Abstract
The primary goals of craniofacial reconstruction include the restoration of the form, function, and facial esthetics, and in the case of pediatric patients, respect for craniofacial growth. The surgeon, however, faces several challenges when attempting a reconstructive cranioplasty. For that reason, craniofacial defect repair often requires sophisticated treatment strategies and multidisciplinary input. In the ideal situation, autologous tissue similar in structure and function to that which is missing can be utilized for repair. In the context of the craniofacial skeleton, autologous cranial bone, or secondarily rib, iliac crest, or scapular bone, is most favorable. Often, this option is limited by the finite supply of available bone. Therefore, alternative strategies to repair craniofacial defects are necessary. In the field of regenerative medicine, tissue engineering has emerged as a promising concept, and several methods of bone engineering are currently under investigation. A growth factor-based approach utilizing bone morphogenetic proteins (BMPs) has demonstrated stimulatory effects on cranial bone and defect repair. When combined with cell-based and matrix-based models, regenerative goals can be optimized. This manuscript intends to review recent investigations of tissue engineering models used for the repair of craniofacial defects with a focus on the role of BMPs, scaffold materials, and novel cell lines. When sufficient autologous bone is not available, safe and effective strategies to engineer bone would allow the surgeon to meet the reconstructive goals of the craniofacial skeleton.
Keywords: Biomimetic, bone morphogenetic protein, craniofacial defect repair, scaffold matrix, three-dimensional scaffold
INTRODUCTION
Craniofacial defect repair
The repair of craniofacial defects employs a multidisciplinary approach and broad treatment strategies. Nevertheless, healing of critical-sized craniofacial defects, whether secondary to tumor, trauma, or congenital disease, poses significant challenges. The major limitation in the repair of such defects lies in the finite supply of autologous tissue (i.e., bone) available. In addition, potential donor site morbidity (e.g., infection, pain, hemorrhage, nerve injury) necessitates continued exploration into alternative forms of bone regeneration and repair.[1,2,3] While various therapies have attempted to solve this clinical dilemma,[4,5,6,7] they are laden with disadvantages.[8,9,10] Ultimately, the objective is to develop a safe and effective strategy to heal defects of the craniofacial skeleton.
The goals of craniofacial reconstruction are four-fold: Restore function, restore form, restore facial esthetics, and respect craniofacial growth (in the case of pediatric patients). A core principle of tissue reconstruction is to replace “like with like.” To this end, the “gold-standard” material for calvarial defect repair is autologous bone. Indeed, the preferred method of cranioplasty is the use of parts of the remaining cranium. Several characteristics of autologous bone are responsible for its desirability. It is progressively incorporated into the existing craniofacial skeleton (osseointegration). Additionally, it demonstrates impressive resistance to infection and is potentially capable of growing in the expanding pediatric craniofacial skeleton. Traditionally, split calvarium[11] and rib[12] have been at the front line of craniofacial skeletal defect repair. Iliac crest[13] and scapula[14] are also suitable options. In the era of free tissue transfer, the use of composite tissue that has both a soft tissue and bony component is gaining popularity.[15] Often, however, defects are significantly large and require more than the available quantity of autologous tissue. This review will describe current research investigating the engineering of bony tissue. Kim et al. detailed soft tissue engineering in the craniofacial skeleton in an excellent article published in the previous issue of the journal.[16]
SPECIFIC CHALLENGES TO THE RECONSTRUCTIVE CRANIOPLASTY
The reconstructive surgeon must consider how to replace bone loss in the craniofacial skeleton for which autologous bone is impractical or not feasible. Similarly, particular attention must be paid to cases where the patient is in the process of growing (e.g., ages 2–5 years old). Alternative strategies are numerous and include bone ceramics, demineralized bone matrix, titanium, and porous polyethylene implants.[4,7,17] Such therapies, however, are associated with several shortcomings including an increased risk of infection, failure over time, and the inability to expand in the growing pediatric craniofacial skeleton. Furthermore, in cases of composite defects (missing skin, bone, and/or dura), or what we have termed “hostile” defects (composite defects in the setting of radiation, cigarette smoking, or scarring from previous cranioplasty attempts), chimeric free flaps containing vascularized rib, scapula, iliac crest or a combination thereof, have been utilized by our group.[15,18] However, such options involve prolonged surgeries attendant with risks such as free flap loss, anesthetic/patient-related risks (e.g., deep venous thrombosis, pulmonary embolism, myocardial infarction), and contour deformities
In the search for improved strategies to replace “like with like,” tissue engineering has emerged as a promising concept within the field of craniofacial surgery. Tissue engineering encompasses the use of a combination of cells, engineered materials, and biochemical and physicochemical factors to improve or replace biological functions. From a practical standpoint, the term denotes applications that repair or replace portions of or whole structural tissues including bone, cartilage, vasculature, solid organs, skin, and mucosa. In depth review of the theory and applications of tissue, engineering has been the subject of several articles.[16,19]
Tissue engineering can be approached in several ways [Figure 1]. These include cell-based, growth factor-based, and scaffold matrix-based therapies.[20] An example of a cell-based therapeutic approach is the engineering of bone by stimulating bone precursor cells to expand and differentiate into osteoblasts. Several strategies to do this have been attempted.[21] For example, our laboratory has successfully engineered bony tissue using high-frequency pulsed electromagnetic fields to induce osteogenic differentiation of murine osteoprogenitor cells.[22]
Figure 1.
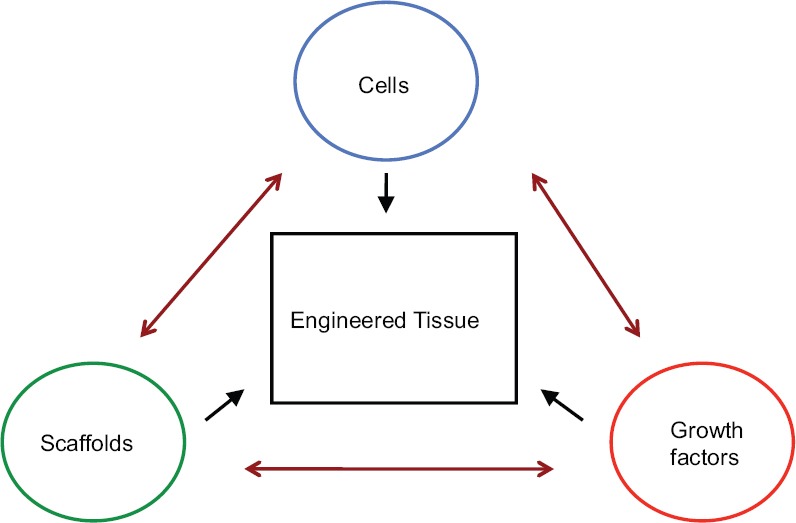
The engineering of tissue is generally approached using cell-based, growth factor-based, or scaffold matrix-based strategies. A combination of two or more strategies can also be employed
Growth factors, including signaling molecules and mitogens, form the basis of growth factor-mediated tissue engineering. An example is the administration of bone morphogenetic protein (BMP) to a critical-sized skeletal defect to stimulate bone production and defect repair. As their name would suggest, several BMP isoforms have demonstrated significant stimulatory effects on bone growth. This paper will review research investigating the use of BMP as a facilitator of bony tissue engineering.
