Abstract
Background:
Quality of life (QoL) studies are well established when accompanying trials in head and neck cancer, but studies on long-term survivors are rare.
Aims:
The aim was to evaluate long-term follow-up patients treated with an intensified multi-modality therapy.
Setting and Design:
Cross-sectional study, tertiary care center.
Patients and Methods:
A total of 135 oral/oropharyngeal cancer survivors having been treated with an effective four modality treatment (intra-arterial induction chemotherapy, radical surgery, adjuvant radiation, concurrent systemic chemotherapy) filled European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 and HN35 questionnaires. Mean distance to treatment was 6.1 (1.3–16.6) years. Results were compared with a reference patient population (EORTC reference manual). In-study group comparison was also carried out.
Statistical Analysis:
One-sample t-test, Mann–Whitney-test, Kruskal–Wallis analysis.
Results:
QoL scores of both populations were well comparable. Global health status, cognitive and social functioning, fatigue, social eating, status of teeth, mouth opening and dryness, and sticky saliva were significantly worse in the study population; pain and need for pain killers, cough, need for nutritional support, problems with weight loss and gain were judged to be significantly less. Patients 1-year posttreatment had generally worse scores as compared to patients with two or more years distance to treatment. Complex reconstructive measures and adjuvant (chemo) radiation were main reasons for significant impairment of QoL.
Conclusion
Subjective disease status of patients following a maximized multi-modality treatment showed an expectable high degree of limitations, but was generally comparable to a reference group treated less intensively, suggesting that the administration of an intensified multi-modality treatment is feasible in terms of QoL/effectivity ratio.
Keywords: Adjuvant chemoradiation, head and neck cancer, intra-arterial induction chemotherapy, oral cancer, quality of life
INTRODUCTION
In social sciences during the middle of the last century, quality of life (QoL) emerged as an important term to describe living conditions. Medicine adopted this parameter, which more and more was given an individual character. Today and in the medical context, it means the subjective well-being of individuals, which can be measured by psychometric means. In 1980, the European Organisation for Research and Treatment of Cancer (EORTC) founded the QoL study group and developed a questionnaire often used in clinical trials in oncology.[1] Meanwhile, the measurement of QoL was integrated in the evaluation of all treatment modalities including palliative care as an additional criterion for success.[2]
Since then, QoL measurement played a prominent role in prospective clinical trials to compare different treatment arms, or to assess the effect of therapies retrospectively. Long-term studies, however, are rare. In head and neck carcinoma which is a world-wide growing cancer entity with still unfavorable outcome, a wide range of treatment modalities has been tried without resounding success. Recently, maximized multi-modality treatment regimens were tested to break resistance and counter distant spread.[3,4] The main problem of these straining, but also effective therapies was compliance due to acute toxicities.
The purpose of this study was to evaluate the long-term QoL in patients after surviving cancer due to an example of intensified multi-modality therapy.
PATIENTS AND METHODS
Totally 135 patients surviving primary oral and oropharyngeal squamous cell cancer [Table 1] were regularly attending the follow-up consultation of the Department of Cranio-Maxillofacial Plastic Surgery of the Goethe-University Frankfurt, Germany, between June and September 2008 and were asked about their QoL during that period. Exclusion criteria were age below 18 years and lacking knowledge of German language. This epidemiologic cross-sectional study was carried out using the questionnaire of the EORTC QLQ-C30 (version 3.0) and its additional disease-specific module QLQ-HN35.
Table 1.
