Abstract
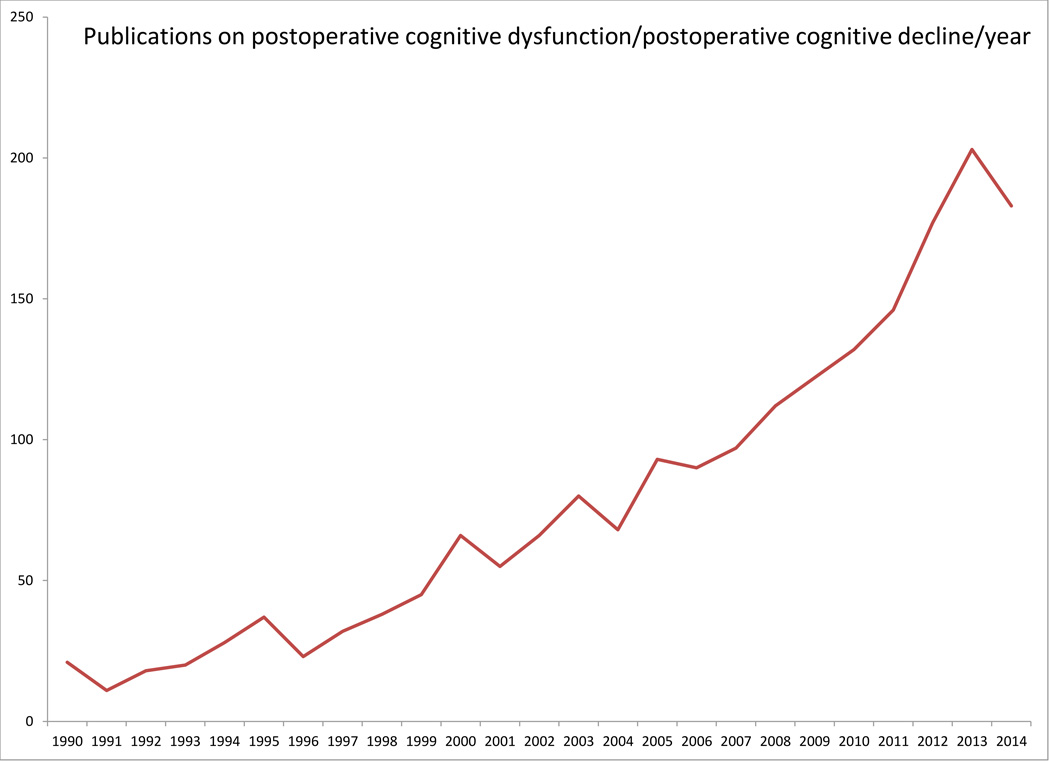
Ever since Bedford’s seminal Lancet case series in 1955, we have known that perioperative care is sometimes followed by significant cognitive dysfunction (1). Although the safety of perioperative care has improved dramatically since 1955, the descriptions of cognitive dysfunction in that case series are eerily similar to the complaints of current patients suffering from post-operative cognitive dysfunction (POCD). POCD remains a common post-operative complication associated with significant morbidity and even mortality, especially among elderly patients. There has been a great deal of interest in and controversy about POCD, from how it is measured, to how long it lasts, to its precise implications for patients. This interest and controversy is reflected partly in the increasing number of papers published on this subject recently (shown in Figure 1). Recent work has also suggested surgery may be associated with cognitive improvement in some patients (2–4), termed Post-Operative Cognitive Improvement (POCI). As the number of surgeries performed worldwide approaches 250 million per year (5) (with an increasing number elderly patients), optimizing postoperative cognitive function and preventing/treating POCD are major public health issues. In this article we review the literature on POCD and POCI, and discuss current research challenges in this area.
Keywords: Post-Operative Cognitive Dysfunction, Post-Operative Cognitive Decline, Post-Operative Cognitive Improvement, Elderly, Anesthesia, Surgery
I. A description of POCD and POCI
What is POCD?
POCD is a syndrome defined by a drop in cognitive performance on a set of neuropsychological tests from before to after surgery. Unlike delirium, this means that POCD cannot be diagnosed unless a patient has undergone formal neuropsychological testing before and after surgery, which typically doesn’t happen outside a research setting. Partly as a result of this, there is no ICD-10 code for POCD, and it is not listed as a diagnosis in the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). However, the utility of the DSM-V as a nosological tool has been questioned recently by many, including the head of the American National Institute of Mental Health (6). Thus, the fact that POCD is not listed in the DMS-V is of questionable import.
Neuropsychological testing for POCD typically includes tests that assess multiple cognitive domains (see Table 1). Individual sub-test scores are then grouped together by factor analysis or by an a priori understanding of which tests measure which cognitive domains (as described in Table 1). Depending on the study, anywhere from 4 to 8 cognitive domains have been used (7,8), although simpler tests such as the mini mental state exam can also detect long term post-operative cognitive changes (9). Post-operative testing is typically performed after the acute effects of surgery and the immediate post-operative period have dissipated; i.e. at least 1 week after surgery (10). A threshold is typically set for either a drop in overall cognitive performance (the mean of the individual cognitive domain scores), or for a drop in performance in a single cognitive domain. Patients scoring below such a pre-set threshold are then defined as having POCD. There is no clear agreement on how low such a POCD cutoff/threshold should be set (e.g. 1 standard deviation (SD), or 1.5, or 2 SD’s) (10). There has also been disagreement over how to classify patients who drop below the pre-set threshold in one domain, but who show cognitive improvement in other domains (11,12). Such patients may even show overall improvement in their composite cognitive index, even though they may meet POCD criteria (if POCD is defined as a drop below threshold in any individual cognitive domain).
Table 1.
Recommended neuropsychological measures for the detection and characterization of POCD
| Core Domain |
Component Cognitive Process |
Measure | Description | Brain regions/circuits involved in task** |
|---|---|---|---|---|
| Global | Multiple | Montreal Cognitive Assessment (MoCA) | The MoCA is a brief cognitive screening measure tapping multiple cognitive domains, including brief assessment of memory and orientation. The screening measure is freely available (www.mocatest.org) and has the advantage of multiple alternate forms, which can help in preventing over-estimation of POCD recovery secondary to simple re-administration practice effects. Administration Time: Variable, with ~15 minute average. (126,127) |
Multiple tasks, n/a. |
| Executive Function | Simple Attention | Digit Span Forward Subtest from Wechsler Adult Intelligence Scale − 3rd Revision (WAIS- III) | The Digit Span Forward subtest from the WAIS-III is a simple auditory-verbal attention task, in which a participant is asked to attend to and immediately repeat a series of serially presented digits that increase in total span as the test progresses. Administration Time: Variable, with ~5–10 minute average. |
Right dorsolateral prefrontal cortex (128) |
| Complex Attention (Working Memory) | Digit Span - Backward Subtest from Wechsler Adult Intelligence Scale − 3rd Revision (WAIS- III) or Letter- Number Sequencing Subtest from WAIS-III | The Digit Span Backward (or Letter-Number Sequencing) subtest from the WAIS-III engages both simple attention and working memory skills. Participants are instructed to attend to a series of verbally presented digits of increasing total length, but rather than respond verbatim participants are instructed to repeat the presented digits in reverse order. In the alternate Letter-Number Sequencing subtest from the WAIS-III participants are presented with randomized series of digits and letters of the alphabet of increasing length and asked to respond to a particular series with all digits in ascending order and all letters in alphabetical order.(128,129) Administration Time: Variable, with ~5–10 minute average. |
right dorsolateral prefrontal cortex (128) | |
| Response Inhibition | Stroop Color Word Test | A time limited test of the ability to inhibit a pre-potent response, known to be sensitive to medial prefrontal lobe dysfunction. The Stroop Color Word Test requires participants to read a series of color words (red, green, blue), then name the color ink of a series of X characters, after which an inhibition trial is given in which the participant is asked to name the color ink of a series of color words that are in opposition to the ink color (e.g., the word blue printed in red ink). The natural tendency of participants is to say the word as printed rather than the ink color; hence the sensitivity of the measure to response inhibition skills. Administration Time: 3–4 minutes. |
Anterior cingulate cortex (ACC), right inferior frontal gyrus, and cerebellum. (130) | |
| Mental Flexibility | Trail Making A & B Test * | The timed Trail Making A subtest requires participants to connect a series of numbered circles distributed on a piece of paper in ascending numerical order, while the Trail Making B subtest has both letters of the alphabet and numbers in circles that then must be connected in alternating ascending order (e.g., 1-A-2-B-3-C…). Trail Making A & B tests should be administered in immediate succession, as the Trail Making A subtest is necessary for familiarization of general subtest B task requirements. Independent administration of Trail Making B test only may result in over-estimation of POCD severity.(131,132) Administration Time: Variable w/ 5 minute timed maximum for each subtest. |
Medial Temporal Lobe (132) | |
| Verbal Fluency | Controlled Oral Word Association Test (COWA) from the Multilingual Aphasia Examination (MAE) * | A lexical verbal fluency task known to be dependent upon pre-Broca area function in the language dominant brain hemisphere. This test also requires retention of task rules for proper performance. Participants say as many words as they can retrieve that start with a particular consonant with 1-minute given for three different consonants (e.g., C, F, L). Participants are asked to not use proper nouns or the same word with different endings (e.g., eat, eating). Administration Time: 3 minutes. |
Posterior part of the left inferior prefrontal cortex (LIPC); category fluency task activates anterior left inferior prefrontal cortex and right inferior prefrontal cortex. (133,134) | |
| Learning & Memory | Auditory- Verbal Learning & Memory | Hopkins Verbal Learning Test, Revised * | An auditory-verbal, list-learning and memory task that involves the presentation of 12 various categorically related item words (e.g., gemstones, furniture, etc.) that are then immediately recalled by the participant. Participants are given the opportunity to learn the list of words over a series of three repeated presentations, then after a 25- minute delay participants are assessed for delayed recall and recognition memory for the primary word list items. Scored items include total recall, delayed recall, percent retention (after delay) and recognition discrimination index. Administration Time: 35 minutes timed (includes 25-minute delay) |
Left ventrolateral prefrontal cortex (135) |
| Visual Learning & Memory | Brief Visuospatial Learning Test, Revised * | A visuospatial learning and memory test analog to the HVLT-R. Six simple line drawings are presented in a 2 × 3 array on a single piece of paper and participants are allowed a brief period to study the figures, after which they are asked to reproduce as many figures in their proper locations as they can on a blank sheet of paper. Three learning trials of the six line drawings are conducted. There is a 25-minute delay, after which participants are asked to recall as many figures in their locations as possible. Delayed recognition for the same line drawings is also conducted. Scored items are the same as the HVLT-R (e.g., total recall, delayed recall, etc.).(136) Administration Time: 45 minutes timed (includes 25-minute delay) |
||
| Visuospatial Functioning | Visuomotor Integration | Digit Symbol Coding Test from the Wechsler Adult Intelligence Scale – 3rd Revision (WAIS- III) | The Digit Symbol Coding Test requires participants to use a symbol/number key at the top of a printed page as a guide to determine the appropriate missing symbols for a large array of unmatched numbers below the test key. The task is timed and the scored response is the total number of correct symbol/number pairs completed by the participant within 120-seconds. Administration Time: 5 minutes. |
Corpus callosum, internal capsule (137); anterior cingulate gyrus, left prefrontal gyrus and inferior parietal lobe (138) |
| Complex Visuospatial Perception | Hooper Visual Organizational Test | HVOT performance is known to be dependent upon bilateral parietal and temporal-occipital cortex functioning and involves participants’ mental integration and naming of common objects presented in a spatially scattered puzzle piece-like format. Proper execution of the task requires participants to mentally rotate and connect partial stimuli pieces into a whole to form a perceptual gestalt. Administration Time: Variable, with ~10–12 minute average. |
Superior parietal lobules, ventral temporal- occipital cortex, and posterior visual association areas, frontal eye fields, left dorsolateral prefrontal cortex (139) | |
| Psychomotor Function | Manual Dexterity & Motor Speed | Lafayette Grooved Pegboard Test | A manipulative dexterity and motor speed test that involves the insertion of small milled key-like pins into randomly rotated matching holes arranged in a 5 × 5 array. Each hand is evaluated separately with a score reflecting the total time to complete insertion of all 25 pegs for each hand. Administration Time: Variable, with ~5 minute average. |
Nigrostriatal dopamine function (140) |
Note: Recommended measures with readily available alternate, equivalent forms are denoted with a “*” symbol.