The third approach to tissue engineering, a matrix-based model, is the newest and the least studied. An understanding that the three-dimensional (3D) structure of the extracellular matrix (ECM) is integral for tissue formation and regeneration has led researchers to make an effort to recreate this environment when attempting to repair tissue defects. Because cell- and growth factor-based approaches often fall short of delivering desired results, matrix-based strategies have become increasingly prevalent. In practice, matrix-based approaches are generally combined with cell- and/or growth factor-based approaches. Whether produced using synthetic or biologic materials, scaffold matrices enhance tissue growth and repair by facilitating delivery and localization of progenitor cells and growth factors to a desired location. Our laboratory is currently evaluating the ability of various peptides and polymers to promote 3D implantation of osteoprogenitor cells into cranial defects.
Just as the use of specific, a cell type or growth factor is related to the reconstructive goal of the situation (e.g., osteoprogenitor cells are better suited for cranial defect repair than chondroblasts), the characteristics of a carrier matrix must be considered. The identification of an ideal carrier matrix depends on several factors. Although recent investigations that combine cell- and/or growth factor-based strategies with matrix-based strategies have demonstrated promising results, many currently available scaffold matrices fall short of an ideal carrier vehicle due to problems with biodegradability, cell viability and retention, and control of release kinetics of growth factors.[23] There is, therefore, an ongoing need to identify novel scaffold platforms that are capable of facilitating bone engineering. The second part of this review will detail scaffold matrices currently under investigation for skeletal tissue regeneration.
THE SCIENCE BEHIND BONE MORPHOGENETIC PROTEINS
Bone morphogenetic proteins are growth factors that play several integral roles in the regulation of cellular behavior, including proliferation and differentiation, during development.[24] BMPs belong to the transforming growth factor beta superfamily. At least 15 isoforms have been identified in humans.[25] The importance of BMPs and their associated signaling pathways is evidenced by the fact that they are expressed by nearly all mammalian cells, both during development and throughout the lifespan of an organism. Originally recognized for their ability to induce bone and cartilage formation, BMPs are now known to regulate many additional processes including embryogenesis, fracture healing, and wound healing. Faulty BMP signaling has been implicated in pathologic states such as oncogenesis, atherosclerosis, and developmental abnormalities.[26,27]
Bone morphogenetic protein-mediated signal transduction is initiated upon the binding of BMP ligands to BMP receptors (BMPRs). There are two major classes of BMPRs known as Type I or Type II. Stimulation of both Type I and Type II BMPRs are requisite for signal transduction.[28] Type I and Type II receptors are structurally similar, comprised of an intracellular serine-threonine kinase domain, a single membrane-spanning domain, and a fairly short extracellular domain. Unlike Type I receptors, Type II receptors are constitutively active. In mammals, seven distinct Type I BMPRs have been identified, three of which bind BMPs.[29] Similarly, three Type II BMPR subtypes bind BMPs in mammals. Each BMP isoform binds to a unique combination of Type I and Type II receptors.[30] Upon ligand binding, the Type II BMPR transphosphorylates glycine-serine domains in the Type I BMPR, activating its receptor kinases. Signal activation ensues and can follow Smad-dependent or Smad-independent pathways [Figure 2].[31] Smad signaling is the predominant mechanism of signal transduction associated with serine/threonine kinase receptors. After ligand stimulation and Type II receptor-mediated activation, Type I receptors phosphorylate receptor-regulated Smads (R-Smads).[28] R-Smads become destabilized and are released into the cytoplasm where they form a complex with common-partner Smads that then translocates into the nucleus. Within the nucleus, the complex interacts with transcription factors, co-activators, and co-repressors to regulate target gene transcription.[30] Smad involvement in osteogenic gene transcription regulation is mediated via the transcription of the osteogenic master gene runt-related transcription factor 2 Runx2 and its transcriptional co-activators Osterix and Dlx5.[32,33] Smad-independent mechanisms that mediate BMP regulation include ERK, JNK, and p38 MAP kinase pathways.[31] Runx2 is also a common final target when these alternate pathways are activated for osteogenic differentiation.
Figure 2.
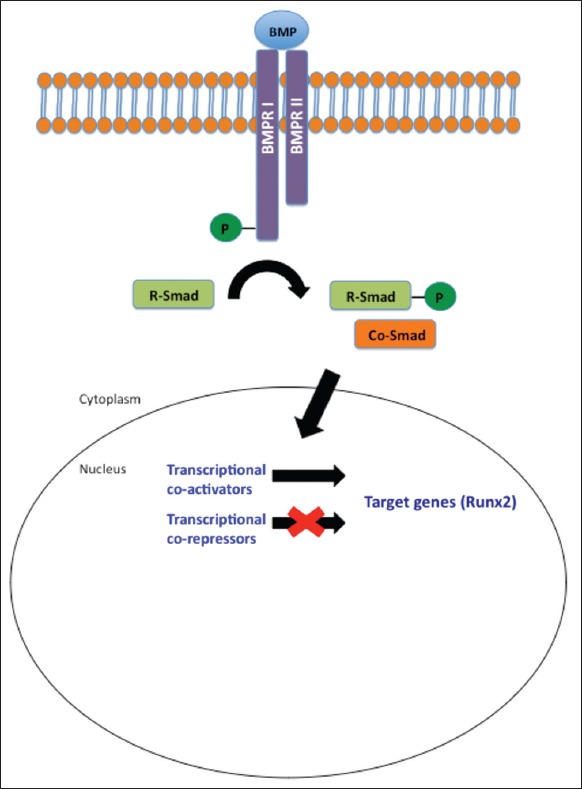
BMP signaling – Activation of BMPR-I and II by BMP initiates a transduction pathway mediated by Smad proteins. Stimulation of transcriptional co-activators and co-repressors within the nucleus facilitate regulated transcription of target genes. BMP: Bone morphogenetic protein, BMPR: BMP receptor
BONE MORPHOGENETIC PROTEINS AND BONE FORMATION
In 1965, a landmark study by Urist demonstrated that demineralized bone can induce de novo bone formation.[34] It was later discovered that BMPs were the agents responsible for these observed effects.[35] Many studies have since confirmed the importance of individual BMP isoforms as bone forming factors.[36,37,38] BMP-2, for example, strongly activates the transcription of Runx2, a protein necessary for osteoblast differentiation in humans.[39] BMP-2 also facilitates expression of cellular markers of osteogenic differentiation, including alkaline phosphatase (ALP) and osteocalcin.[40] Support for the role of BMP signaling in bone formation has also come from studies investigating the disruption of BMP-mediated processes.[41] Reduced BMP activity due to overexpression of native BMP antagonists impairs bone formation and fracture healing. In knockout models, animals deficient in BMP demonstrate abnormal bony development and healing.[42] Defects of BMPRs also have clinical consequences. Brachydactyly and proximal symphalangism have been associated with mutations in BMPRs.[43,44]