Patient characteristics
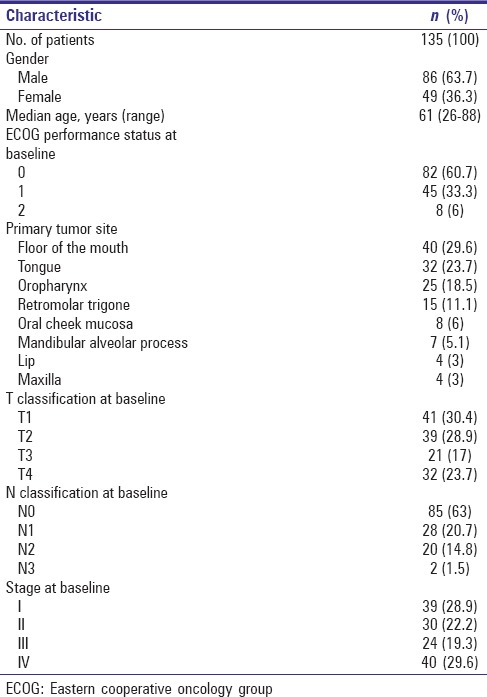
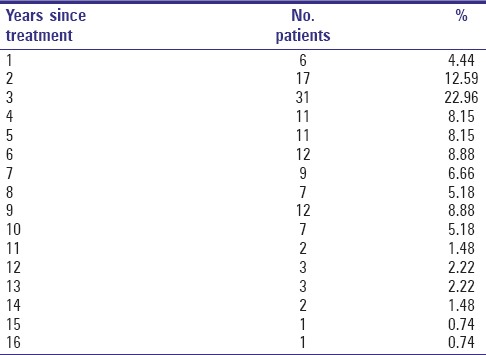
Treatment of the study patients has been carried out during a period between 1992 and 2007 [Table 2]; mean observation time since beginning of treatment was 6.1 years (±3.51; range 1.3–16.6). Fifty-nine interviewed patients (43.7%) survived longer than 5 years.
Table 2.
Distribution of patients per year since treatment
Summary of actually delivered treatment modalities can be seen in Table 3.
Table 3.
Delivered treatment modalities
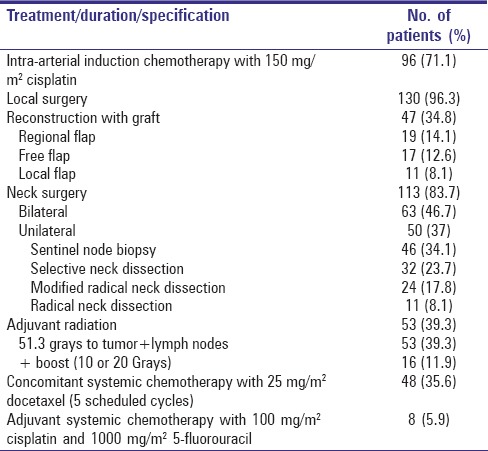
Eight patients with primary squamous cell carcinoma of the oral cavity and the oropharynx survived after a multi-modality treatment reported earlier.[5] They received three cycles of adjuvant systemic 100 mg/m2 cisplatin bolus infusion and 120-h continuous infusion of 1000 mg/m2 5-fluorouracil following radical surgery.
Hundred and twenty-seven patients with primary oral and oropharyngeal cancer survived after an intensified multi-modality treatment consisting of three parts: (1) Intra-arterial induction chemotherapy with cisplatin given to the primary, with concomitant systemic sodium thiosulfate for neutralization, followed by (2) surgery and (3) adjuvant chemoradiation with weekly doses of docetaxel.[4,6]
Treatment was initiated with three cycles of intra-arterial induction highdose cisplatin at 150 mg/m2 given on an inpatient basis (hospital admission for 4–6 days due to health care system guidelines), 3 weeks apart.
On day 1 of each neoadjuvant chemotherapy cycle, patients received hyperhydration and other supportive measures as described elsewhere.[7] Using a transfemoral approach, a 4-french catheter containing a coaxial micro-catheter for superselective visualization of the tumor-feeding vessel by means of fluoroscopy and contrast medium was inserted, and cisplatin (medac GmbH, Wedel, Germany) at a dose of 150 mg/m2 (maximum absolute dose, 300 mg) and diluted in 500 ml of 0.9% saline solution was infused at controlled pressure (2 ml/s). For analgesia, 0.1–0.3 mg of fentanyl was given intravenously, and in case of perfusion of the maxillary artery with occasional tooth ache, 5–15 mg of mepivacain was injected into the perfused artery. 10 s after initiation of the cisplatin infusion, a concomitant intravenous infusion of 9 g/m² of sodium thiosulfate was started and continued for the duration of intra-arterial cisplatin administration. After completion of chemoperfusion, hyperhydration and supportive treatment were resumed and continued until day 2.