Most patients typically improve their performance on these tests over repeated testing sessions due to a learning or practice effect, which makes a drop in performance all the more striking. However, this also makes it difficult to determine the true level of post-operative performance drop, because the observed post-operative performance may thus reflect both post-operative deficits and practice-related improvements. Practice effects can be mitigated by using cognitive tests with equivalent alternate forms, such that one form is used at pre-surgical baseline and alternate forms are used for subsequent post-operative evaluation (see Table 1 for tests with available alternate forms). In addition to practice effects, each cognitive test has its own inherent test-retest variability, which can affect the interpretation of any post-operative cognitive change (13). Simple change scores in performance from a patient’s pre-surgical baseline do not take practice effects or other issues like test-retest reliability, floor/ceiling effects, or regression to the mean into account. Reliable change index (RCI) methods has been developed that can account for these effects (14,15). The RCI is typically defined as the pre- to post-test change in a study subject minus the average pre- to post-test change among control subjects, divided by the standard deviation of the change in pre- to post-test scores among non-surgical controls (16). The RCI method may have higher sensitivity and specificity for detecting POCD than other statistical methods (16). However, unless there are published test-retest data over an equivalent time interval in age matched individuals, the RCI method requires collecting data from non-surgical controls at the same times as surgical patients.
The RCI method also allows one to define clinical significance thresholds for POCD, because the RCI method generates z-scores with an assumed normal distribution. An RCI score range of +1.645, relative to a normal distribution, means that 90% of obtained change scores would fall within this range. Outliers would thus fall either in the upper or lower 5% tails of the distribution by chance. Early Alzheimer’s disease (also known as mild cognitive impairment, or MCI) is often diagnosed by a less stringent measure of cognitive decline relative to normative performance expectations (e.g., z-score drop of a at least 1.5, (17)). Thus, the strict −1.645 RCI criterion for clinical change significance may be excessively stringent, and may miss POCD cases that are functionally relevant to patients. There is currently no consensus among neuropsychologists of statistical method (such as an RCI) and/or threshold should be used, and how many cognitive domains must show a decline to make the diagnosis of POCD (10).
Although POCD has been defined by the statistical results of cognitive tests, multiple investigators have found that is also associated with impairments in quality of life (18), increased exit from the work force (19), and increased mortality after surgery (19,20). Thus, POCD can be conceptualized as a lack of resilience in the face of perioperative stress (21), which is associated with impairments in multiple aspects of life. This conceptual model raises the question of whether POCD may also be associated with impairments in social relationships, increased physical frailty, decreased sexual interest and/or performance, and deficits in other aspects of life- these are important questions to be addressed by future studies (Table 3).
Table 3.
Important Questions for Future Research, Suggestions and Challenges
| Research Question | Suggested Study Design and Methods* | Issues/Challenges |
|---|---|---|
| -What aspects of aging are most closely tied to POCD risk? | Cohort Design, with cognitive testing and geriatric evaluations | Important to have involvement of geriatricians |
| Are certain patients (ie the elderly, or those with less cognitive reserve) at higher risk for POCD after general vs regional anesthesia? | RCT of general vs regional anesthesia with cognitive testing, stratified by age group and/or other variables | Patient recruitment, importance of minimizing sedation in the regional arm to allow for a true comparison of general vs regional anesthesia |
| -Is POCD associated with an increased long term Risk of dementia? | Prospective Cohort with long term follow up, cognitive and MCI/AD/dementia screening | Need to enroll patients who may already have MCI or other baseline cognitive deficits. Large samples sizes needed to obtain sufficient power for clinically relevant effects; see (167) and (28) for discussion of this issue. |
| -What is the relationship between central neuroinflammation, cerebrovascular white matter disease, AD pathology (and/or perioperative changes in these pathologic processes) and POCD? | Cohort Design, Cognitive Testing, neuroimaging, and CSF AD and inflammatory biomarker studies | Patient Recruitment |
| -Is POCD associated with altered functional brain connectivity? | Cohort Design, cognitive testing and functional MRI scans | Patient Recruitment, MRI safety issues may exclude some elderly patients with pacemakers/AICDs, metal joint replacements, etc. |
| -Are there CSF biomarkers of POCD? | Cohort Design, Cognitive Testing and CSF sampling | Patient Recruitment |
| -Would SSRI treatment prevent POCD, improve cognitive trajectory, affect and mood, and/or improve quality of life in patients with POCD? | RCT, cognitive testing, quality of life measurement, depression/anxiety and affect rating scales | Possible Side Effects from Treatment |
| -Would preconditioning with ischemia or anesthetic agents help prevent POCD? | RCT | Patient recruitment |
| -Would physical and/or cognitive prehabilitation reduce the incidence or severity of POCD, and/or improve quality of life after surgery in the elderly? | RCT | Blinding difficult if not impossible; need to ensure patient participation in prehabilitation interventions. |
| -How long does POCI last? Does it depend on surgery type? To what extent does POCI reflect surgically-induced improvements in underlying disease processes, quality of life, and/or mental health? | Cohort design | Careful statistical analysis required to separate true POCI from test practice effects. |
For prospective study designs, it is important that all testing be completed both before and after surgery.
How long does POCD last?
Aside from this disagreement over how POCD diagnosis is defined, it is also unclear how long it may last. This issue is difficult to address for several reasons. First, it is ethically unreasonable and practically impossible to randomize patients to surgery and anesthesia (vs. placebo treatment). Without a non-surgical control group, though, it is unclear how much of the cognitive dysfunction in surgical patients is truly due to anesthesia, surgery and perioperative care (22). The initial rapid drop in cognition seen in patients with POCD occurs much more rapidly than normal age related cognitive decline (20,23).
Matched cohort study designs can attempt to provide non-surgical control groups for comparison, but such study designs are non-randomized and thus potentially confounded by the fact that surgical patients may be intrinsically different than non-surgical controls. Nonetheless, several studies have compared the incidence of cognitive dysfunction in surgical patients and non-surgical controls (23,24). In the International Study of Post-Operative Cognitive Dysfunction (ISPOCD), statistically significant differences in the incidence of cognitive dysfunction were found between surgical patients and non-surgical controls at 1 week and 3 months after surgery (23), but no difference was seen at 1 year after surgery. In a prospective matched cohort study, however, greater cognitive dysfunction was seen in surgical patients than non-surgical controls even at 1 year after surgery (24). A retrospective study by Avidan and colleagues found no difference in the cognitive decline trajectory between non-cardiac surgical patients and matched controls over a period of up to several years (median of 3.1 years of follow-up in surgical patients, (25)). However, the two largest studies to examine this issue both found that patients who have gone through anesthesia and surgery are at an increased risk of developing dementia years later (26,27). Taken together, these data suggest that POCD after non-cardiac surgery typically lasts months or even up to a year, but does not exclude the possibility that it may last longer in some cases. Whether perioperative care and/or POCD are linked to a long term risk of developing dementia remains an important question for future prospective studies ((28), Table 3).
After cardiac surgery Newman and colleagues found an overall cognitive trajectory of decline up to 5 years later (7). Interestingly, cognitive dysfunction in the early post-operative period was a predictor of cognitive decline 5 years later (7), raising the possibility that long-term cognitive decline after cardiac surgery may be caused by insults sustained during the perioperative period. This view is challenged, however, by data from Selnes and McKann et al, who found no difference in the long term cognitive trajectory (at 3 or 6 years after surgery) in patients with coronary artery disease (CAD) who underwent cardiac surgery versus control patients with CAD who did not undergo cardiac surgery (8,29,30). Similarly, CAD patients who underwent off-pump CABG had similar cognitive outcomes as CAD patients who underwent percutaneous coronary intervention (31). Thus, there is clearly long term cognitive decline that occurs over years in older patients with CAD, but this long term decline appears to be largely due to patient factors (such as pre-existing neurovascular disease) rather than procedural factors (such as cardiac surgery, cardiopulmonary bypass, or anesthesia itself, (32)). This interpretation need not imply that the mechanisms of cognitive decline are identical in CAD patients treated surgically versus medically, though (33). Taken together, these data suggest that as in the case POCD after non-cardiac surgery POCD after cardiac surgery may last from weeks to several months. However, the current data does not rule out the possibility that POCD after cardiac surgery may last longer in some cases.
In asking how long POCD may last, it is important to note that POCD is a syndrome rather than a disease caused by a single underlying pathophysiologic process. In this sense, POCD is more akin to a fever than influenza. Although a recent study suggested that POCD is independent of surgical procedure or anesthetic drug choice on a population level (34), there are likely some patients who experience POCD due to specific intraoperative or perioperative factors, and the duration of POCD likely depends on its specific etiology. For example, the 5th patient in Bedford’s case report was described as “an intelligent and active man- mentally normal in every way” prior to surgery, but after he was “unable to recognize his relations and remained unaware of his surroundings” even up to 18 months after surgery (1). This severe post-operative cognitive dysfunction was likely related to the fact that this patient’s “blood pressure fell to unrecordable levels for about 15 minutes” during surgery (1). There is considerable evidence that prolonged cerebral hypoperfusion can cause cerebral ischemic damage and result in life long neurocognitive deficits. Thus, it is likely that this patient’s post-operative cognitive dysfunction was caused by intraoperative hypotension, and that it lasted the rest of this patient’s life. Although this is a somewhat extreme case (intraoperative periods of undetectable blood pressure lasting over 15 minutes are currently extremely rare, except in deep hypothermic circulatory arrest cases), this example makes the point that the duration of POCD depends on its etiology. This should come as no surprise. The length, severity and outcome of any syndrome depends on its cause; a fever caused by a cold virus is likely to have a shorter duration and better outcome than one caused by gram negative rod sepsis.
We believe that the question of how long POCD lasts, as opposed to how long other types of cognitive deficits may last, is largely irrelevant for individual patients though. If a patient is suffering from cognitive decline, in most cases he or she (and family members) are unlikely to care what percentage of the cognitive decline may be attributable to perioperative care, versus what percentage may have occurred in the absence of perioperative care. Furthermore, there is no way to calculate these percentages on an individual patient basis, and even if we could calculate these percentages on an individual patient basis, it would be therapeutically irrelevant: there is currently no specific treatment for POCD that differs from that for any other age-related cognitive disorder. Nonetheless, patients may ask preoperatively what their risk of POCD is, and how long it may last. At a population-level we believe that current data suffice to tell patients that most cases of POCD resolve within months after both non-cardiac and cardiac surgery, although it is impossible to tell how long any individual case of POCD will last. It is important to emphasize during pre-operative counseling that POCD has more than one potential underlying cause, and if it develops, the course and prognosis will depend on its cause(s).
What is POCI?