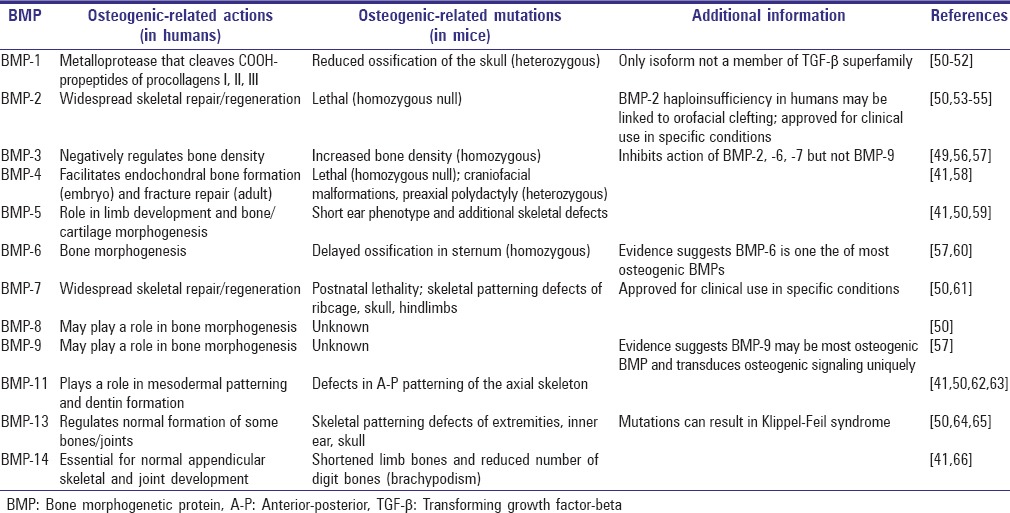
Despite a similar structure and overall function, specific BMP isoforms have distinct roles within an organism.[45,46,47,48] For example, BMP-2, -4, and -7 mediate cartilage regeneration while BMP-7, -8, and -15 are believed to facilitate growth of reproductive tissue during the early stages of development. Furthermore, studies have demonstrated that BMP-12 and -13 affect tendon healing. BMP isoforms may have both stimulatory and inhibitive effects. For example, although many isoforms have a strongly positive effect on bone development, BMP-3 is a negative regulator of osteogenesis.[49] Luu et al. comprehensively studied the role of BMP isoforms in osteogenic differentiation of mesenchymal stem cells (MSCs).[37] HEK293, C2C12, and C3H10T1/2 cells were infected with recombinant adenoviruses expressing 14 BMP isoforms (AdBMP). Expression levels of ALP and osteocalcin were measured in vitro and cells infected with AdBMPs were also implanted into the quadriceps of nude mice to assess in vivo heterotopic bone formation. The authors found that the BMPs with the greatest osteogenic potential are BMP-2, -6, and -9, and to a lesser extent BMP-4 and -7.[37] Interestingly, relatively little is known about BMP-9, but it demonstrates the greatest degree of osteogenic activity. Table 1 summarizes the osteogenic effects of BMP isoforms.[50]
Table 1.
Osteogenic-related effects of BMPs
The successful use of BMPs to repair critical-sized craniofacial defects has been demonstrated.[42] Recombinant human BMP-7 (rhBMP-7) implanted into a growing bone model of calvarial defects in infant mini-pigs demonstrated superior bone induction compared to autologous bone graft.[67] In addition, several studies describe the use of rhBMP-2 in human cranial vault, cleft, and mandible reconstruction.[42] Though promising, strategies that utilize rhBMP therapy are associated with several shortcomings including the requirement of supraphysiologic concentrations, difficulty of production, and high cost.[68]
In response to these challenges, alternate modes of BMP delivery have been explored. An example is the use of the adenoviral vector technology. The theoretical basis of this form of gene therapy consists of the delivery of recombinant BMP DNA to cells in the defect site. This facilitates synthesis and secretion of endogenous BMPs by engineered cells. Reapplication of osteoinductive signaling factors is no longer necessary as cells infected with adenoviral vectors containing coding regions of human BMP continuously supply the extracellular environment with required factors for bone growth.[69]
The concept of combining multiple strategies to successfully engineer tissue is now being utilized by several scientists. As our understanding of the biologic properties and behavior of scaffold matrices expands, it is likely that the addition of an appropriate scaffold to cell- and/or growth factor-based strategies will augment skeletal reconstruction. The following sections detail scaffolds that have been tested in osseous defect repair and highlight current efforts using these biomaterials.
SCAFFOLD BIOMIMICRY
Significant recent focus has been placed on the investigation of 3D biomimetic scaffolds for the purpose of tissue engineering. This stems from the attempt to circumvent shortcomings associated with current tissue regeneration as well as from a better understanding of the structural implications of native ECM. Scaffold development allows for the production of complex 3D structures, which are utilized for the delivery of progenitor cells and growth factors found in bone.[70] Approaching the craniofacial repair in such a manner would permit for a restoration of native tissue function, while minimizing such limitations as supply constraints, donor site morbidity, and complex anatomical form.
Scaffolds for bone engineering aim to serve as a template analogous to the ECM of bone,[71,72] and as such should possess characteristics that make them conducive to in vivo implantation. In addition to being biocompatible, scaffolds must demonstrate the capacity to support cellular adhesion and proliferation.[71] Previous studies have indicated that adhesion capacity is largely governed by pore size and interconnectivity, recommending a range of 200–350 μm as ideal for cellular attachment.[73] Scaffolds should also possess mechanical properties that are representative of surrounding tissue[74] and exhibit a degree of biodegradability to permit the eventual ingrowth of autologous tissue.[72]
Polymer-based scaffolds
As Type I collagen comprises a significant proportion of bone ECM, much focus has been placed on biodegradable polymers as potential materials for biomimetic scaffold construction. These materials can be characterized as naturally occurring or synthetically derived, and are meant to act as a proxy for the collagenous matrix of native bone.[72] Natural polymers harvested from plant or animal sources have been used to construct scaffolds because they exhibit a high degree of biocompatibility and low toxicity.[75] The two most promising natural polymers for bony tissue regeneration are chitosan and silk fibroin. Other natural polymers including collagen, starch, and agarose have also been investigated for bony tissue engineering. Because of relatively weak mechanical properties, however, these polymers are better suited for soft tissue applications.
