurg. 1997;86:286–8. doi: 10.3171/jns.1997.86.2.0286. [DOI] [PubMed] [Google Scholar]
- 29.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. 0020Nat Med;1998(4):619–22. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 30.Aszterbaum M, Epstein J, Oro A, Douglas V, LeBoit PE, Scott MP, et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med. 1999;5:1285–91. doi: 10.1038/15242. [DOI] [PubMed] [Google Scholar]
- 31.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 33.Wolfe CM, Green WH, Cognetta AB Jr, Hatfield HK. Basal cell carcinoma rebound after cessation of vismodegib in a nevoid basal cell carcinoma syndrome patient. Dermatol Surg. 2012;38:1863–6. doi: 10.1111/j.1524-4725.2012.02513.x. [DOI] [PubMed] [Google Scholar]
- 34.Ally MS, Wysong A, Tang JY, Aasi S. Comment on basal cell carcinoma rebound after cessation of vismodegib in an individual with basal cell nevus syndrome. Dermatol Surg. 2013;39:1413–4. doi: 10.1111/dsu.12250. [DOI] [PubMed] [Google Scholar]
- 35.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–4. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ally MS, Tang JY, Joseph T, Thompson B, Lindgren J, Raphael MA, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150:542–5. doi: 10.1001/jamadermatol.2013.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chidambaram A, Goldstein AM, Gailani MR, Gerrard B, Bale SJ, DiGiovanna JJ, et al. Mutations in the human homologue of the Drosophila patched gene in Caucasian and African-American nevoid basal cell carcinoma syndrome patients. Cancer Res. 1996;56:4599–601. [PubMed] [Google Scholar]
- 38.Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–8. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 39.Reifenberger J, Arnold N, Kiechle M, Reifenberger G, Hauschild A. Coincident PTCH and BRCA1 germline mutations in a patient with nevoid basal cell carcinoma syndrome and familial breast cancer. J Invest Dermatol. 2001;116:472–4. doi: 10.1046/j.1523-1747.2001.01279-2.x. [DOI] [PubMed] [Google Scholar]
- 40.Savino M, d’Apolito M, Formica V, Baorda F, Mari F, Renieri A, et al. Spectrum of PTCH mutations in Italian nevoid basal cell-carcinoma syndrome patients: Identification of thirteen novel alleles. Hum Mutat. 2004;24:441. doi: 10.1002/humu.9289. [DOI] [PubMed] [Google Scholar]
- 41.Fan Z, Li J, Du J, Zhang H, Shen Y, Wang CY, et al. A missense mutation in PTCH2 underlies dominantly inherited NBCCS in a Chinese family. J Med Genet. 2008;45:303–8. doi: 10.1136/jmg.2007.055343. [DOI] [PubMed] [Google Scholar]
- 42.Pastorino L, Ghiorzo P, Nasti S, Battistuzzi L, Cusano R, Marzocchi C, et al. Identification of a SUFU germline mutation in a family with Gorlin syndrome. Am J Med Genet A. 2009;149A:1539–43. doi: 10.1002/ajmg.a.32944. [DOI] [PubMed] [Google Scholar]