While much of the focus has been on patients who experience a worsening of cognitive performance after anesthesia and surgery, the same neuropsychological testing demonstrates that some patients improve their cognitive performance after their procedure. This enhancement may reflect a genuine improvement in cognitive function, or simply the slowing or reversal of a preoperative deterioration. In some cases, post-operative cognitive improvement (POCI) can be directly attributed to the goals of the specific surgery itself, e.g. the post-operative restoration of cerebral perfusion after carotid endarterectomy (35), the removal of a brain lesion (36), or the surgical treatment of obesity and metabolic syndrome (3,4). However, even in these patients, practice effects (i.e., the tendency for test performance to improve with repeat testing) may also significantly contribute to the probability of mistakenly concluding that a subject has post-operative cognitive improvement (37).
More generally, the use of multiple sensitive tests, performed at different times, results in great measurement variability. The ISPOCD group examined this variability and uncovered cognitive improvement in 4.2–8.7% of patients after 1 week and in 5.0–7.8% after 3 months (38). However, in the same population, these investigators found a 3–6 times greater incidence of POCD, leading them to conclude that the observed “improvement” merely reflected the unpredictable variability inherent in precise neuropsychological testing. While there may be a subset of patients who improve after surgery, it appears to be a much smaller population than those who experience dysfunction (38).
It is hard to imagine that the factors associated with anesthesia and surgery themselves (e.g. fasting, stress, anesthesia, tissue damage, blood loss) would confer a cognitive benefit to patients. Thus, how do we explain patients who do appear to experience genuine cognitive improvement after anesthesia and surgery? In a meta-analysis evaluating cognitive function before and after coronary artery bypass grafting (CABG) surgery, there was evidence of cognitive improvement in multiple neuropsychological tests (39). CABG patients also demonstrate improved physical, social, and emotional function six months and one year after surgery, including less anxiety and depression (40). It is likely that the improvement in the cognitive function of CABG patients stems from this generalized improvement in overall health and quality of life, especially given the known negative effects of depression on cognitive performance (41,42). Moreover, a successful surgery sometimes enables patients to taper or discontinue cognition-clouding medications (e.g., medications for pain, sleep, or anxiety) that were used preoperatively, and may thus allow patients to improve their overall functioning. In line with this idea, even among patients who had POCD at 6 weeks after surgery, increased ability to perform instrumental activities of daily living at 6 weeks after surgery was associated improved cognition at 1 year (2).
How long does POCI last?
It is unclear how long cognitive improvement would last if it results from a generalized improvement in health postoperatively. The increases in performance described above began to appear at three months, and continued throughout the first year after cardiac surgery (39). In bariatric surgery, improvements were seen two and three years later (3,4). We hypothesize that postoperative cognitive improvement will last as long as a patient’s general health and quality of life remain improved after surgery, and this is an important question for future study (Table 3).
A comparison between POCD and other medically-related/induced cognitive disorders
The phenomenon of POCD has a parallel in the cognitive changes associated with cancer and chemotherapy (reviewed in (43)). Although some of the classical psychological sequelae of cancer diagnosis (including depression, anxiety, fatigue) are independently associated with alterations in cognitive function, it remains difficult to untangle the pathophysiology of CRCI since several chemotherapeutic agents have direct neurotoxic actions (reviewed in (44)) and modulate interactions between the immune system and brain (45). While cancer-related cognitive impairment (CRCI) appears to occur more frequently than POCD, the two syndromes share many of the same characteristics including demographics, biological factors and time courses. As in the case of POCD, older patients and those with lower levels of pretreatment cognitive reserve experience the greatest reductions in performance from CRCI (46,47). These impairments occur in multiple cognitive domains, reach a nadir shortly after cancer treatment, and then gradually return toward baseline (48). Additionally, many of the proposed mechanisms of “chemo-brain” are the same ones proposed in the POCD literature (discussed below). Patients subjectively report greater deficits than are seen in objective neuropsychological testing in both POCD and CRCI (49,50), which suggests that the neurocognitive tests utilized in CRCI and POCD testing do not pick up the full intensity of the cognitive impairments that patients themselves experience. Unfortunately, many of the same methodological problems (e.g. inability to randomize, variation between studies in criteria used to define cognitive impairment, practice-related effects) affect studies on both POCD and CRCI.
II. Who is at risk for developing POCD?
Modifiable risk factors
Several studies have examined risk factors for POCD (see Tables 2a and 2b for a list of modifiable and non-modifiable POCD risk factors, respectively). Interestingly, several studies have found that either lighter anesthetic depth or careful anesthetic depth monitoring can lower POCD rates (51,52), which suggests that POCD may be due to excessive anesthetic drug exposure in some cases. In line with these findings, a prior study found an increased rate of POCD one week after general vs regional anesthesia (p=0.06 overall, p=0.04 in an as treated analysis (53)), although there was no difference in POCD rates 3 months after surgery (p=0.93). However, Silbert and colleagues recently found no difference in POCD rates between patients who underwent extracorporeal shock wave lithotripsy under either general or spinal anesthesia (54); if anything, there was a surprising trend towards increased POCD risk in the spinal anesthesia group (p=0.06, and the trial was stopped early). This suggests that general anesthesia does not increase POCD risk. These findings are particularly difficult to reconcile with those discussed above because more than 90% of the patients in the spinal anesthesia group did not receive any intravenous sedation (54), in contrast to many other studies in which patients randomized to receive regional anesthesia also received large doses of intravenous sedation (53). However, the Silbert et al. study (54) was nearly 4 fold smaller than the Rasmussen et al. study (53). Further, the patients in the spinal anesthesia group were also 3 years older on average than those in the general anesthesia group in the Silbert et al. study (54). A meta-analysis performed on this subject in 2010 found a non-statistically significant trend towards an increased risk of POCD after general versus regional anesthesia (OR 1.34, 95% CI 0.93–1.95%, p=0.26) (55). Another meta-analysis performed in 2011 also found a slight, but non-significant trend towards a decreased risk of POCD after regional vs general anesthesia (standardized difference in means −0.08, −0.17 to 0.01, p=0.094, (56)). Future studies will be necessary to better understand whether there is a relationship between general anesthesia and POCD risk, and whether certain subgroups of patients (such as older patients, those with more cerebrovascular disease, or those with less cognitive reserve for other reasons) are at higher risk of developing POCD after general vs regional anesthesia (Table 3).
Table 2A.
Potentially Modifiable risk factors for POCD
| Risk Factor | Effect Size | Study Design | Reference |
|---|---|---|---|
| Bispectral index (EEG) guided anesthetic care (versus routine care) | Odds ratio (OR) 0.92 (0.66–1.29) at 1 week p=0.06; OR 0.62 (0.39–0.97) at 3 months p=0.02 18.1% v. 23.9% at 7 days p=0.062; 8% v. 10.3% at 3 months p=0.372 |
Randomized Controlled Trial (RCT) RCT |
(117) (52) |
| Fentanyl dosage | Low (10 mcg/kg) v. High Dose Fentanyl (50 mcg/kg), POCD rates 23.6% v. 13.7% at 1 week, respectively, p= 0.03. N.S. at 3 and 12 months. | RCT | (143) |
| Ketamine Treatment | 2 SD drop in overall cognition in 7/26 ketamine group vs 21/26 patients, P<0.001 | RCT | (108) |
| Lidocaine v. No Lidocaine | POCD 18.6% v. 40% p=0.028 Neurocognitive Deficit 45.8% v. 40.7% at 10 weeks p=0.577; 35.2% v. 37.7% at 25 weeks p=0.710 45.5% vs 45.7%, p=0.97 |
RCT RCT RCT |
(144) (145) (66) |
| Magnesium Sulfate Infusion | Multivariate OR for Low Dose 0.09 (0.02, 0.50) p=0.01; OR for High Dose 0.45 (0.16, 1.33) p=0.15 44.4% vs 44.9%, p=0.93 |
RCT RCT |
(146) (107) |
| Piracetam v. No Piracetam | Overall Cognitive Function Preop 0.06±1.02 v. - 0.06±0.99 Postop −0.65±0.93 v. −1.38± 1.11 p<0.0005 | RCT | (147) |
| Intraoperative Steroid Treatment | No v. Low v. High Dose Dexamethasone POCD 22.3% v. 20.6% v. 31.4% p=0.003 Relative Risk (RR) 1.87 (0.90–3.88) at 1 month p = 0.09; RR 1.98 (0.61–6.40) at 1 year p = 0.24 |
RCT RCT |
(111) (110) |
| Post-operative delirium* | Multivariate OR 9.58 (4.62–19.9) p<0.001 POCD v. No POCD** 1.5% v. 1.1% at discharge p=0.046; 6.7% v. 5.6% at 3 months p=0.373 Delirium v. No Delirium MMSE Scores: 24.1 v. 27.4 at 1 month p<0.001; 25.2 v. 27.2 at 1 year p<0.001 |
RCT Prospective Cohort Study Prospective Cohort Study |
(117) (20) (9) |
| Post-operative infection* | Univariate OR 2.17 (1.50–3.15) p=0.001 | RCT | (117) |
| Post-operative respiratory complication* | Univariate OR 1.69 (1.01–2.89) p=0.02 | RCT | (117) |
| Metabolic Syndrome* | POCD v. No POCD 43.3% v. 26.7% p<0.02 |
Prospective Cohort Study | (129) |
| Cigarette Abuse | Multivariate OR 2.04 (1.11–3.74) p=0.022 N.S. |
RCT Prospective Cohort study |
(148) (23) |
| Diabetes* | Multivariate OR 2.34 (1.22–4.51) p=0.01 POCD v. No POCD 40% v. 19.2% p=0.021 Multivariate linear regression, parameter estimate 0.031 (−0.111, 0.172) p=0.671 |
Prospective Cohort Study Prospective Cohort Study RCT |
(149) (104) (66) |
| Duration of anesthesia | OR 1.1 (1.0–1.3), p=0.01 POCD v. No POCD at 3 months 215.0±92.8 v. 211.5±103.2 minute duration, p=0.52, POCD v. No POCD 5.6±1.5 v. 5.0±1.2 p=0.026 POCD v. No POCD 4.6±1.5 v. 3.8±0.8 p=0.001 |
Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study |
(23) (20) (104) (150) |
| Benzodiazepines before surgery | OR 0.4 (0.2–1.0) p=0.03 | Prospective Cohort Study | (23) |
| Duration of hospital stay | POCD v. No POCD 6.6±16.3 v. 4.8±5.9 at discharge p=0.0003; Multivariate OR 1.03 (1.00–1.05) at 3 months p=0.2479 |
Prospective Cohort Study | (20) |
| Duration of Surgery | POCD v. No POCD 4.7±0.9 v. 4.2±1.0 p=0.01 |
Prospective Cohort Study | (104) |
| Anesthetic Type (general vs regional) | Mean Difference −0.08 (–0.17–0.01) p= 0.094 General vs. non-general anesthesia, OR 1.34 (0.95- 1.93), p=0.26 |
Meta-Analysis Meta-analysis |
(56) (55) |
| Bispectral Index and Cerebral Oxygenation | 1 week Mild Fisher’s exact test p=0.018 1 week Moderate, Pearson’s p=0.037 1 week Severe, Fisher’s exact test p=0.12 |
RCT | (24) |
| Monitoring | 12 week Mild Chi Square Test p=0.02 12 week Moderate Chi Square Test p=0.85 12 week Severe Chi Square Test p=0.65 1 Year Mild Chi Square Test p=0.015 1 Year Moderate Chi Square Test p=0.02 1 Year Severe Fisher’s Exact Test p=0.36 |
||
| Postoperative Copeptin Levels* | OR 28.814 (7.131–116.425) p<0.001 | Prospective Cohort Study | (104) |
| Peripheral Inflammatory Markers* | S-100β Standardized Mean Difference 1.377 (0.423–2.331) p=0.005; IL-6 Standardized Mean Difference 1.614 (0.603– 2.624) p=0.002 |
Meta-Analysis | (151) |
| Off pump v. On pump Cardiac Surgery | Standardized Cognitive Change Score 0.19 v. 0.13 at 3 months p=0.03; 0.19 v. 0.12 at 1 year p=0.09 Off Pump v. On Pump v. Non-Surgical Cardiac Comparison v. Healthy Heart Comparison MMSE 27.7±2.0 v. 27.6±2.4 v. 27.9±2.0 v. 28.5±1.9 at baseline p<0.01 28.5±1.8 v. 27.4±2.5 v. 28.0±2.3 v. 28.6±1.7 at 6 years p<0.01 62% vs 53% at post op day 4, p=0.50; 39% v. 14% at 3 months p=0.04 |
RCT Prospective longitudinal study RCT |
(152) (8) (153) |
| Perfusion Pressure (in Cardiac Surgery) | MMSE Score drop 48 h after surgery, in Low Pressure v. High Pressure: 3.9±6.5 v. 1.1±1.9 p = 0.012 | RCT | (154) |
| rS02 Desaturation Score >3000 | Multivariate OR 2.22 (1.11–4.45) p=0.024 | RCT | (148) |
| Hemodilution (in CPB cases) | Age x hemodilution interaction, p=0.03 | RCT, stopped early | (155) |
| Hyperglycemia (i.e. glucose >200 mg/dL at any point during CPB cases) | Associated with POCD in non-diabetic patients, N=380, OR=1.85 [95% CI 1.12–3.04], p=0.017; N.S. (p=0.81) in diabetic patients, N=145 |
Retrospective analysis of pooled data from multiple prior prospective studies | (156) |
| Slow rewarming vs normal rewarming (in CPB cases) | Multivariate linear regression variable estimate 0.35, p=0.047 (favoring slow rewarming) | RCT | (157) |
| Continuous Cell Saver use (in CPB cases) | 6% vs 15% in controls, p=0.038 16.7% vs 15.9% in controls, relative risk: 1.05, 95% CI: 0.58 to 1.90 at 3 months). |
RCT RCT |
(158) (159) |
| Embolic Load (in CPB cases) | No correlation between embolic load measured by transcranial doppler ultrasound and POCD at 1 week (P=0.617) or at 3 months (P=0.110), N=356 patients. | Pooled analysis of data from 2 other RCTs. | (102) |
| Alpha stat vs pH stat blood gas management (in CPB cases) | 27% versus 44%, p = 0.047 | RCT | (160) |
| Hypothermia vs normothermia (in CPB cases) | Multivariate odds ratio 1.15 [95% Confidence Interval, 1.01, 1.31] p=0.042, for POCD at hospital discharge after intraoperative normothermia vs mild hypothermia. N.S. difference for POCD at 6 weeks after surgery. Hypothermia vs normothermia, relative risk for POCD at 1 week after surgery = 0.77, P=0.048. Hypothermia vs normothermia, relative risk for POCD at 5 years after surgery =0.66, p=0.16 |
Retrospective analysis of pooled data from 2 prior trials. RCT RCT RCT |
(161) (162) (163) (164) |
Partially modifiable risk factor.