Natural polymers
Chitosan
Chitosan is a deacetylated derivative of chitin, a polymer found in the exoskeleton of crustaceans that exhibits hydrophilic surface properties.[75,76,77] The hydrophilic nature of chitosan promotes cellular adhesion and proliferation.[78] 3D sponges formulated from chitosan have been shown to be osteoconductive and to promote in vitro and in vivo bone growth.[79,80] Although chitosan also promotes osteoinduction, concern regarding its mechanical strength has been raised, particularly for load bearing applications.[78] In an attempt to remedy this, hybrid scaffolds that combine chitosan with other natural and synthetic polymers have been developed. Some of these copolymer scaffolds have demonstrated pore sizes that range from 100 to 300 μm, which is ideal for promoting cellular adhesion. Additionally, a significant increase in the compressive modulus of chitosan-based copolymers was noted compared to scaffolds comprised of chitosan in isolation, potentially allowing for greater bony defect repair.[77,78]
Fibroin
Fibroin is an insoluble protein produced by spiders, the larvae of the silkworm of the mulberry tree (Bombyx mori), and several moth and insect species found in silk. Silk fibroin, which has been used to make surgical suture, is unique in its high mechanical strength and biodegradability.[75,81] These features have made it desirable for use in 3D scaffolds for bone engineering. Additionally, it can be processed into a variety of forms such as sponges, nano woven nets, and injectable gels,[82] allowing for customization depending on the application. While the majority of focus has centered on fibroin derived from the mulberry silkworm, the presence of nonpolar sequences of alanine and glycine in this construct may impede cell growth and proliferation.[75,81] Tasar (Antheraea mylitta) and Eri (Philosamia ricini) silk have recently been investigated due to their increased ability to mediate cellular adhesion.[81] Through the controlled blending of various silk fibroins, scaffolds can be adjusted to alter degradation rate, mechanical strength, and cellular adhesion. Fibroin scaffolds have demonstrated the ability to support cellular adhesion and proliferation experimentally, as an increase in the number of MSCs within scaffolds has been noted at 14 days and a maintenance in the number of MSCs within scaffolds at 28 days in in vitro models.[81] Fibroin scaffolds have also been shown to promote osteoprogenitor cell differentiation, as evidenced by elevated ALP activity and increased expression of Runx2 and osteocalcin in MSCs grown in these scaffolds and exposed to osteogenic medium.[81] Increased bone formation using fibroin-based scaffolds has also been demonstrated in the in vivo setting using a critical-sized calvarial defect model.[82]
Synthetic polymers
While natural polymers have many characteristics that are attractive for use in 3D scaffold construction, synthetic polymers offer tighter control over the production process and, therefore, may demonstrate more reproducible outcomes in production. An additional advantage of synthetic polymers is that they can be more readily manipulated to change mechanical properties such as the degradation rate and porosity depending on application need.[72,75,83]
Poly(lactic acid) and poly(glycolic acid)
Poly(α-hydroxy esters), which include poly(lactic acid) (PLA) and poly(glycolic acid) (PGA), are commonly used biodegradable synthetic polymers in the production of 3D scaffold matrices. PLA and PGA contain ester linkages within their polymer backbone, allowing gradual degradation via hydrolysis.[84] While structurally related, PLA and PGA demonstrate divergence with respect to several physical and chemical traits, lending differently to the potential use for tissue engineering. Differences in hydrophilicity between the two polymers result in variation in the ability to promote cellular adhesion, as well as the time needed for degradation.[75] Combining different quantities of the polymers result in the formation of copolymer blend scaffolds consisting of poly(lactic-co-glycolic acid) (PLGA). This allows for increased control of mechanical properties.[72] Additionally, alteration of the crystalline nature and molecular weight of substrate polymers further modifies mechanical properties of PLGA scaffolds.[85] Many investigators utilize solvent casting/particulate leaching to prepare PLGA scaffolds. Lin et al. demonstrated the ability to improve upon these characteristics utilizing a porogen additive in the synthesis process, making PLGA scaffolds more conducive for bone engineering.[86] Although there are few studies on the efficacy of PLGA scaffolds in calvarial defect models, Breitbart et al. demonstrated adequate bone growth within PGA scaffolds in such defect models.[87]
Poly(e-caprolactone)
Poly(e-caprolactone) (PCL) is an aliphatic polyester with hydrophobic characteristics that result in a longer time to degradation compared to PLA and PGA.[75] Although an increased hydrophobic potential is useful for long-term applications including bony reconstruction, it has been shown to have a negative impact on cellular adhesion. PCL scaffolds coated with polydopamine demonstrate a five-fold increase in cellular adhesion compared to uncoated PCL scaffolds.[88] PCL is also used for the production of shape-memory polymer (SMP) scaffolds, which allow for conformal expansion through controlled heating. Utilizing SMP scaffolds for the purpose of craniofacial reconstruction would allow for the repair of irregularly shaped defects by creating a tight interface between the implant and autologous tissue thereby maximizing osseointegration.[88] PCL-SMP scaffolds have demonstrated the ability to support osteoblastic function both in vitro and in vivo.[88,89]
Calcium phosphate-based ceramic scaffolds
Ceramics are inorganic, nonmetallic solids that have also been the subject of significant research searching for suitable bone graft substitutes. They are generally composed of calcium phosphate (CaP)-based materials such as hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP). HA is a naturally occurring mineral form of calcium apatite that has the chemical formula Ca10 (PO4)6 (OH)2. It comprises the inorganic phase of native bone and, therefore, significant focus has been placed on its utility as an osteoimplant. HA, β-TCP and other CaP-based biomaterials such as biphasic calcium phosphate (BCP), which combines HA + β-TCP, are biocompatible and osteoconductive.[70] By varying the crystallinity of HA and the ratio of HA to β-TCP in BCP scaffolds, the degradation rate of the scaffolds can be tailored to specific applications.[73,90] Mechanical properties of CaP-based scaffolds are further affected by porosity and grain size; lowering both results in greater stiffness, compressive strength, and resistance to fracture.[90] Thus, by adjusting the composition as well as the production methods, ceramic scaffolds can be created with varying degrees of mechanical properties. Employing a polymer replica method allows for the production of porous 3D BCP scaffolds, which demonstrate pore size and interconnectivity that facilitate bone formation in vivo. Disadvantages of using ceramic scaffolds include a relatively slow rate of degradation and brittleness.[90] These shortcomings have been attenuated through the addition of zinc oxide or silicone dioxide, resulting in a 2.5-fold increase in compressive strength due to an increase in type one collagen production.[70,73] The addition of these impurities has also demonstrated positive regulation of angiogenesis and osteoblast differentiation.[73]
Composite scaffolds