Routine laboratory studies were performed on alternate days after the start of neoadjuvant treatment, and toxicity was graded using World Health Organization criteria.[8] Acute toxicity of this treatment was reported in several papers (e.g., Kovács 2004).[9]
Surgery was the second step of this multi-modality program (3–4 weeks after neoadjuvant treatment). Since significant downstaging of the tumor was not considered a realistic aim of induction chemotherapy, all resections were intended to include tumor-free margins based on the tumor extension prior to therapy (which was also recorded on photographs). Deep infiltration was assessed by comparison of pretherapeutic and presurgical CTs and intraoperative palpation. Surgical treatment was carried out according to the guidelines of the German-Austrian-Swiss Cooperative Group on tumors of the maxillofacial region,[10] with two important modifications. First, patients with N0 disease after baseline staging including PET underwent only ipsilateral suprahyoid neck dissection (a selective neck dissection including levels I and IIa), irrespective of the localization and size of the primary tumor. Second, in cases of lymph node involvement at baseline on whichever side of the neck, a Type III modified radical neck dissection was performed. If the histological examination of the dissection material revealed a positive finding despite baseline N0 classification, a lower neck dissection that included levels IIb to V was performed as soon as possible to eventually result in a modified radical neck dissection. Radical neck dissections were carried out in cases of fixed lymph nodes.
Beginning in March 2000, sentinel node dissection (SND) was performed instead of suprahyoid neck dissection in cases of clinical N0 status. In case of positive sentinel lymph nodes, a modified radical neck dissection was performed 1-week after SND.
In case of positive surgical margins (invasive microscopic cancer at the resection margins), an additional resection at the respective site(s) was carried out. The classification of neck levels and types of operations followed the proposal of the Committee for Neck Dissection Classification of the American Head and Neck Society.[11]
The reconstructive measures were not disturbed by induction chemotherapy. In this surviving population, they were composed of myocutaneous flaps (14.1%), microsurgical free flaps (12.6%), local flaps (8.1%), and closure following folding, rotation or expansion (65.2%).
The last treatment step (obligatory for stage 3 and 4, optional for stage 2 patients) involved weekly irradiation of the primary and lymphatic drainage area and concurrent systemic administration of docetaxel (Aventis Pharma S.A., Antony Cedex, France).[12] Before starting chemoradiation, patients were required to complete any dental procedure including surgery and tooth extraction, and to demonstrate completely healed surgical wounds. Mucositis prophylaxis included frequent rinsing of the mouth with dexpanthenol and camomile tea. Using thermal plastic masks for immobilization and 3-D planning (HELAX TMS) according to ICRU 50, radiotherapy was administered with a 6 MeV linear accelerator in daily fractions of 1.9 Gy on 5 days a week to a total dose of 51.3 Gy. If microscopic local tumor residues were detected at the surgical margin at primary surgery, an additional boost of 10 Gy was delivered; in case of infiltration of a surgical margin at the additional resection, a boost of 20 Gy (5×/week, 2.0 Gy/day) was delivered to these selected local areas, respectively. The target volume was defined as the pretreatment tumor site and the bilateral regional lymph node areas including the submental, submandibular, pharyngeal and retropharyngeal lymph nodes as well as the lower cervical and supraclavicular regions, depending on tumor localization and stage. Because of the specific surgical procedure used in patients with T1–2 N0 tumors (suprahyoidal neck dissection), the lower neck was not irradiated in these patients. The target volume was treated with a rotating field technique combined with lateral and ventral portals using multileaf collimators. The allowed radiation dose to the spinal cord was 36 Gy. The slightly higher daily dose of boost radiation was justified with the smaller target volume.