Delirium during hospital stay.
N.S. = Not Significant, CPB= Cardiopulmonary Bypass.
Table 2B.
Non-Modifiable risk factors for POCD
| Risk Factor | POCD vs no POCD | Study Design | Reference |
|---|---|---|---|
| Age | Multivariate Odds Ratio (OR) 1.04 (1.01–1.08), p=0.01 Multivariate OR 1.03 (0.99–1.06) p=0.1 Age of patients with POCD 51.9±17.3 v. no POCD 49.4±16.5, measured at discharge p=0.027 OR 1.03 (1.01–1.06) p=0.013 OR 1.151 (1.030–1.285) p=0.003 Multivariate OR 1.34 (1.01–1.78) p=0.043 Multivariate OR 0.95 (0.71–1.26) p=0.70 Multivariate linear regression parameter estimate (for continuous cognitive change score) - 0.009 (−0.012, −0.005) p<0.001 |
Randomized Controlled Trial (RCT) Prospective Observational Study Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study RCT RCT |
(117) (149) (20) (67) (104) (150) (148) (66) |
| Educational Level | Multivariate OR 0.98 (0.91–1.07) p=0.67 Multivariate OR 0.84 (0.76–0.93) at 3 months p=0.0031 OR 0.9 (0.83–0.98) p=0.021 Multivariable linear regression model, parameter estimate: 0.012 (−0.002, 0.027) p=0.098 |
Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study RCT |
(149) (20) (67) (66) |
| Type of surgery | Minimally invasive 4% v. 34%, Intraabdominal/thoracic 21% v. 14%, Orthopedic 11% v. 16% at discharge p=0.001 Congenital disease 10 v. 42 Valvular disease 32% v. 54% Aorta disease 14% v. 20% Tumor 2% v. 2%; p=0.051 overall N.S. |
Prospective Cohort Study Prospective Cohort Study Prospective Cohort Study |
(20) (150) (23) |
| Genetic risk alleles | CRP 1059G/C SNP OR 0.37 (0.16–0.78) p=0.013; SELP 1087G/A SNP OR 0.51 (0.30–0.85) p=0.011 | Prospective Cohort Study | (67) |
| Left Hippocampal Volume | POCD v. No POCD 2.26±0.21 v. 2.45±0.15 p<0.01 | Prospective Cohort Study | (165) |
| Right Hippocampal Volume | POCD v. No POCD 2.49±0.11 v. 2.62±0.20 p<0.05 | Prospective Cohort Study | (165) |
| Middle Cerebral Artery Velocity | Left MCA POCD v. No POCD 42.5±5.5 v. 54.3±4.4 p<0.1; Left v. Right MCA POCD 42.5±5.5 v. 56.3±4.5 p<0.05** | Prospective Cohort Study | (166) |
| Preoperative Renal Insufficiency | Multivariate OR 0.18 (0.04–0.75) p=0.019 | RCT | (148) |
| Prior Stroke | Multivariate OR 0.30 (0.11, 0.84) p=0.02 | RCT | (146) |
Values estimated from the bar graph in Fig. 1 of the paper
Numerous studies have also examined whether specific anesthetic drugs are tied to POCD risk. Although basic science studies have suggested differential neurotoxicity between inhaled/volatile anesthetics versus intravenous agents (reviewed in (28)), there is a paucity of well-controlled clinical studies examining this issue. For example, higher rates of cognitive decline (as measured by the MMSE) were found on one, two and three days after surgery among 2000 patients randomized to receive inhaled versus intravenous anesthesia (57). However, there was no MMSE test score difference between groups by 10 days after surgery in this study, there was no attempt made to ensure equivalent anesthetic depth in both groups, and patients in the inhaled group likely received a significant anesthetic overdose (58). There was no difference in POCD rates between patients randomized to sevoflurane vs desflurane in another study, although patients who received desflurane awakened faster and had higher satisfaction scores (59). Patients who received spinal anesthesia and isoflurane had a higher incidence of POCD than patients who received spinal anesthesia alone or spinal anesthesia plus desflurane in a pilot study (60). It is somewhat unusual to use spinal and inhaled anesthesia together in the United States, though, which makes it hard to apply these findings to typical clinical practice. These findings are also challenged by the results of Kanbak et al, who found that isoflurane use was associated with improved neurocognitive function after cardiac surgery (as compared to desflurane or sevoflurane) in a similar size pilot randomized controlled trial (61). Schoen and colleagues found that sevoflurane administration (as compared to propofol-based intravenous anesthesia) was associated with improved cognition within one week after on-pump cardiac surgery (62). In summary, the available evidence is insufficient to determine whether any specific anesthetic agent is associated with a reduced risk of POCD.
Several recent papers have also examined whether specific anesthetic drugs may be associated with less cognitive decline after surgery in patients with mild cognitive impairment (MCI, an early stage of Alzheimer’s disease). One recent study found increased rates of MCI progression two years later in spine surgery patients who were randomized to receive sevoflurane vs propofol or epidural anesthesia (N=60 per group, (63)). Another study among MCI patients found no difference in POCD rates among MCI patients randomized to receive sevoflurane (N=99) vs propofol anesthesia (N=101) for radical rectal resection, although there was an increased rate of severe POCD in the sevoflurane treated patients (64). Taken together, these studies raise the possibility that propofol anesthesia (as compared to sevoflurane anesthesia) might be associated with improved post-operative cognition in patients with MCI, but further studies are necessary to examine this issue.
Non-modifiable risk factors
One non-modifiable risk factor for POCD consistently found in multiple studies is increased age (7,20,23,51,54); POCD was present in one study more than twice as often in patients age 60 and over as in those below 60 (20). This association makes intuitive sense in the setting of the failed resilience model discussed above. Older patients often have more neurovascular disease risk factors, more cerebral white matter damage and less cognitive reserve (65), which may place them at a higher risk of cognitive dysfunction after the stress of surgery, anesthesia and perioperative care. Aside from age, two other non-modifiable risk factors for POCD that have been found across multiple studies are less years of prior education and lower preoperative cognitive test scores (20,66,67), which also fits with the idea that patients with less cognitive reserve are at higher risk of developing POCD (see Table 2B). <<AU: Please check placement of Table 2B mentioned in text and revise as needed.>>
Although age itself is non-modifiable, age is also frequently associated with frailty, which is at least partly modifiable (68–71). Numerous studies in recent years have focused on physical “prehabilitation” to decrease post-operative complications and/or improve post-operative physical function (72–75), but to the best of our knowledge no study has evaluated whether physical and/or cognitive prehabilitation might decrease the risk of POCD. The plasticity of the human brain in response to both physical (76) and cognitive (77) exercise suggest that such interventions may help prevent and/or treat POCD. The degree to which an aging brain possesses the plasticity to benefit from such interventions is unclear, though (78), making this is an important area for future research (Table 3).
III. What Causes POCD?
Animal models
Animal studies have suggested the POCD may be caused be either excessive neuroinflammation after surgery (79), a failure to resolve inflammation (80,81), blood-brain barrier/endothelial dysfunction (82–84), and/or pre-existing Alzheimer’s disease pathology (reviewed in (28)). Activation of the innate immune system is increasingly appreciated as an underlying factor in several neurodegenerative conditions, including Alzheimer’s disease. Using different preclinical models of non-CNS surgery, neuroinflammation has been repeatedly associated with behavioral dysfunction and memory deficits. Up-regulation of systemic proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukins (IL)-1, IL-6, chemokines and damage-associated molecular patters (DAMPs) like high-mobility group box 1 (HMGB1) have been shown to activate bone-marrow derived macrophages and contribute to the overall brain pathology after aseptic trauma (80,81,85,86). Several mechanisms including humoral, cellular and neuronal pathways have been proposed in this bidirectional communication between the immune system and the brain following surgery. Notably, strategies aimed at mitigating the excessive inflammatory milieu have been promising in limiting surgery-induced cognitive decline in several rodent models and may offer novel insights for future interventional studies in humans.