More recently, scientists have started to combine ceramic and polymer-based materials into a single composite scaffold. While ceramic and polymer-based scaffolds can individually mimic specific aspects of autologous bone, they cannot mimic all components of native ECM. Additionally, both have specific advantages and disadvantages with which they are associated. Polymeric scaffolds, for example, allow for fine control over structural design and degradation rate. Concerns exist regarding whether their mechanical properties are suitable for load bearing applications. Conversely, ceramic-based scaffolds provide optimal biocompatibility, osteoconduction and offer superior mechanical properties. They are limited by relatively low compressive strength and an extended degradation time.[90]
Therefore, due to inherent limitations when using monophasic 3D constructs, composite scaffolds that homogenize the organic and inorganic aspects of bone are becoming popular options for bone engineering. For example, the addition of CaP nanoparticles to organic polymers confers a superior compressive modulus and yield strength compared to polymer-only scaffolds.[91] Additionally, the incorporation of polymeric fibers in CaP ceramics decreases scaffold brittleness and allows better control of degradation rate. Indeed, the combination of CaP nanoparticles with a variety of natural or synthetic polymers allows for a superior degradation rate, compression module (i.e., stiffness or rigidity), and biocompatibility.[70,73,91,92]
Polymer-calcium phosphate composites
Due to initial promising results but inherent material limitations, natural and synthetic polymers have been prepared with HA, β-TCP, or BCP in an effort to improve tissue engineering results. Both chitosan and silk fibroin polymers combined with HA and β-TCP result in greater than a two-fold increase in compression modulus over polymeric-only scaffolds.[93] Furthermore, in vitro osteoblastic function within composite scaffolds surpasses behavior within polymer-only scaffolds as measured by osteocalcin production. In vivo implantation of osteoblasts in calvarial defect models further corroborates this, demonstrating superior bone growth in composite scaffolds.[94] Similar results have also been demonstrated when collagen-derived scaffolds include HA.[95,96]
Poly(lactic acid)-calcium phosphate and poly(glycolic acid)-calcium phosphate
Poly(lactic acid)-, PGA-, and PLGA-based composite scaffolds have also generated interest for potential in bone engineering. As with polymer-only scaffolds, production can be accomplished through a variety of methods.[97,98,99] One study found that using a gas forming/particulate leaching technique to produce PLGA-HA composite scaffolds resulted in a 99% increase in compression modulus and a 1331% increase in tensile strength over conventional production methods.[99] In vivo implantation of PLGA-HA scaffolds demonstrates increased de novo bone formation compared to polymer-only scaffolds.[100]
Poly(e-caprolactone)-calcium phosphate composites
The addition of β-TCP to PCL results in a significant increase in compressive modulus, therefore improving its capacity for load-bearing applications. The presence of β-TCP also reduces the hydrophobic nature of PCL.[101,102] In vitro studies have demonstrated that these composite scaffolds are capable of supporting cellular adhesion and proliferation up to 28 days as well as osteogenic differentiation of stem cells.[103] These results were more pronounced in composite scaffolds than in polymeric-only counterparts. Enhanced bone engineering using these composite scaffolds has also been noted in subcutaneous implant and calvarial defect models.[101,102,103]
Poly(1,8-octanediol-co citric acid)
Poly(1,8-octanediol-co citric acid) (POC) is a citric acid-based elastomer that has been combined with HA and β-TCP for the purpose of bone engineering. This concept is based on the notion that a combination scaffold such as POC-TCP contains elements that are representative of different aspects of native bone. POC constitutes the organic aspect of bone and TCP is analogous to the inorganic phase of bone [Figure 3]. POC is an interesting polymer choice because recent research has shown that citrate plays a large role in bone's unique stability, strength, and resistance to fracture by regulating apatite nanocrystal growth.[104,105,106] Therefore, this biodegradable polymer may be an optimum synthetic scaffold for regenerating bone due to its capacity to release citric acid and oligomers thereof that may help the crystallization process during polymer degradation. Additionally, research has shown that polymer-ceramic composites are compatible with soft and hard tissue and exhibit minimal inflammatory response while promoting cellular processes for bone formation.[107,108] Furthermore, adhesion proteins are frequently used to enhance attachment and proliferation of cells in polymer-only scaffolds; however, POC-based composite scaffolds have demonstrated increased adhesion in the absence of such modifications, thus potentially simplifying large-scale production. POC composite scaffolds containing HA and β-TCP are capable of supporting bone production.[70,92]
Figure 3.
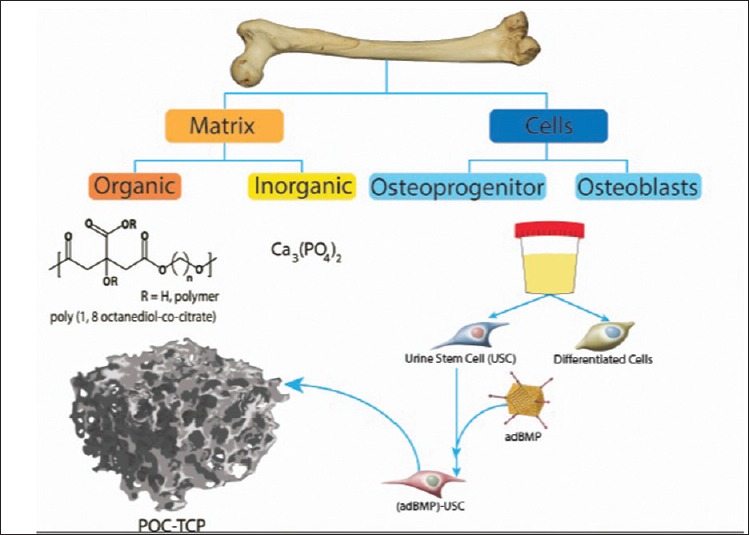
Native bone is recapitulated using substitutes for organic, inorganic, and cellular components of bone AdBMP-9-infected USCs are seeded into a POC-TCP scaffold matrix. POC: Poly(1,8-octanediol-co-citrate), TCP: Tricalcium phosphate, Ca3(PO4)2, USC: Urine-derived stem cell, AdBMP-9: Adenovirus expressing bone morphogenetic protein-9
FUTURE DIRECTIONS AND CONCLUDING REMARKS
In designing novel scaffolds, one must consider the ideal characteristics of an effective scaffold. An effective scaffold is nontoxic and nonimmunogenic, recapitulates the environment of the ECM, allows for cellular migration and tissue ingrowth, handles well, and importantly, allows for diffusion of biological factors freely into and out of the defect.[109] Eventually, our goal is to fabricate customized biomimetic implants using 3D printing of scaffolds that will house differentiating cells induced to form target tissue type. Biomimicry in the context of bone healing can be accomplished using such cells in a 3D system.
While an understanding of constituent materials is important for assessing scaffold viability for tissue engineering, so too is the selection of appropriate cells and growth factors. Many cell types are currently under investigation for the purpose of tissue engineering. However, the acquisition of many of these cells can be challenging. This is particularly true for primary progenitor cells. Again recognizing the principle of replacing “like with like,” primary calvarial cells have been isolated in an effort to repair cranial defects. Despite the time-consuming and labor-intensive processes required for isolation of these cells, their lifespan, and therefore use, is limited. To overcome these challenges, our laboratory has employed methods to safely and efficiently reversibly immortalize primary calvarial and other mesenchymal progenitor cell lines.[110,111] This was accomplished with the large T/SV40 antigen system. Immunohistochemical, immunofluorescence, and flow cytometry data demonstrated that the immortalization process did not alter the physical and morphological properties of the primary cell population. Additionally, infection of these immortalized calvarial cells (iCALs) with BMP-containing adenoviral vectors demonstrated the strong osteogenic potential of these cells in vitro. These findings were corroborated in vivo. Immortalized calvarial progenitor cells were infected ex vivo with adenoviruses expressing BMP or a control protein and were then implanted into the quadriceps of male nude mice (stem cell implantation model). Ectopic bone formation was superior in mice treated with BMP-infected cells compared to control.[110] Further investigations to comprehensively test the effect of various BMPs and matrix scaffolds on iCALs are underway.