Concomitant chemotherapy was given on an inpatient basis. Docetaxel (25 mg/m2) was administered as an intravenous infusion over 60 min on day 2 of each weekly cycle of radiotherapy for a maximum of five cycles. To prevent edema and hypersensitivity reactions, the patients received oral dexamethasone 4 mg bid and oral cimetidine 300 mg daily for 3 days, starting the day before each administration of docetaxel. If a hypersensitivity reaction occurred, chemotherapy was stopped and prednisolone 250 mg and clemastine 2 mg were administered intravenously. Toxicities were graded according to the National Cancer Institute Common Toxicity Criteria v. 2.0.[13] and have been reported in several papers (e.g., Kovács et al. 2005).[12]
Before commencement of the study, local research ethics committee approval was obtained. The results of the questionnaires were evaluated to obtain “scores” according to the instructions of the EORTC, and were compared to QoL scores of a reference population of 2929 head and neck cancer patients who were examined by the QoL Group of the EORTC.[14] Reference data provide one potential reference point against which a population may be compared that was treated e.g., with an exceptionally intensified treatment regimen like that of the presented study, to look for unexpected long-term toxicity.
In a second step, values were subjected to an in-study group comparison: The effect of time since beginning of treatment, sex, tumor localization, reconstruction measures, neck surgery, and adjuvant (chemo) radiation on the QoL was examined.
Descriptive statistics were given as means and standard deviations; comparisons of means with constant values were carried out with the one-sample t-test (study versus reference population), comparisons of two groups were carried out with the Mann–Whitney-test, of more than two groups using the Kruskal–Wallis analysis (in-study group comparisons).
RESULTS
The QoL scores of both populations were well comparable, and most of the parameters were similar or not significantly different [Table 4].
Table 4.
Comparison of QoL-values
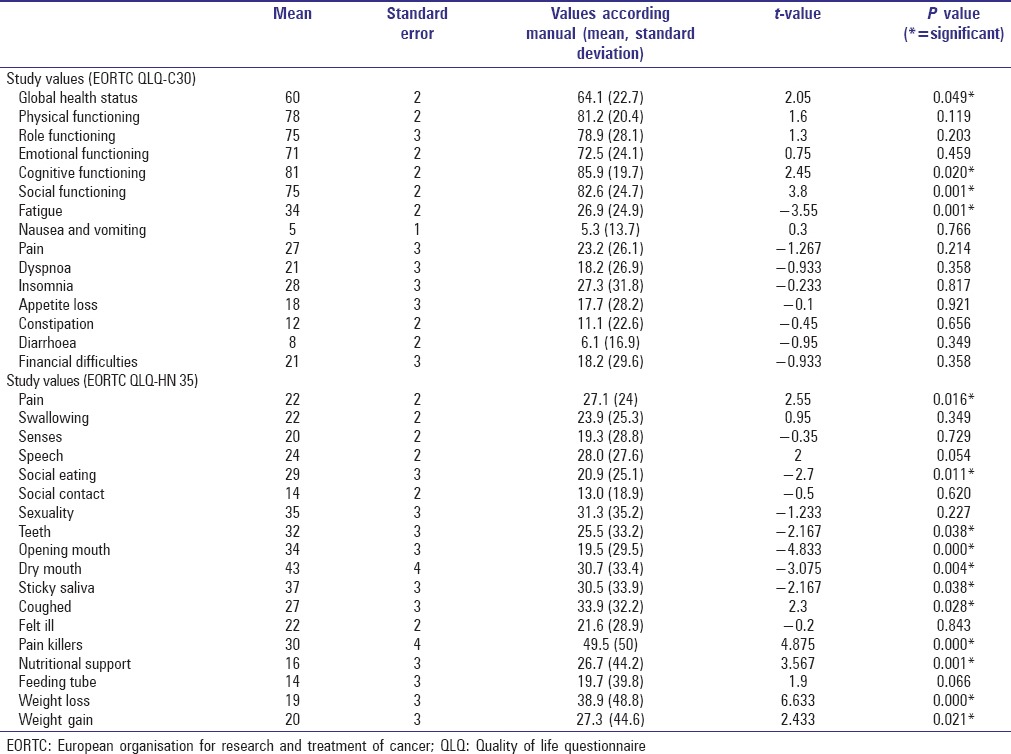
The general conditions global health status, cognitive and social functioning were significantly worse in the study population. From the symptomatic scores, fatigue was also significantly worse. In the head and neck module, social eating, status of the teeth, opening of the mouth, dryness of the mouth and sticky saliva were stated to be significantly worse as compared to the reference group while pain and need for pain killers, cough, need for nutritional support, problems with weight loss and gain were judged to be significantly less in the study population.