Ongoing clinical studies are starting to uncover a potential role for blood and CSF inflammatory biomarkers in the pathophysiology of POCD and this represent a burgeoning area of research for the field. However, it remains unclear to what extent the findings in animal models translate to human patients. Although mice are useful for modeling spatial memory deficits after anesthesia and surgery, mice lack the ability to perform more complex human cognitive functions such as executive function. Thus, mouse models may be useful for understanding some of the memory deficits seen in human POCD, but they cannot recapitulate the full spectrum of cognitive deficits seen in our patients. Also, it is challenging to model the temporal course of human POCD in rodents: preclinical studies often report acute cognitive changes in hippocampal-dependent cognitive tasks but provide limited evidence for longer-lasting neurocognitive dysfunction. Combining anesthesia/surgical trauma with known risk factors for POCD (i.e. aging, infection, metabolic syndrome) in rodents may provide a more relevant model of human POCD (87–89). Further, given the significant differences between the mouse and human immune systems and inflammatory mechanisms (90), it is unclear to what extent the neuroinflammatory mechanisms seen in mouse models are involved in human POCD.
One recent animal study also suggested that post-operative memory deficits may be due to anesthetic-induced up-regulation of the alpha 5-subunit containing GABA-A receptors in the hippocampus (91). These alpha 5 containing GABA receptors inhibit long term potentiation (LTP, a cellular correlate of learning and memory). Thus, upregulation of these receptors would be predicted to cause learning and memory deficits, which could play a role in contributing to human POCD and/or post-operative delirium. However, it is unclear whether anesthetic drugs and/or inflammatory mediators that impinge on LTP function also cause a sustained upregulation of alpha 5 GABA-A receptors in humans.
Human studies
The mouse studies described above provide useful hypotheses about what might cause POCD, which can then be investigated in human studies. Indeed, surgery is associated with a central neuroinflammatory response in humans (92–96). It remains unclear, though, whether this central neuroinflammatory response is associated with POCD. To the best of our knowledge, there are also no human studies that have demonstrated an association between the presence or levels of central anti-inflammatory mediators and the presence or duration of POCD. Pre-existing AD pathology (as measured by CSF biomarkers) has been associated with increased perioperative decline in some cognitive tests but not in others (97), and patients with mild cognitive impairment (MCI, a prodromal stage of AD) have larger cognitive deficits in some tests after perioperative care than surgical patients without MCI (98,99). These studies suggest that pre-existing AD pathology may be associated with POCD, but the full extent of this relationship remains to be elucidated. One recent pilot study also found that the extent of pre-existing white matter damage (as measured by MRI) was a predictor of post-operative executive function deficits in patients who underwent knee arthroplasty (100). Neuroimaging studies have also shown that cardiac surgery in particular is associated with an increase in “silent strokes” or white matter hyperintensities seen on magnetic resonance imaging, although the total burden of white matter hyperintensities after surgery does not correlate with the presence of POCD ((101,102), reviewed in (32)). Clearly, understanding the relationship between central neuroinflammation, cerebrovascular white matter disease, AD pathology (and/or perioperative changes in these processes) and POCD is a major question in the field.
Numerous human studies have failed to find plasma biomarkers associated with POCD (11,103), although one recent study found that higher plasma levels of copeptin (a peptide co-released with arginine vasopressin from the hypothalamus) were associated with increased risk of both postoperative delirium and POCD (104). Remarkably, plasma copeptin levels were a better predictor of POCD risk than age in this study (104); it will be important to replicate these results. The failure of numerous other studies to find plasma biomarkers of POCD may be due to differential protein and cytokine expression between the cerebrospinal fluid and plasma (94). Future human studies combining CSF biomarker measurements, cognitive testing, and functional neuroimaging both before and after perioperative care will be necessary to further elucidate the pathophysiology of POCD, and to fully ascertain whether anesthesia and surgery are associated with an acceleration of AD pathology (28,105).
IV. How can we prevent or treat POCD?
POCD Prevention Studies
Although we are likely only in the infancy of understanding the etiologies of POCD, its detrimental impact on patients mandate that clinicians do everything in their power to prevent patients from developing POCD and to treat POCD once it does develop. Numerous investigators have attempted to prevent POCD with interventions ranging from intraoperative lidocaine (106), magnesium (107), ketamine (108), complement suppression (109), or even high dose dexamethasone (110) treatment in randomized controlled trials. Lidocaine treatment had no effect on preventing POCD overall (106), although in a secondary analysis, lidocaine infusion appeared to have a beneficial effect on cognition in non-diabetic patients. Similarly, intraoperative magnesium treatment had no effect on preventing POCD overall, although there was a trend towards a detrimental effect of magnesium treatment on cognition in heavier patients (107). Complement suppression with the monoclonal antibody pexelizumab (which inhibits complement factor C5) also had no effect on the rate of POCD, although it was associated with improved visuospatial cognition (109).
Surprisingly, ketamine treatment (0.5 mg/kg) on anesthetic induction was associated with a decrease in POCD occurrence after cardiac surgery (108). Ketamine treatment was also associated with a reduction in serum CRP levels in this study (108), leading the authors to propose that ketamine decreases POCD incidence by decreasing inflammation. However, high-dose dexamethasone (i.e. 1 mg/kg on anesthetic induction) did not decrease POCD rates in patients undergoing cardiac surgery in the DECS trial (110), which was almost 4 times the size of the ketamine trial (108). Higher dose dexamethasone (0.2 mg/kg) was actually associated with increased POCD rates (as compared to 0.1 mg/kg dexamethasone or placebo treatment) in a trial of patients undergoing microvascular decompression for facial spasms (111). Taken together, these results could imply that while inflammation may play a role in POCD, not all drugs with anti-inflammatory activity have the same effects on POCD. Alternatively, the efficacy of ketamine in reducing POCD (108) may be due to its effects on neurotransmitter receptors or other biological processes unrelated to inflammation; in addition to blocking the NMDA receptor, ketamine modulates signaling via a number of other receptors (reviewed in (112)).
While the failure of numerous single drug therapies for POCD prevention are disappointing, these failures are not surprising if POCD is viewed as a syndrome of brain dysfunction caused by diverse factors rather than a single disease caused by a specific etiology. Further, the complexity of the human brain itself is staggering- it contains over 80 billion neurons (each of which make thousands of synaptic connections to other neurons), and over 80 billion interneurons (113). The human brain also expresses more than 80% of the human genome (114), a higher percentage than any other organ. Considering the complexity of the human brain, and the diverse factors that may contribute to POCD, it is less surprising that several single drug interventions failed to reduce the incidence of POCD.
Preventing POCD may thus require a multi-component intervention that addresses the diverse factors that contribute to its genesis. A recent pilot study suggested that remote ischemic preconditioning may decrease cognitive deficits after cardiac surgery (115). Since remote ischemic preconditioning has a plethora of biological effects (116), this study is consistent with the idea that preventing POCD will require interventions that work more broadly than a single drug.
Similarly, Ballard et al examined the effect of a combined intervention to decrease POCD rates, which included intraoperative bispectral index monitoring (to optimally titrate anesthetic depth) and cerebral oxygen saturation monitoring (to titrate cerebral oxygen delivery). This combined intervention reduced POCD incidence at multiple time points (24) (see table 2A for statistics). However, there is mixed evidence whether bispectral (BIS) index monitoring alone can decrease POCD rates (52,117), and the authors of a systematic review also concluded that there is insufficient evidence to recommend the use of cerebral oximetry monitoring on its own to decrease POCD rates after cardiac surgery (118). Taken together, these studies may mean that the combined use of BIS and cerebral oximetry monitoring may help decrease POCD rates, but that either monitor alone may have a lesser or non-significant effect. Future studies will be necessary to examine this hypothesis by evaluating these interventions in isolation and together.
POCD Treatment Studies
Very few randomized controlled studies have examined whether any intervention can treat or improve POCD once it has already occurred. Such studies are challenging to conduct since most cases of POCD resolve spontaneously within months (see section I), although they would still be important given the association between POCD and decreased quality of life (18), early exit from the work force (19) and premature mortality (19,20). However, these studies would be neither unprecedented nor impossible- a similar challenge occurs in depression treatment trials, in which there is a high response even among patients receiving placebo within weeks to months (119). One randomized trial examined the use of the acetylcholinesterase inhibitor donepezil in patients who displayed cognitive decline (0.5 SD drop in at least one cognitive domain) 1 year after cardiac surgery. Donepezil improved some aspects of memory performance in these patients, which is consistent with the role of acetylcholinesterase inhibitors in improving memory in patients with early stage Alzheimer’s disease. However, donepezil treatment had no effect on the overall cognitive index in these patients with cognitive decline 1 year after cardiac surgery (120).
Interestingly, the antidepressant and selective serotonin reuptake inhibitor (SSRI) citalopram was used to successfully treat POCD in one case report (121). This finding is generally in line with the pleiotropic biological roles of serotonin including its ability to promote neuroplasticity (122). If POCD is a syndrome of failed resilience or a lack of neuroplasticity after perioperative care that occurs in patients with pre-existing neurovascular disease or “silent strokes”, then the established efficacy of SSRI’s in improving neurologic outcomes after stroke (123) suggest that they may be useful in treating POCD as well. SSRI’s also have been shown to quality of life in depressed elderly patients(124) and improve affect and mood even in non-depressed, healthy individuals (125), which further suggests that SSRI’s may be efficacious in reducing POCD-associated quality of life impairments. More work will be necessary to determine whether SSRI’s improve cognition, quality of life, and other outcomes in patients with POCD (Table 3).
V. Conclusions and Future Directions
POCD is a syndrome that occurs more frequently in patients age 60 and older, and is associated with early exit from the work force (19), decreased quality of life (18), and premature mortality (19,20). It typically lasts for weeks to months (23), although rare cases may last considerably longer (1). Based on this understanding, we believe that patients at high risk for POCD (i.e. those with multiple risk factors listed in tables 2a and 2b, such as elderly patients) should be counselled preoperatively about the risk of POCD, just as we counsel patients preoperatively about numerous other risks of perioperative care. This counselling could allow patients to make cognitively demanding decisions before surgery/anesthesia, and/or to ensure that they will have loved ones or others to help them with cognitively demanding tasks for the first weeks to months after surgery/anesthesia. Additional help and assistance may help these patients recover better from surgery in general; after all, patients with POCD who may not be able to remember what they ate for breakfast that morning are also unlikely to be able to remember whether they took their medicine that morning.
The long term sequelae of POCD discussed above also mandate that we try to prevent it, and that we develop effect treatments for it once it has occurred. The failure of numerous intervention trials to prevent POCD, and our general lack of understanding of what causes POCD, argue that developing a better understanding of the etiology of POCD may be essential for developing strategies to prevent it. We have conceptualized POCD as a failure of resilience in the face of perioperative stress, which suggests that strategies to improve physical and cognitive resilience in the elderly may help prevent POCD and improve overall recovery after surgery.
Figure 1.