Human urine-derived stem cells (USCs) have also been identified as an exciting option for cell-based engineering.[112] USCs are easily isolatable from human urine, and single clones are capable of expanding to yield a large population. These cells express a combination of pericyte and MSC markers and have multipotent differential capacity. Indeed, when cultured in osteogenic medium, USCs differentiate down an osteogenic lineage. Because of their promising features, we are currently investigating the ability of AdBMP-9-infected USCs to proliferate and differentiate within a scaffold matrix composed of POC-TCP [Figure 3]. Early in vitro data have demonstrated success with this strategy [Figure 4]. BMP-9, which is thought to be the most osteogenic of the BMP isoforms, has facilitated progression of USCs cultured in scaffolds toward bone forming cells. The current phase of the study will incorporate the implantation of scaffolds containing osteogenically stimulated USCs into craniofacial defect sites to assess whether autologous bone growth occurs in vivo.
Figure 4.
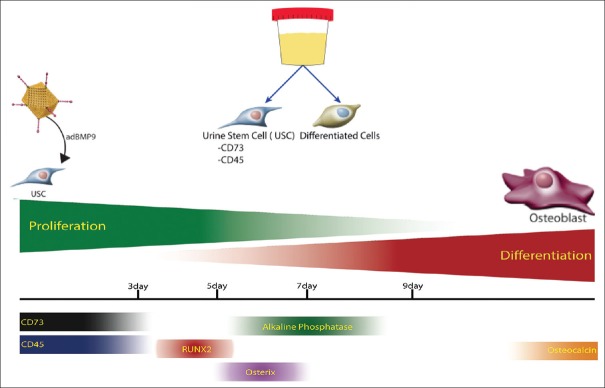
Early evidence suggests that the addition of AdBMP-9 to USCs facilitates induction toward an osteogenic lineage. AdBMP-9: Adenovirus expressing bone morphogenetic protein-9, USCs: Urine-derived stem cells
For the challenging reconstructive cranioplasty, the future will see the application of innovative therapeutic options in an effort to restore the form, function, and appearance of the pediatric and adult craniofacial skeleton. One strategy is the harvest of additional autologous tissue with novel flap design and tissue rearrangement. However, these attempts often fall short and subject patients to additional morbidity. Within the last decade, scientists have made significant advances in elucidating the biology and methodology of tissue engineering. There must continue to be an increase in the study of progenitor cell lines, growth factors and signaling molecules, and scaffold materials that promote 3D bone healing in order to have the ability to engineer deficient tissue. Given the complex nature of tissue repair, the most successful strategies of tissue regeneration are likely to employ a combination approach.
ACKNOWLEDGMENTS
The authors apologize to the investigators whose original work was not cited due to space constraints. The authors thank the members of the Molecular Oncology Laboratory at the University of Chicago Medical Center for their scientific and technical input.
Footnotes
Source of Support: The reported work was supported in part by research grants from the National Institute of Dental and Craniofacial Research (RRR; #1K08DE020140-01) and The American Society of Maxillofacial Surgeons/Maxillofacial Surgeons Foundation (RRR).
Conflict of Interest: None declared.
REFERENCES
- 1.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–24. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 2.Bishop GB, Einhorn TA. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721–7. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613–29. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Acarturk TO, Hollinger JO. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006;118:862–73. doi: 10.1097/01.prs.0000232385.81219.87. [DOI] [PubMed] [Google Scholar]
- 5.Smartt JM, Jr, Karmacharya J, Gannon FH, Ong G, Jackson O, Bartlett SP, et al. Repair of the immature and mature craniofacial skeleton with a carbonated calcium phosphate cement: Assessment of biocompatibility, osteoconductivity, and remodeling capacity. Plast Reconstr Surg. 2005;115:1642–50. doi: 10.1097/01.prs.0000161466.74294.1e. [DOI] [PubMed] [Google Scholar]
- 6.Sargent LA, Fulks KD. Reconstruction of internal orbital fractures with Vitallium mesh. Plast Reconstr Surg. 1991;88:31–8. doi: 10.1097/00006534-199107000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hurvitz KA, Kobayashi M, Evans GR. Current options in head and neck reconstruction. Plast Reconstr Surg. 2006;118:122e–33. doi: 10.1097/01.prs.0000237094.58891.fb. [DOI] [PubMed] [Google Scholar]
- 8.Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J Plast Reconstr Aesthet Surg. 2010;63:1615–23. doi: 10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Gosain AK, Chim H, Arneja JS. Application-specific selection of biomaterials for pediatric craniofacial reconstruction: Developing a rational approach to guide clinical use. Plast Reconstr Surg. 2009;123:319–30. doi: 10.1097/PRS.0b013e318193478c. [DOI] [PubMed] [Google Scholar]
- 10.Chim H, Gosain AK. Biomaterials in craniofacial surgery: Experimental studies and clinical application. J Craniofac Surg. 2009;20:29–33. doi: 10.1097/SCS.0b013e318190dd9e. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal A, Garg LN. Split calvarial bone graft for the reconstruction of skull defects. J Surg Tech Case Rep. 2011;3:13–6. doi: 10.4103/2006-8808.78465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsalanty ME, Genecov DG. Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr. 2009;2:125–34. doi: 10.1055/s-0029-1215875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oppenheimer AJ, Tong L, Buchman SR. Craniofacial bone grafting: Wolff's law revisited. Craniomaxillofac Trauma Reconstr. 2008;1:49–61. doi: 10.1055/s-0028-1098963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turan A, Kostakoglu N, Tuncel U, Gökçe E, Markoç F. Scapular bone grafts: Good options for craniofacial defects? Ann Plast Surg. 2014 doi: 10.1097/SAP.0000000000000357. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Lee JC, Kleiber GM, Pelletier AT, Reid RR, Gottlieb LJ. Autologous immediate cranioplasty with vascularized bone in high-risk composite cranial defects. Plast Reconstr Surg. 2013;132:967–75. doi: 10.1097/PRS.0b013e31829f4b59. [DOI] [PubMed] [Google Scholar]
- 16.Kim RY, Fasi AC, Feinberg SE. Soft tissue engineering in craniomaxillofacial surgery. Ann Maxillofac Surg. 2014;4:4–8. doi: 10.4103/2231-0746.133064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosain AK, Persing JA. Biomaterials in the face: Benefits and risks. J Craniofac Surg. 1999;10:404–14. doi: 10.1097/00001665-199909000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Fong AJ, Lemelman B, Lam SK, Reid RR, Gottlieb LJ. Reconstructive approach to hostile cranioplasty: The university of chicago experience. Plast Reconstr Surg. 2014;134:18–9. doi: 10.1016/j.bjps.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Griffith LG, Naughton G. Tissue engineering – Current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 20.Bancroft GN, Mikos AG. Bone tissue engineering by cell transplantation. In: Ikada Y, Oshima N, editors. Tissue Engineering for Therapeutic Use. New York: Elvesier; 2001. [Google Scholar]
- 21.Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. Cell-based bone tissue engineering. PLoS Med. 2007;4:e9. doi: 10.1371/journal.pmed.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teven CM, Greives M, Natale RB, Su Y, Luo Q, He BC, et al. Differentiation of osteoprogenitor cells is induced by high-frequency pulsed electromagnetic fields. J Craniofac Surg. 2012;23:586–93. doi: 10.1097/SCS.0b013e31824cd6de. [DOI] [PubMed] [Google Scholar]