Time since baseline of therapy played no significant role for a change in QoL but patients 1-year posttreatment had generally worse scores as compared to patients with 2 or more years distance to treatment. Only exception was social eating (P = 0.043) and need of nutritional support (0.011) which was significantly worse for patients after 4 years distance to treatment.
Men showed significantly worse scores in cognitive (P = 0.01) and social functioning (P = 0.02) and had more financial difficulties (P < 0.01).
Tumor localization had the following impact: Social contact was significantly worse after having cancer of the floor of the mouth as compared to the tongue (P < 0.01), sticky saliva and need of feeding tubes were significantly worse in patients with oropharyngeal cancer as compared to the tongue (P < 0.01) or retromolar trigone (P < 0.01), respectively.
Between the surgical reconstruction methods there has been no significant difference in the general scores; however, in the head and neck module, scores for swallowing (distant compared with free flap and no reconstruction, each P = 0.02), need of feeding tubes (distant compared to local flap and no reconstruction, P = 0.04 and 0.01, respectively; free flap compared to no reconstruction P = 0.03), social eating (distant flap compared to no reconstruction P < 0.01) and social contact (free compared to local flap and no reconstruction, P = 0.01 and 0.03, respectively; distant compared to local flap and no reconstruction, P = 0.02 and 0.05, respectively) were significantly worse for the more extensive reconstructive measures.
The laterality of neck surgery had no significant effect on the QoL except for the opening of the mouth where unilateral or bilateral neck surgery resulted in worse scores as compared to no neck surgery (P < 0.01 and 0.02, respectively). Modified radical neck dissection, however, was significantly worse as compared to the other modalities for swallowing, speech, social eating, social contact, sexuality, mouth opening and dryness, sticky saliva, need of feeding tube and weight loss (range of P between 0.01 and < 0.001).
Adjuvant radiation resulted in worse QoL as compared to patients with no radiation. Emotional and social functioning, appetite, swallowing, senses, speech, social eating and contact, sexuality, opening of the mouth, and quality of saliva were impaired or in the case of pain, mouth dryness, and need of feeding tubes intensified (range P between 0.04 and < 0.001). Patients with concomitant systemic chemotherapy showed very similar impairments of their QoL.
Intra-arterial induction chemotherapy had no verifiable effect on QoL because possible long-term toxicities were covered by the impairment caused by the other modalities. In the eight patients with adjuvant systemic chemotherapy, none of the mentioned damaging effects could be observed; sticky saliva was found significantly less in these patients as compared to the rest of the study population (P = 0.03).
DISCUSSION
The described four-modality treatment achieved high survival rates; at a median follow-up of 4 years, the 5-year survival rate for patients receiving all four modalities was 80%; among patients with advanced disease (stage III and IV), survival was 83 and 59%, respectively[12] and after a median observation time of 5 years, the final absolute survival of patients treated with curative intention but without completing the protocol in every case, was 62% (especially, 70% and 50% for patients with operable stages III and IV, respectively).[4] This convincing effect was paid quite dearly with a loss of the global health status and impairment of cognitive and social functioning when compared to a reference population of head and neck cancer patients not treated so extensively.