POCD publications by year
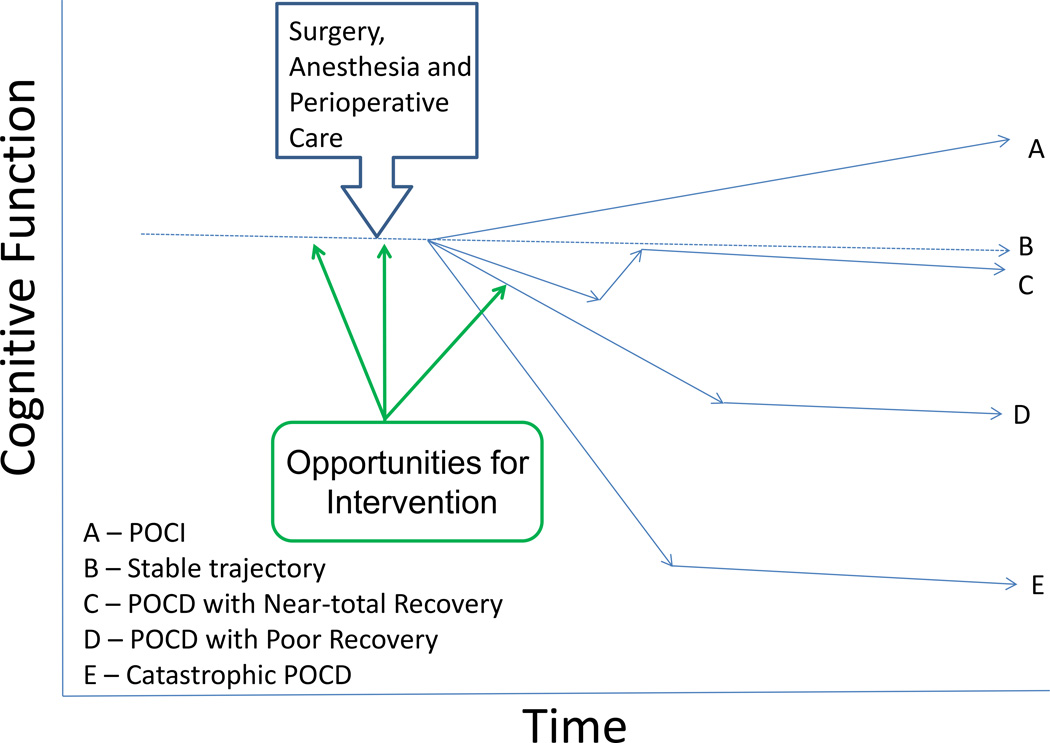
Figure 2.
Potential Cognitive Trajectories Following Surgery
Key Point Sentences.
POCD is a syndrome of cognitive dysfunction following anesthesia and surgery, which likely has myriad causes.
As an increasing number of elderly patients undergo surgery and anesthesia each year, optimizing post-operative cognitive function and preventing/treating POCD are major public health issues.
POCD is associated with impaired quality of life, increased exit from the work force, and increased mortality after surgery.
POCD can be conceptualized as a lack of cognitive resilience in the face of perioperative stress.
Acknowledgments
The authors are not supported by, nor maintain any financial interest in, any commercial activity that may be associated with the topic of this article. Dr Miles Berger acknowledges grant funding from the International Anesthesia Research Society and the National Institute of Health (T32 #GM08600).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–263. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- 2.Fontes MT, Swift RC, Phillips-Bute B, Podgoreanu MV, Stafford-Smith M, Newman MF, Mathew JP. Neurologic Outcome Research Group of the Duke Heart C. Predictors of cognitive recovery after cardiac surgery. Anesth Analg. 2013;116:435–442. doi: 10.1213/ANE.0b013e318273f37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Crosby RD, Mitchell JE, Gunstad J. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg. 2014;207:870–876. doi: 10.1016/j.amjsurg.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alosco ML, Spitznagel MB, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, Gunstad J. Improved memory function two years after bariatric surgery. Obesity (Silver Spring) 2014;22:32–38. doi: 10.1002/oby.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–144. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 6.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 7.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 8.Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88:445–454. doi: 10.1016/j.athoracsur.2009.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funder KS, Steinmetz J, Rasmussen LS. Methodological issues of postoperative cognitive dysfunction research. Semin Cardiothorac Vasc Anesth. 2010;14:119–122. doi: 10.1177/1089253210371520. [DOI] [PubMed] [Google Scholar]
- 11.McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852–859. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avidan MS, Xiong C, Evers AS. Postoperative cognitive decline: the unsubstantiated phenotype. Anesthesiology. 2010;113:1246–1248. doi: 10.1097/ALN.0b013e3181f696f5. author reply 8–50. [DOI] [PubMed] [Google Scholar]
- 13.Levine AJ, Miller EN, Becker JT, Selnes OA, Cohen BA. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clin Neuropsychol. 2004;18:373–384. doi: 10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 14.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–61. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 16.Lewis MS, Maruff P, Silbert BS, Evered LA, Scott DA. The sensitivity and specificity of three common statistical rules for the classification of post-operative cognitive dysfunction following coronary artery bypass graft surgery. Acta Anaesthesiol Scand. 2006;50:50–57. doi: 10.1111/j.1399-6576.2006.00893.x. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 18.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF. Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med. 2006;68:369–375. doi: 10.1097/01.psy.0000221272.77984.e2. [DOI] [PubMed] [Google Scholar]
- 19.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–555. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 20.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 21.Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112:440–451. doi: 10.1093/bja/aet420. [DOI] [PubMed] [Google Scholar]
- 22.Avidan MS, Evers AS. Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimers Dis. 2011;24:201–216. doi: 10.3233/JAD-2011-101680. [DOI] [PubMed] [Google Scholar]
- 23.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 24.Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, Lowery D, Corbett A, Wesnes K, Katsaiti E, Arden J, Amoako D, Prophet N, Purushothaman B, Green D. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One. 2012;7:e37410. doi: 10.1371/journal.pone.0037410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avidan MS, Searleman AC, Storandt M, Barnett K, Vannucci A, Saager L, Xiong C, Grant EA, Kaiser D, Morris JC, Evers AS. Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111:964–970. doi: 10.1097/ALN.0b013e3181bc9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen PL, Yang CW, Tseng YK, Sun WZ, Wang JL, Wang SJ, Oyang YJ, Fuh JL. Risk of dementia after anaesthesia and surgery. Br J Psychiatry. 2014;204:188–193. doi: 10.1192/bjp.bp.112.119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement. 2014;10:196–204. doi: 10.1016/j.jalz.2013.05.1766. [DOI] [PubMed] [Google Scholar]
- 28.Berger M, Burke J, Eckenhoff R, Mathew J. Alzheimer’s disease, anesthesia, and surgery: a clinically focused review. J Cardiothorac Vasc Anesth. 2014;28:1609–1623. doi: 10.1053/j.jvca.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Selnes OA, Grega MA, Bailey MM, Pham LD, Zeger SL, Baumgartner WA, McKhann GM. Cognition 6 years after surgical or medical therapy for coronary artery disease. Ann Neurol. 2008;63:581–590. doi: 10.1002/ana.21382. [DOI] [PubMed] [Google Scholar]
- 30.Selnes OA, Grega MA, Bailey MM, Pham L, Zeger S, Baumgartner WA, McKhann GM. Neurocognitive outcomes 3 years after coronary artery bypass graft surgery: a controlled study. Ann Thorac Surg. 2007;84:1885–1896. doi: 10.1016/j.athoracsur.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 31.Sauer AM, Nathoe HM, Hendrikse J, Peelen LM, Regieli J, Veldhuijzen DS, Kalkman CJ, Grobbee DE, Doevendans PA, van Dijk D, Octopus Study G. Cognitive outcomes 7.5 years after angioplasty compared with off-pump coronary bypass surgery. Ann Thorac Surg. 2013;96:1294–1300. doi: 10.1016/j.athoracsur.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 32.McDonagh DL, Berger M, Mathew JP, Graffagnino C, Milano CA, Newman MF. Neurological complications of cardiac surgery. Lancet Neurol. 2014;13:490–502. doi: 10.1016/S1474-4422(14)70004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartels K, McDonagh DL, Newman MF, Mathew JP. Neurocognitive outcomes after cardiac surgery. Curr Opin Anaesthesiol. 2013;26:91–97. doi: 10.1097/ACO.0b013e32835bf24c. [DOI] [PubMed] [Google Scholar]
- 34.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 35.Hemmingsen R, Mejsholm B, Vorstrup S, Lester J, Engell HC, Boysen G. Carotid surgery, cognitive function, and cerebral blood flow in patients with transient ischemic attacks. Ann Neurol. 1986;20:13–19. doi: 10.1002/ana.410200104. [DOI] [PubMed] [Google Scholar]
- 36.Raeder MB, Helland CA, Hugdahl K, Wester K. Arachnoid cysts cause cognitive deficits that improve after surgery. Neurology. 2005;64:160–162. doi: 10.1212/01.WNL.0000148724.61966.A4. [DOI] [PubMed] [Google Scholar]
- 37.Casey JE, Ferguson GG, Kimura D, Hachinski VC. Neuropsychological improvement versus practice effect following unilateral carotid endarterectomy in patients without stroke. J Clin Exp Neuropsychol. 1989;11:461–470. doi: 10.1080/01688638908400906. [DOI] [PubMed] [Google Scholar]
- 38.Rasmussen LS, Siersma VD, Ispocd G. Postoperative cognitive dysfunction: true deterioration versus random variation. Acta Anaesthesiol Scand. 2004;48:1137–1143. doi: 10.1111/j.1399-6576.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 39.Cormack F, Shipolini A, Awad WI, Richardson C, McCormack DJ, Colleoni L, Underwood M, Baldeweg T, Hogan AM. A meta-analysis of cognitive outcome following coronary artery bypass graft surgery. Neurosci Biobehav Rev. 2012;36:2118–2129. doi: 10.1016/j.neubiorev.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Lindquist R, Dupuis G, Terrin ML, Hoogwerf B, Czajkowski S, Herd JA, Barton FB, Tracy MF, Hunninghake DB, Treat-Jacobson D, Shumaker S, Zyzanski S, Goldenberg I, Knatterud GL, Investigators PCBS. Comparison of health-related quality-of-life outcomes of men and women after coronary artery bypass surgery through 1 year: findings from the POST CABG Biobehavioral Study. Am Heart J. 2003;146:1038–1044. doi: 10.1016/S0002-8703(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 41.Rabbitt P, Donlan C, Watson P, McInnes L, Bent N. Unique and interactive effects of depression, age, socioeconomic advantage, and gender on cognitive performance of normal healthy older people. Psychol Aging. 1995;10:307–313. doi: 10.1037//0882-7974.10.3.307. [DOI] [PubMed] [Google Scholar]
- 42.Norman S, Troster AI, Fields JA, Brooks R. Effects of depression and Parkinson’s disease on cognitive functioning. J Neuropsychiatry Clin Neurosci. 2002;14:31–36. doi: 10.1176/jnp.14.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saykin AJ, Ahles TA, McDonald BC. Mechanisms of chemotherapy-induced cognitive disorders: neuropsychological, pathophysiological, and neuroimaging perspectives. Semin Clin Neuropsychiatry. 2003;8:201–216. [PubMed] [Google Scholar]
- 45.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 46.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 48.Falleti MG, Sanfilippo A, Maruff P, Weih L, Phillips KA. The nature and severity of cognitive impairment associated with adjuvant chemotherapy in women with breast cancer: a meta-analysis of the current literature. Brain Cogn. 2005;59:60–70. doi: 10.1016/j.bandc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Johnson T, Monk T, Rasmussen LS, Abildstrom H, Houx P, Korttila K, Kuipers HM, Hanning CD, Siersma VD, Kristensen D, Canet J, Ibanaz MT, Moller JT, Investigators I. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 50.Correa DD, Hess LM. Cognitive function and quality of life in ovarian cancer. Gynecol Oncol. 2012;124:404–409. doi: 10.1016/j.ygyno.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 52.Radtke FM, Franck M, Lendner J, Kruger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):98–105. doi: 10.1093/bja/aet055. [DOI] [PubMed] [Google Scholar]
- 53.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, Jolles J, Papaioannou A, Abildstrom H, Silverstein JH, Bonal JA, Raeder J, Nielsen IK, Korttila K, Munoz L, Dodds C, Hanning CD, Moller JT, Investigators I. Does anaesthesia cause postoperative cognitive dysfunction? A randomised study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 54.Silbert BS, Evered LA, Scott DA. Incidence of postoperative cognitive dysfunction after general or spinal anaesthesia for extracorporeal shock wave lithotripsy. Br J Anaesth. 2014;113:784–791. doi: 10.1093/bja/aeu163. [DOI] [PubMed] [Google Scholar]
- 55.Mason SE, Noel-Storr A, Ritchie CW. The impact of general and regional anesthesia on the incidence of post-operative cognitive dysfunction and post-operative delirium: a systematic review with meta-analysis. J Alzheimers Dis. 2010;22(Suppl 3):67–79. doi: 10.3233/JAD-2010-101086. [DOI] [PubMed] [Google Scholar]