- 23.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009;31:1825–35. doi: 10.1007/s10529-009-0100-8. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 25.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18:545–59. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleuming SA, He XC, Kodach LL, Hardwick JC, Koopman FA, Ten Kate FJ, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–55. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 27.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 28.Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 30.Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–71. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 31.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 32.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–63. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 33.Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T. Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem. 2012;151:247–54. doi: 10.1093/jb/mvs004. [DOI] [PubMed] [Google Scholar]
- 34.Urist MR. Bone: Formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 35.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 36.Tasli PN, Aydin S, Yalvaç ME, Sahin F. Bmp 2 and bmp 7 induce odonto-and osteogenesis of human tooth germ stem cells. Appl Biochem Biotechnol. 2014;172:3016–25. doi: 10.1007/s12010-013-0706-0. [DOI] [PubMed] [Google Scholar]
- 37.Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25:665–77. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 38.He TC. Distinct osteogenic activity of BMPs and their orthopaedic applications. J Musculoskelet Neuronal Interact. 2005;5:363–6. [PubMed] [Google Scholar]
- 39.Bae SC, Lee KS, Zhang YW, Ito Y. Intimate relationship between TGF-beta/BMP signaling and runt domain transcription factor, PEBP2/CBF. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S48–55. [PubMed] [Google Scholar]
- 40.Bain G, Müller T, Wang X, Papkoff J. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun. 2003;301:84–91. doi: 10.1016/s0006-291x(02)02951-0. [DOI] [PubMed] [Google Scholar]
- 41.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 42.Chenard KE, Teven CM, He TC, Reid RR. Bone morphogenetic proteins in craniofacial surgery: Current techniques, clinical experiences, and the future of personalized stem cell therapy. J Biomed Biotechnol 2012. 2012 doi: 10.1155/2012/601549. 601549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Süring K, et al. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proc Natl Acad Sci U S A. 2003;100:12277–82. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seemann P, Schwappacher R, Kjaer KW, Krakow D, Lehmann K, Dawson K, et al. Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J Clin Invest. 2005;115:2373–81. doi: 10.1172/JCI25118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14:1126–35. doi: 10.1016/j.joca.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Hu J, Chen YX, Wang D, Qi X, Li TG, Hao J, et al. Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev Biol. 2004;276:158–71. doi: 10.1016/j.ydbio.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Derynck R, Feng XH. TGF-beta receptor signaling. Biochim Biophys Acta. 1997;1333:F105–50. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 48.Luther G, Wagner ER, Zhu G, Kang Q, Luo Q, Lamplot J, et al. BMP-9 induced osteogenic differentiation of mesenchymal stem cells: Molecular mechanism and therapeutic potential. Curr Gene Ther. 2011;11:229–40. doi: 10.2174/156652311795684777. [DOI] [PubMed] [Google Scholar]
- 49.Bahamonde ME, Lyons KM. BMP3: To be or not to be a BMP. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S56–62. [PubMed] [Google Scholar]
- 50.Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: A critical review. Cell Signal. 2011;23:609–20. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science. 1996;271:360–2. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–95. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 54.Sahoo T, Theisen A, Sanchez-Lara PA, Marble M, Schweitzer DN, Torchia BS, et al. Microdeletion 20p12.3 involving BMP2 contributes to syndromic forms of cleft palate. Am J Med Genet A. 2011;155A:1646–53. doi: 10.1002/ajmg.a.34063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg. 2007;77:626–31. doi: 10.1111/j.1445-2197.2007.04175.x. [DOI] [PubMed] [Google Scholar]
- 56.Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–8. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 57.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–20. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 58.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 59.King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol. 1994;166:112–22. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- 60.Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–39. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 61.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–20. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 62.Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A. Induction of reparative dentin formation by ultrasound-mediated gene delivery of growth/differentiation factor 11. Hum Gene Ther. 2003;14:591–7. doi: 10.1089/104303403764539369. [DOI] [PubMed] [Google Scholar]
- 63.Gamer LW, Wolfman NM, Celeste AJ, Hattersley G, Hewick R, Rosen V. A novel BMP expressed in developing mouse limb, spinal cord, and tail bud is a potent mesoderm inducer in Xenopus embryos. Dev Biol. 1999;208:222–32. doi: 10.1006/dbio.1998.9191. [DOI] [PubMed] [Google Scholar]
- 64.Shen B, Bhargav D, Wei A, Williams LA, Tao H, Ma DD, et al. BMP-13 emerges as a potential inhibitor of bone formation. Int J Biol Sci. 2009;5:192–200. doi: 10.7150/ijbs.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, de Bock CE, et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel-Feil syndrome. Hum Mutat. 2008;29:1017–27. doi: 10.1002/humu.20741. [DOI] [PubMed] [Google Scholar]
- 66.Buxton P, Edwards C, Archer CW, Francis-West P. Growth/differentiation factor-5 (GDF-5) and skeletal development. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S23–30. [PubMed] [Google Scholar]
- 67.Springer IN, Açil Y, Kuchenbecker S, Bolte H, Warnke PH, Abboud M, et al. Bone graft versus BMP-7 in a critical size defect - Cranioplasty in a growing infant model. Bone. 2005;37:563–9. doi: 10.1016/j.bone.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, et al. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–79. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 69.Breyer B, Jiang W, Cheng H, Zhou L, Paul R, Feng T, et al. Adenoviral vector-mediated gene transfer for human gene therapy. Curr Gene Ther. 2001;1:149–62. doi: 10.2174/1566523013348689. [DOI] [PubMed] [Google Scholar]
- 70.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaky SH, Hangadora CK, Tudares MA, Gao J, Jensen A, Wang Y, et al. Poly (glycerol sebacate) elastomer supports osteogenic phenotype for bone engineering applications. Biomed Mater. 2014;9:025003. doi: 10.1088/1748-6041/9/2/025003. [DOI] [PubMed] [Google Scholar]
- 72.Gentile P, Chiono V, Carmagnola I, Hatton PV. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–59. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tevlin R, McArdle A, Atashroo D, Walmsley GG, Senarath-Yapa K, Zielins ER, et al. Biomaterials for craniofacial bone engineering. J Dent Res. 2014;93:1187–95. doi: 10.1177/0022034514547271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arahira T, Todo M. Effects of proliferation and differentiation of mesenchymal stem cells on compressive mechanical behavior of collagen/ß-TCP composite scaffold. J Mech Behav Biomed Mater. 2014;39:218–30. doi: 10.1016/j.jmbbm.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Chaudhuri JB. 3D Cell Culture: Methods and Protocols. In: Haycock JW, editor. Totowa: Humana Press; 2010. [Google Scholar]