The distribution of significantly impaired findings were similar to that of a large Indian tertiary care center.[15] Two studies compared their QoL results with scores of a general population,[16,17] which does not seem to be adequate; therefore, scores of a reference population of head and neck cancer patients were chosen for the present study to be more appropriate.
In comparison with this reference population and by in-study comparison, the long-time survivors reported loss of QoL concerning fatigue, status of the teeth, opening of the mouth, dryness of the mouth and saliva conditions typically associated with (chemo) radiation. These results are supported by Pourel et al.[16] who found mainly emotional and social functioning and fatigue being impaired by radiation, and others.[18] Especially xerostomia is only very slowly improving following treatment.[19] The results for modified radical neck dissection pointed in the same direction because patients who underwent this type of neck surgery due to higher clinical stage generally got radiation, too. An improvement over time as observed by Shah et al.[20] could not be seen. Free or distant flaps necessary in case of larger defects due to bulky tumors also caused damage to QoL. It can be said that higher stages urging for intensified treatment comprising (chemo) radiation resulted in higher problems with QoL.[21]
Surprisingly, preoperative chemoradiation followed by radical surgery was found to have QoL results comparable to other therapies.[22] This possibly could be explained by the removal of radiated tissue. Less surprising in this context were results which suggested similar QoL whether the patients choose primary chemoradiation or surgery with postoperative radiation because radiation is acknowledged as the main cause for a loss of QoL.[23,24,25] In contrast, chemotherapy as induction or as single adjuvant modality had no detectable or actual effect on QoL.
There has been no significant change of QoL over time, but values were better with 2 or more years distance to treatment as compared to 1-year distance. This corresponds to many other findings of long-term studies after surgical and/or (chemo) radiation treatment.[18,26,27,28]
However, there were scores in the present study which did not improve like social eating and need of nutritional support, which were worse after 4 years and more; Magné et al.[27] observed that financial and psychological problems after concomitant twice-a-day radiotherapy and chemotherapy in patients with unresectable cancer did not improve, either. Other studies even found general deterioration of QoL after 10 years by 15% when compared with years 1 and 2 following treatment.[29] It might be asked, however, whether this correlates with general aging because mean age of head and neck cancer patients is quite high.
Recently, research turns to prediction of QoL; e.g., were pretreatment predictors of depression such factors like smoking at diagnosis, more than 14 alcoholic drinks per week, T3 or T4 status, and more than three medications.[30] The results of the present study can be used as predictors, too, proceeding like Pierre et al. 2013 did: High tumor stage which needs a more complex treatment, and tumor localizations which involve tongue suspension are correlated with worse QoL.[31]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 2.Leiberich P, Averbeck M, Grote-Kusch M, Schroeder A, Olbrich E, Kalden JR. The quality of life of tumor patients as a multidimensional concept. Z Psychosom Med Psychoanal. 1993;39:26–37. [PubMed] [Google Scholar]
- 3.Schuller DE, Grecula JC, Agrawal A, Rhoades CA, Orr DA, Young DC, et al. Multimodal intensification therapy for previously untreated advanced resectable squamous cell carcinoma of the oral cavity, oropharynx, or hypopharynx. Cancer. 2002;94:3169–78. doi: 10.1002/cncr.10571. [DOI] [PubMed] [Google Scholar]