- 56.Guay J. General anaesthesia does not contribute to long-term post-operative cognitive dysfunction in adults: A meta-analysis. Indian J Anaesth. 2011;55:358–363. doi: 10.4103/0019-5049.84850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai Y, Hu H, Liu P, Feng G, Dong W, Yu B, Zhu Y, Song J, Zhao M. Association between the apolipoprotein E4 and postoperative cognitive dysfunction in elderly patients undergoing intravenous anesthesia and inhalation anesthesia. Anesthesiology. 2012;116:84–93. doi: 10.1097/ALN.0b013e31823da7a2. [DOI] [PubMed] [Google Scholar]
- 58.Deiner S, Baxter MG. Cognitive dysfunction after inhalation versus intravenous anesthesia in elderly patients. Anesthesiology. 2012;117:676–678. doi: 10.1097/ALN.0b013e31826256a8. author reply 8. [DOI] [PubMed] [Google Scholar]
- 59.Rortgen D, Kloos J, Fries M, Grottke O, Rex S, Rossaint R, Coburn M. Comparison of early cognitive function and recovery after desflurane or sevoflurane anaesthesia in the elderly: a double-blinded randomized controlled trial. Br J Anaesth. 2010;104:167–174. doi: 10.1093/bja/aep369. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Tian M, Zhen Y, Yue Y, Sherman J, Zheng H, Li S, Tanzi RE, Marcantonio ER, Xie Z. The effects of isoflurane and desflurane on cognitive function in humans. Anesth Analg. 2012;114:410–415. doi: 10.1213/ANE.0b013e31823b2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanbak M, Saricaoglu F, Akinci SB, Oc B, Balci H, Celebioglu B, Aypar U. The effects of isoflurane, sevoflurane, and desflurane anesthesia on neurocognitive outcome after cardiac surgery: a pilot study. Heart Surg Forum. 2007;10:E36–E41. doi: 10.1532/HSF98.20061076. [DOI] [PubMed] [Google Scholar]
- 62.Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund-Adib B, Berger KU, Hueppe M, Heringlake M. Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth. 2011;106:840–850. doi: 10.1093/bja/aer091. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, Zhou J, Liu G, Gao M. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel-group study. Am J Med Sci. 2013;345:355–360. doi: 10.1097/MAJ.0b013e31825a674d. [DOI] [PubMed] [Google Scholar]
- 64.Tang N, Ou C, Liu Y, Zuo Y, Bai Y. Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. J Int Med Res. 2014;42:1252–1261. doi: 10.1177/0300060514549781. [DOI] [PubMed] [Google Scholar]
- 65.Griebe M, Amann M, Hirsch JG, Achtnichts L, Hennerici MG, Gass A, Szabo K. Reduced functional reserve in patients with age-related white matter changes: a preliminary FMRI study of working memory. PLoS One. 2014;9:e103359. doi: 10.1371/journal.pone.0103359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, Blumenthal JA, Newman MF. Neurologic Outcome Research Group of the Duke Heart C. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–887. doi: 10.1161/STROKEAHA.108.531236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford-Smith M, Mackensen GB, Rinder CS, Blumenthal JA, Schwinn DA, Newman MF, Team PI. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49:1934–1942. doi: 10.1016/j.jacc.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 68.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–965. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 69.Binder EF, Schechtman KB, Ehsani AA, Steger-May K, Brown M, Sinacore DR, Yarasheski KE, Holloszy JO. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 70.Mohandas A, Reifsnyder J, Jacobs M, Fox T. Current and future directions in frailty research. Popul Health Manag. 2011;14:277–283. doi: 10.1089/pop.2010.0066. [DOI] [PubMed] [Google Scholar]
- 71.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, Sherrington C, Lord SR, Kurrle SE. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, Carli F. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 73.Jaggers JR, Simpson CD, Frost KL, Quesada PM, Topp RV, Swank AM, Nyland JA. Prehabilitation before knee arthroplasty increases postsurgical function: a case study. J Strength Cond Res. 2007;21:632–634. doi: 10.1519/R-19465.1. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen PR, Andreasen J, Asmussen M, Tonnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Serv Res. 2008;8:209. doi: 10.1186/1472-6963-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furze G, Dumville JC, Miles JN, Irvine K, Thompson DR, Lewin RJ. “Prehabilitation” prior to CABG surgery improves physical functioning and depression. Int J Cardiol. 2009;132:51–58. doi: 10.1016/j.ijcard.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 79.Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol. 2011;70:986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su X, Feng X, Terrando N, Yan Y, Chawla A, Koch LG, Britton SL, Matthay MA, Maze M. Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of the metabolic syndrome. Mol Med. 2012;18:1481–1490. doi: 10.2119/molmed.2012.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bartels K, Ma Q, Venkatraman TN, Campos CR, Smith L, Cannon RE, Podgoreanu MV, Lascola CD, Miller DS, Mathew JP. Effects of deep hypothermic circulatory arrest on the blood brain barrier in a cardiopulmonary bypass model--a pilot study. Heart Lung Circ. 2014;23:981–984. doi: 10.1016/j.hlc.2014.04.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hu N, Guo D, Wang H, Xie K, Wang C, Li Y, Wang C, Wang C, Yu Y, Wang G. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014;1551:13–24. doi: 10.1016/j.brainres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 84.He HJ, Wang Y, Le Y, Duan KM, Yan XB, Liao Q, Liao Y, Tong JB, Terrando N, Ouyang W. Surgery upregulates high mobility group box-1 and disrupts the blood-brain barrier causing cognitive dysfunction in aged rats. CNS Neurosci Ther. 2012;18:994–1002. doi: 10.1111/cns.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, Young WL, Maze M. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–536. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vacas S, Degos V, Tracey KJ, Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014;120:1160–1167. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Terrando N, Yang T, Ryu JK, Newton PT, Monaco C, Feldmann M, Ma D, Akassoglou K, Maze M. Stimulation of the alpha 7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol Med. 2014 doi: 10.2119/molmed.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng X, Degos V, Koch LG, Britton SL, Zhu Y, Vacas S, Terrando N, Nelson J, Su X, Maze M. Surgery results in exaggerated and persistent cognitive decline in a rat model of the Metabolic Syndrome. Anesthesiology. 2013;118:1098–1105. doi: 10.1097/ALN.0b013e318286d0c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433–454. [PubMed] [Google Scholar]
- 91.Zurek AA, Yu J, Wang DS, Haffey SC, Bridgwater EM, Penna A, Lecker I, Lei G, Chang T, Salter EW, Orser BA. Sustained increase in alpha5GABAA receptor function impairs memory after anesthesia. J Clin Invest. 2014;124:5437–5441. doi: 10.1172/JCI76669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yeager MP, Lunt P, Arruda J, Whalen K, Rose R, DeLeo JA. Cerebrospinal fluid cytokine levels after surgery with spinal or general anesthesia. Reg Anesth Pain Med. 1999;24:557–562. doi: 10.1016/s1098-7339(99)90049-4. [DOI] [PubMed] [Google Scholar]
- 93.Tang JX, Baranov D, Hammond M, Shaw LM, Eckenhoff MF, Eckenhoff RG. Human Alzheimer and inflammation biomarkers after anesthesia and surgery. Anesthesiology. 2011;115:727–732. doi: 10.1097/ALN.0b013e31822e9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Reis HJ, Teixeira AL, Kalman J, Bogats G, Babik B, Janka Z, Teixeira MM, Palotas A. Different inflammatory biomarker patterns in the cerebro-spinal fluid following heart surgery and major non-cardiac operations. Curr Drug Metab. 2007;8:639–642. doi: 10.2174/138920007781368845. [DOI] [PubMed] [Google Scholar]
- 96.Reinsfelt B, Westerlind A, Blennow K, Zetterberg H, Ricksten SE. Open-heart surgery increases cerebrospinal fluid levels of Alzheimer-associated amyloid beta. Acta Anaesthesiol Scand. 2013;57:82–88. doi: 10.1111/j.1399-6576.2012.02769.x. [DOI] [PubMed] [Google Scholar]
- 97.Xie Z, McAuliffe S, Swain CA, Ward SA, Crosby CA, Zheng H, Sherman J, Dong Y, Zhang Y, Sunder N, Burke D, Washicosky KJ, Tanzi RE, Marcantonio ER. Cerebrospinal Fluid Abeta to Tau Ratio and Postoperative Cognitive Change. Ann Surg. 2013;258:364–369. doi: 10.1097/SLA.0b013e318298b077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kline RP, Pirraglia E, Cheng H, De Santi S, Li Y, Haile M, de Leon MJ, Bekker A. Surgery and brain atrophy in cognitively normal elderly subjects and subjects diagnosed with mild cognitive impairment. Anesthesiology. 2012;116:603–612. doi: 10.1097/ALN.0b013e318246ec0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bekker A, Lee C, de Santi S, Pirraglia E, Zaslavsky A, Farber S, Haile M, de Leon MJ. Does mild cognitive impairment increase the risk of developing postoperative cognitive dysfunction? Am J Surg. 2010;199:782–788. doi: 10.1016/j.amjsurg.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Price CC, Tanner JJ, Schmalfuss I, Garvan CW, Gearen P, Dickey D, Heilman K, McDonagh DL, Libon DJ, Leonard C, Bowers D, Monk TG. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120:601–613. doi: 10.1097/ALN.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cook DJ, Huston J, 3rd, Trenerry MR, Brown RD, Jr, Zehr KJ, Sundt TM., 3rd Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389–1395. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez RA, Rubens FD, Wozny D, Nathan HJ. Cerebral emboli detected by transcranial Doppler during cardiopulmonary bypass are not correlated with postoperative cognitive deficits. Stroke. 2010;41:2229–2235. doi: 10.1161/STROKEAHA.110.590513. [DOI] [PubMed] [Google Scholar]
- 103.Gerriets T, Schwarz N, Bachmann G, Kaps M, Kloevekorn WP, Sammer G, Tschernatsch M, Nottbohm R, Blaes F, Schonburg M. Evaluation of methods to predict early long-term neurobehavioral outcome after coronary artery bypass grafting. Am J Cardiol. 2010;105:1095–1101. doi: 10.1016/j.amjcard.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 104.Dong S, Li CL, Liang WD, Chen MH, Bi YT, Li XW. Postoperative plasma copeptin levels independently predict delirium and cognitive dysfunction after coronary artery bypass graft surgery. Peptides. 2014;59:70–74. doi: 10.1016/j.peptides.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 105.Terrando N, Eriksson LI, Eckenhoff RG. Perioperative neurotoxicity in the elderly: summary of the 4th international workshop. Anesth Analg. 2015;120:649–652. doi: 10.1213/ANE.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 106.Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, Blumenthal JA, Newman MF. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke. 2009;40:880–887. doi: 10.1161/STROKEAHA.108.531236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathew JP, White WD, Schinderle DB, Podgoreanu MV, Berger M, Milano CA, Laskowitz DT, Stafford-Smith M, Blumenthal JA, Newman MF. Neurologic Outcome Research Group of The Duke Heart C. Intraoperative magnesium administration does not improve neurocognitive function after cardiac surgery. Stroke. 2013;44:3407–3413. doi: 10.1161/STROKEAHA.113.002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, Hudetz AG, Pagel PS, Warltier DC. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand. 2009;53:864–872. doi: 10.1111/j.1399-6576.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- 109.Mathew JP, Shernan SK, White WD, Fitch JC, Chen JC, Bell L, Newman MF. Preliminary report of the effects of complement suppression with pexelizumab on neurocognitive decline after coronary artery bypass graft surgery. Stroke. 2004;35:2335–239. doi: 10.1161/01.STR.0000141938.00524.83. [DOI] [PubMed] [Google Scholar]