- 76.Di Martino A, Sittinger M, Risbud MV. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials. 2005;26:5983–90. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Jiang T, Abdel-Fattah WI, Laurencin CT. In vitro evaluation of chitosan/poly(lactic acid-glycolic acid) sintered microsphere scaffolds for bone tissue engineering. Biomaterials. 2006;27:4894–903. doi: 10.1016/j.biomaterials.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 78.Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26:3919–28. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 79.Seol YJ, Lee JY, Park YJ, Lee YM, Young-Ku, Rhyu IC, et al. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol Lett. 2004;26:1037–41. doi: 10.1023/B:BILE.0000032962.79531.fd. [DOI] [PubMed] [Google Scholar]
- 80.Muzzarelli RA, Mattioli-Belmonte M, Tietz C, Biagini R, Ferioli G, Brunelli MA, et al. Stimulatory effect on bone formation exerted by a modified chitosan. Biomaterials. 1994;15:1075–81. doi: 10.1016/0142-9612(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 81.Panda NN, Biswas A, Pramanik K, Jonnalagadda S. Enhanced osteogenic potential of human mesenchymal stem cells on electrospun nanofibrous scaffolds prepared from eri-tasar silk fibroin. J Biomed Mater Res B Appl Biomater. 2014 doi: 10.1002/jbm.b.33272. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 82.Riccio M, Maraldi T, Pisciotta A, La Sala GB, Ferrari A, Bruzzesi G, et al. Fibroin scaffold repairs critical-size bone defects in vivo supported by human amniotic fluid and dental pulp stem cells. Tissue Eng Part A. 2012;18:1006–13. doi: 10.1089/ten.TEA.2011.0542. [DOI] [PubMed] [Google Scholar]
- 83.Temple JP, Hutton DL, Hung BP, Huri PY, Cook CA, Kondragunta R, et al. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317–25. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 84.Ishaug SL, Yaszemski MJ, Bizios R, Mikos AG. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28:1445–53. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- 85.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–86. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 86.Lin HR, Kuo CJ, Yang CY, Shaw SY, Wu YJ. Preparation of macroporous biodegradable PLGA scaffolds for cell attachment with the use of mixed salts as porogen additives. J Biomed Mater Res. 2002;63:271–9. doi: 10.1002/jbm.10183. [DOI] [PubMed] [Google Scholar]
- 87.Breitbart AS, Grande DA, Kessler R, Ryaby JT, Fitzsimmons RJ, Grant RT. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg. 1998;101:567–74. doi: 10.1097/00006534-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 88.Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS, Grunlan MA. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014;10:4597–605. doi: 10.1016/j.actbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 89.Schantz JT, Hutmacher DW, Lam CX, Brinkmann M, Wong KM, Lim TC, et al. Repair of calvarial defects with customised tissue-engineered bone grafts II. Evaluation of cellular efficiency and efficacy in vivo . Tissue Eng. 2003;9(Suppl 1):S127–39. doi: 10.1089/10763270360697030. [DOI] [PubMed] [Google Scholar]
- 90.Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 91.Rogel MR, Qiu H, Ameer GA. The role of nanocomposites in bone regeneration. J Mater Chem. 2008;18:4233–41. [Google Scholar]
- 92.Chung EJ, Sugimoto MJ, Ameer GA. The role of hydroxyapatite in citric acid-based nanocomposites: Surface characteristics, degradation, and osteogenicity in vitro . Acta Biomater. 2011;7:4057–63. doi: 10.1016/j.actbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 93.Qi XN, Mou ZL, Zhang J, Zhang ZQ. Preparation of chitosan/silk fibroin/hydroxyapatite porous scaffold and its characteristics in comparison to bi-component scaffolds. J Biomed Mater Res A. 2014;102:366–72. doi: 10.1002/jbm.a.34710. [DOI] [PubMed] [Google Scholar]
- 94.Chesnutt BM, Yuan Y, Buddington K, Haggard WO, Bumgardner JD. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in vivo . Tissue Eng Part A. 2009;15:2571–9. doi: 10.1089/ten.tea.2008.0054. [DOI] [PubMed] [Google Scholar]
- 95.Villa MM, Wang L, Huang J, Rowe DW, Wei M. Bone tissue engineering with a collagen-hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J Biomed Mater Res B Appl Biomater. 2015;103:243–53. doi: 10.1002/jbm.b.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gleeson JP, Plunkett NA, O’Brien FJ. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur Cell Mater. 2010;20:218–30. doi: 10.22203/ecm.v020a18. [DOI] [PubMed] [Google Scholar]
- 97.Ngiam M, Liao S, Patil AJ, Cheng Z, Chan CK, Ramakrishna S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone. 2009;45:4–16. doi: 10.1016/j.bone.2009.03.674. [DOI] [PubMed] [Google Scholar]
- 98.Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, et al. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. doi: 10.1371/journal.pone.0011177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim SS, Sun Park M, Jeon O, Yong Choi C, Kim BS. Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:1399–409. doi: 10.1016/j.biomaterials.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 100.Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR. In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng. 2007;13:619–27. doi: 10.1089/ten.2006.0102. [DOI] [PubMed] [Google Scholar]
- 101.Liao HT, Lee MY, Tsai WW, Wang HC, Lu WC. Osteogenesis of adipose-derived stem cells on polycaprolactone-ß-tricalcium phosphate scaffold fabricated via selective laser sintering and surface coating with collagen type I. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1811. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 102.Yeo A, Wong WJ, Teoh SH. Surface modification of PCL-TCP scaffolds in rabbit calvaria defects: Evaluation of scaffold degradation profile, biomechanical properties and bone healing patterns. J Biomed Mater Res A. 2010;93:1358–67. doi: 10.1002/jbm.a.32633. [DOI] [PubMed] [Google Scholar]
- 103.Baykan E, Koc A, Eser Elcin A, Murat Elcin Y. Evaluation of a biomimetic poly(e-caprolactone)/ß-tricalcium phosphate multispiral scaffold for bone tissue engineering: In vitro and in vivo studies. Biointerphases. 2014;9:029011. doi: 10.1116/1.4870781. [DOI] [PubMed] [Google Scholar]
- 104.Hu YY, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci U S A. 2010;107:22425–9. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Costello LC, Franklin RB, Reynolds MA, Chellaiah M. The important role of osteoblasts and citrate production in bone formation: “Osteoblast citration” as a new concept for an old relationship. Open Bone J. 2012;4 doi: 10.2174/1876525401204010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Davies E, Müller KH, Wong WC, Pickard CJ, Reid DG, Skepper JN, et al. Citrate bridges between mineral platelets in bone. Proc Natl Acad Sci U S A. 2014;111:E1354–63. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baler K, Ball JP, Cankova Z, Hoshi RA, Ameer GA, Allen JB. Advnaced nanocomposites for bone regeneration. Biomater Sci. 2014;2:1355–66. doi: 10.1039/c4bm00133h. [DOI] [PubMed] [Google Scholar]
- 108.Qiu H, Yang J, Kodali P, Koh J, Ameer GA. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials. 2006;27:5845–54. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 109.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One. 2007;2:e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shenaq DS, Teven CM, Seitz IA, Rastegar F, Greives MR, He TC, et al. Characterization of reversibly immortalized calvarial mesenchymal progenitor cells. J Craniofac Surg. 2015 doi: 10.1097/SCS.0000000000001717. In press [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang E, Bi Y, Jiang W, Luo X, Yang K, Gao JL, et al. Conditionally immortalized mouse embryonic fibroblasts retain proliferative activity without compromising multipotent differentiation potential. PLoS One. 2012;7:e32428. doi: 10.1371/journal.pone.0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells. 2013;31:1840–56. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]