- 4.Kovács AF. Maximized combined modality treatment of an unselected population of oral and oropharyngeal cancer patients. Final results of a pilot study compared with a treatment-dependent prognosis index. J Craniomaxillofac Surg. 2006;34:74–84. doi: 10.1016/j.jcms.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Kovács AF, Ghahremani MT, Stefenelli U, Bitter K. Postoperative chemotherapy with cisplatin and 5-fluorouracil in cancer of the oral cavity and the oropharynx – long-term results. J Chemother. 2003;15:495–502. doi: 10.1179/joc.2003.15.5.495. [DOI] [PubMed] [Google Scholar]
- 6.Kovács AF, Schiemann M, Turowski B. Combined modality treatment of oral and oropharyngeal cancer including neoadjuvant intra-arterial cisplatin and radical surgery followed by concurrent radiation and chemotherapy with weekly docetaxel-Three year results of a pilot study. J Craniomaxillofac Surg. 2002;30:112–20. doi: 10.1054/jcms.2002.0283. [DOI] [PubMed] [Google Scholar]
- 7.Kovács AF, Turowski B, Ghahremani MT, Loitz M. Intra-arterial chemotherapy as neoadjuvant treatment of oral cancer. J Craniomaxillofac Surg. 1999;27:302–7. doi: 10.1054/jcms.1999.0900. [DOI] [PubMed] [Google Scholar]
- 8.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–14. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Kovács AF. Intra-arterial induction high-dose chemotherapy with cisplatin for oral and oropharyngeal cancer: Long-term results. Br J Cancer. 2004;90:1323–8. doi: 10.1038/sj.bjc.6601674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bier J. Definitions for radical surgical approach in squamous cell cancers of the oral cavity. German-Austrian-Swiss Study Group for Tumors of the Maxillofacial Region (DÖSAK) Dtsch Z Mund Kiefer Gesichtschir (German Journal for Oral and Maxillofacial Surgery) 1982;6:369–72. [Google Scholar]
- 11.Robbins KT, Denys D. Committee for Neck Dissection Classification, American Head and Neck Society. The American Head and Neck society's revised classification for neck dissection. In: Johnson JT, Shaha AR, editors. Proceedings of the 5th International Conference in Head and Neck Cancer. Madison: Omnipress; 2000. pp. 365–71. [Google Scholar]
- 12.Kovács AF, Mose S, Böttcher HD, Bitter K. Multimodality treatment including postoperative radiation and concurrent chemotherapy with weekly docetaxel is feasible and effective in patients with oral and oropharyngeal cancer. Strahlenther Onkol. 2005;181:26–34. doi: 10.1007/s00066-005-1272-3. [DOI] [PubMed] [Google Scholar]
- 13.Seegenschmiedt MH. Interdisciplinary documentation of treatment side effects in oncology. Present status and perspectives. Strahlenther Onkol. 1998;174(Suppl 3):25–9. [PubMed] [Google Scholar]
- 14.Scott NW, Fayers PM, Aaronson NK, Bottomley A, de Graeff A, Groenvold M, et al. Brussels: EORTC Quality of Life Department: EORTC; 2008. EORTC QLQ-C30 Reference Values; pp. 118–310. [Google Scholar]
- 15.Chaukar DA, Walvekar RR, Das AK, Deshpande MS, Pai PS, Chaturvedi P, et al. Quality of life in head and neck cancer survivors: A cross-sectional survey. Am J Otolaryngol. 2009;30:176–80. doi: 10.1016/j.amjoto.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Pourel N, Peiffert D, Lartigau E, Desandes E, Luporsi E, Conroy T. Quality of life in long-term survivors of oropharynx carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:742–51. doi: 10.1016/s0360-3016(02)02959-0. [DOI] [PubMed] [Google Scholar]
- 17.Funk GF, Karnell LH, Christensen AJ. Long-term health-related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138:123–33. doi: 10.1001/archoto.2011.234. [DOI] [PubMed] [Google Scholar]
- 18.Infante-Cossio P, Torres-Carranza E, Cayuela A, Hens-Aumente E, Pastor-Gaitan P, Gutierrez-Perez JL. Impact of treatment on quality of life for oral and oropharyngeal carcinoma. Int J Oral Maxillofac Surg. 2009;38:1052–8. doi: 10.1016/j.ijom.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Braam PM, Roesink JM, Raaijmakers CP, Busschers WB, Terhaard CH. Quality of life and salivary output in patients with head-and-neck cancer five years after radiotherapy. Radiat Oncol. 2007;2:3. doi: 10.1186/1748-717X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S, Har-El G, Rosenfeld RM. Short-term and long-term quality of life after neck dissection. Head Neck. 2001;23:954–61. doi: 10.1002/hed.1138. [DOI] [PubMed] [Google Scholar]
- 21.Thomas L, Moore EJ, Olsen KD, Kasperbauer JL. Long-term quality of life in young adults treated for oral cavity squamous cell cancer. Ann Otol Rhinol Laryngol. 2012;121:395–401. doi: 10.1177/000348941212100606. [DOI] [PubMed] [Google Scholar]
- 22.Klug C, Neuburg J, Glaser C, Schwarz B, Kermer C, Millesi W. Quality of life 2-10 years after combined treatment for advanced oral and oropharyngeal cancer. Int J Oral Maxillofac Surg. 2002;31:664–9. doi: 10.1054/ijom.2002.0301. [DOI] [PubMed] [Google Scholar]
- 23.Mowry SE, Ho A, Lotempio MM, Sadeghi A, Blackwell KE, Wang MB. Quality of life in advanced oropharyngeal carcinoma after chemoradiation versus surgery and radiation. Laryngoscope. 2006;116:1589–93. doi: 10.1097/01.mlg.0000233244.18901.44. [DOI] [PubMed] [Google Scholar]
- 24.Boscolo-Rizzo P, Maronato F, Marchiori C, Gava A, Da Mosto MC. Long-term quality of life after total laryngectomy and postoperative radiotherapy versus concurrent chemoradiotherapy for laryngeal preservation. Laryngoscope. 2008;118:300–6. doi: 10.1097/MLG.0b013e31815a9ed3. [DOI] [PubMed] [Google Scholar]
- 25.Payakachat N, Ounpraseuth S, Suen JY. Late complications and long-term quality of life for survivors (>5 years) with history of head and neck cancer. Head Neck. 2013;35:819–25. doi: 10.1002/hed.23035. [DOI] [PubMed] [Google Scholar]
- 26.Rogers SN, Hannah L, Lowe D, Magennis P. Quality of life 5-10 years after primary surgery for oral and oro-pharyngeal cancer. J Craniomaxillofac Surg. 1999;27:187–91. doi: 10.1016/s1010-5182(99)80049-3. [DOI] [PubMed] [Google Scholar]
- 27.Magné N, Marcy PY, Chamorey E, Guardiola E, Pivot X, Schneider M, et al. Concomitant twice-a-day radiotherapy and chemotherapy in unresectable head and neck cancer patients: A long-term quality of life analysis. Head Neck. 2001;23:678–82. doi: 10.1002/hed.1095. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Wahab M, Abitbol A, Lewin A, Troner M, Hamilton K, Markoe A. Quality-of-life assessment after hyperfractionated radiation therapy and 5-fluorouracil, cisplatin, and paclitaxel (Taxol) in inoperable and/or unresectable head and neck squamous cell carcinoma. Am J Clin Oncol. 2005;28:359–66. doi: 10.1097/01.coc.0000158837.47450.81. [DOI] [PubMed] [Google Scholar]
- 29.Mehanna HM, Morton RP. Deterioration in quality-of-life of late (10-year) survivors of head and neck cancer. Clin Otolaryngol. 2006;31:204–11. doi: 10.1111/j.1749-4486.2006.01188.x. [DOI] [PubMed] [Google Scholar]
- 30.Moubayed SP, Sampalis JS, Ayad T, Guertin L, Bissada E, Gologan OE, et al. Predicting Depression and Quality of Life among Long-term Head and Neck Cancer Survivors. Otolaryngol Head Neck Surg. 2015;152:91–7. doi: 10.1177/0194599814557772. [DOI] [PubMed] [Google Scholar]
- 31.Pierre CS, Dassonville O, Chamorey E, Poissonnet G, Ettaiche M, Santini J, et al. Long-term quality of life and its predictive factors after oncologic surgery and microvascular reconstruction in patients with oral or oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2014;271:801–7. doi: 10.1007/s00405-013-2592-z. [DOI] [PubMed] [Google Scholar]