- 110.Ottens TH, Dieleman JM, Sauer AM, Peelen LM, Nierich AP, de Groot WJ, Nathoe HM, Buijsrogge MP, Kalkman CJ, van Dijk D, Group DEfCSS. Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology. 2014;121:492–500. doi: 10.1097/ALN.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 111.Fang Q, Qian X, An J, Wen H, Cope DK, Williams JP. Higher dose dexamethasone increases early postoperative cognitive dysfunction. J Neurosurg Anesthesiol. 2014;26:220–225. doi: 10.1097/ANA.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 112.Potter DE, Choudhury M. Ketamine: repurposing and redefining a multifaceted drug. Drug Discov Today. 2014;19:1848–1854. doi: 10.1016/j.drudis.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 113.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 114.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, Daly BD, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SG, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hudetz JA, Patterson KM, Iqbal Z, Gandhi SD, Pagel PS. Remote Ischemic Preconditioning Prevents Deterioration of Short-Term Postoperative Cognitive Function After Cardiac Surgery Using Cardiopulmonary Bypass: Results of a Pilot Investigation. J Cardiothorac Vasc Anesth. 2014 doi: 10.1053/j.jvca.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 116.Gill R, Kuriakose R, Gertz ZM, Salloum FN, Xi L, Kukreja RC. Remote ischemic preconditioning for myocardial protection: update on mechanisms and clinical relevance. Mol Cell Biochem. 2015 doi: 10.1007/s11010-014-2312-z. [DOI] [PubMed] [Google Scholar]
- 117.Chan MT, Cheng BC, Lee TM, Gin T, Group CT. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42. doi: 10.1097/ANA.0b013e3182712fba. [DOI] [PubMed] [Google Scholar]
- 118.Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg. 2013;116:663–676. doi: 10.1213/ANE.0b013e318277a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, Hargreaves R, Hietala J, Lines C, Beebe K, Reines S. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry. 2006;59:216–223. doi: 10.1016/j.biopsych.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 120.Doraiswamy PM, Babyak MA, Hennig T, Trivedi R, White WD, Mathew JP, Newman MF, Blumenthal JA. Donepezil for cognitive decline following coronary artery bypass surgery: a pilot randomized controlled trial. Psychopharmacol Bull. 2007;40:54–62. [PubMed] [Google Scholar]
- 121.Yap KK, Joyner P. Post-operative cognitive dysfunction after knee arthroplasty: a diagnostic dilemma. Oxford Medical Case Reports. 2014;3:60–62. doi: 10.1093/omcr/omu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, Bejot Y, Deltour S, Jaillard A, Niclot P, Guillon B, Moulin T, Marque P, Pariente J, Arnaud C, Loubinoux I. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 124.Heiligenstein JH, Ware JE, Jr, Beusterien KM, Roback PJ, Andrejasich C, Tollefson GD. Acute effects of fluoxetine versus placebo on functional health and well-being in late-life depression. Int Psychogeriatr. 1995;(7 Suppl):125–137. doi: 10.1017/s1041610295002407. [DOI] [PubMed] [Google Scholar]
- 125.Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson RC, Terpstra J, Turner RA, Reus VI. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- 126.Hewitt J, Williams M, Pearce L, Black A, Benson E, Tarrant M, Chakrabati M, Stechman MJ, Moug SJ, McCarthy K. The prevalence of cognitive impairment in emergency general surgery. Int J Surg. 2014;12:1031–1035. doi: 10.1016/j.ijsu.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 127.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 128.Aleman A, van’t Wout M. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex disrupts digit span task performance. Neuropsychobiology. 2008;57:44–48. doi: 10.1159/000129666. [DOI] [PubMed] [Google Scholar]
- 129.Hudetz JA, Patterson KM, Amole O, Riley AV, Pagel PS. Postoperative cognitive dysfunction after noncardiac surgery: effects of metabolic syndrome. J Anesth. 2011;25:337–344. doi: 10.1007/s00540-011-1137-0. [DOI] [PubMed] [Google Scholar]
- 130.Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Nagase T, Nouchi R, Fukushima A, Kawashima R. Regional gray and white matter volume associated with Stroop interference: evidence from voxel-based morphometry. Neuroimage. 2012;59:2899–2907. doi: 10.1016/j.neuroimage.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 131.Vazzana R, Bandinelli S, Lauretani F, Volpato S, Lauretani F, Di Iorio A, Abate M, Corsi AM, Milaneschi Y, Guralnik JM, Ferrucci L. Trail Making Test predicts physical impairment and mortality in older persons. J Am Geriatr Soc. 2010;58:719–7123. doi: 10.1111/j.1532-5415.2010.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oosterman JM, Vogels RL, van Harten B, Gouw AA, Poggesi A, Scheltens P, Kessels RP, Scherder EJ. Assessing mental flexibility: neuroanatomical and neuropsychological correlates of the Trail Making Test in elderly people. Clin Neuropsychol. 2010;24:203–219. doi: 10.1080/13854040903482848. [DOI] [PubMed] [Google Scholar]
- 133.Audenaert K, Brans B, Van Laere K, Lahorte P, Versijpt J, van Heeringen K, Dierckx R. Verbal fluency as a prefrontal activation probe: a validation study using 99mTc-ECD brain SPET. Eur J Nucl Med. 2000;27:1800–1808. doi: 10.1007/s002590000351. [DOI] [PubMed] [Google Scholar]
- 134.Wood AG, Saling MM, Abbott DF, Jackson GD. A neurocognitive account of frontal lobe involvement in orthographic lexical retrieval: an fMRI study. Neuroimage. 2001;14:162–169. doi: 10.1006/nimg.2001.0778. [DOI] [PubMed] [Google Scholar]
- 135.Strangman GE, O’Neil-Pirozzi TM, Goldstein R, Kelkar K, Katz DI, Burke D, Rauch SL, Savage CR, Glenn MB. Prediction of memory rehabilitation outcomes in traumatic brain injury by using functional magnetic resonance imaging. Arch Phys Med Rehabil. 2008;89:974–981. doi: 10.1016/j.apmr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 136.Kane KD, Yochim BP. Construct validity and extended normative data for older adults for the Brief Visuospatial Memory Test, Revised. Am J Alzheimers Dis Other Demen. 2014;29:601–606. doi: 10.1177/1533317514524812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gawryluk JR, Mazerolle EL, Beyea SD, D’Arcy RC. Functional MRI activation in white matter during the Symbol Digit Modalities Test. Front Hum Neurosci. 2014;8:589. doi: 10.3389/fnhum.2014.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Forn C, Rocca MA, Bosca I, Casanova B, Sanjuan A, Filippi M. Analysis of “task-positive” and “task-negative” functional networks during the performance of the Symbol Digit Modalities Test in patients at presentation with clinically isolated syndrome suggestive of multiple sclerosis. Exp Brain Res. 2013;225:399–407. doi: 10.1007/s00221-012-3380-5. [DOI] [PubMed] [Google Scholar]
- 139.Moritz CH, Johnson SC, McMillan KM, Haughton VM, Meyerand ME. Functional MRI neuroanatomic correlates of the Hooper Visual Organization Test. J Int Neuropsychol Soc. 2004;10:939–947. doi: 10.1017/s1355617704107042. [DOI] [PubMed] [Google Scholar]
- 140.Bohnen NI, Kuwabara H, Constantine GM, Mathis CA, Moore RY. Grooved pegboard test as a biomarker of nigrostriatal denervation in Parkinson’s disease. Neurosci Lett. 2007;424:185–189. doi: 10.1016/j.neulet.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 142.Ropper AH. Cogito ergo sum by MRI. N Engl J Med. 2010;362:648–649. doi: 10.1056/NEJMe0909667. [DOI] [PubMed] [Google Scholar]
- 143.Silbert BS, Scott DA, Evered LA, Lewis MS, Kalpokas M, Maruff P, Myles PS, Jamrozik K. A comparison of the effect of high- and low-dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology. 2006;104:1137–1145. doi: 10.1097/00000542-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 144.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–1141. doi: 10.1097/00000539-200211000-00002. table of contents. [DOI] [PubMed] [Google Scholar]
- 145.Mitchell SJ, Merry AF, Frampton C, Davies E, Grieve D, Mills BP, Webster CS, Milsom FP, Willcox TW, Gorman DF. Cerebral protection by lidocaine during cardiac operations: a follow-up study. Ann Thorac Surg. 2009;87:820–825. doi: 10.1016/j.athoracsur.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 146.Mack WJ, Kellner CP, Sahlein DH, Ducruet AF, Kim GH, Mocco J, Zurica J, Komotar RJ, Haque R, Sciacca R, Quest DO, Solomon RA, Connolly ES, Heyer EJ. Intraoperative magnesium infusion during carotid endarterectomy: a double-blind placebo-controlled trial. J Neurosurg. 2009;110:961–967. doi: 10.3171/2008.9.17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holinski S, Claus B, Alaaraj N, Dohmen PM, Kirilova K, Neumann K, Uebelhack R, Konertz W. Cerebroprotective effect of piracetam in patients undergoing coronary bypass burgery. Med Sci Monit. 2008;14:PI53–PI57. [PubMed] [Google Scholar]
- 148.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, 3rd, Rodriguez AL, Magovern CJ, Zaubler T, Freundlich K, Parr GV. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. discussion −5. [DOI] [PubMed] [Google Scholar]
- 149.McDonagh DL, Mathew JP, White WD, Phillips-Bute B, Laskowitz DT, Podgoreanu MV, Newman MF. Neurologic Outcome Research G. Cognitive function after major noncardiac surgery, apolipoprotein E4 genotype, and biomarkers of brain injury. Anesthesiology. 2010;112:852–859. doi: 10.1097/ALN.0b013e3181d31fd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu T, Bo L, Wang J, Zhao Z, Xu Z, Deng X, Zhu W. Risk factors for early postoperative cognitive dysfunction after non-coronary bypass surgery in Chinese population. J Cardiothorac Surg. 2013;8:204. doi: 10.1186/1749-8090-8-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Peng LY, Xu LW, Ouyang W. Role of Peripheral Inflammatory Markers in Postoperative Cognitive Dysfunction (POCD): A Meta-Analysis. Plos One. 2013:8. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Dijk D, Jansen EW, Hijman R, Nierich AP, Diephuis JC, Moons KG, Lahpor JR, Borst C, Keizer AM, Nathoe HM, Grobbee DE, De Jaegere PP, Kalkman CJ, Octopus Study G. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287:1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 153.Kok WF, van Harten AE, Koene BM, Mariani MA, Koerts J, Tucha O, Absalom AR, Scheeren TW. A pilot study of cerebral tissue oxygenation and postoperative cognitive dysfunction among patients undergoing coronary artery bypass grafting randomised to surgery with or without cardiopulmonary bypass*. Anaesthesia. 2014;69:613–622. doi: 10.1111/anae.12634. [DOI] [PubMed] [Google Scholar]
- 154.Siepe M, Pfeiffer T, Gieringer A, Zemann S, Benk C, Schlensak C, Beyersdorf F. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011;40:200–207. doi: 10.1016/j.ejcts.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 155.Mathew JP, Mackensen GB, Phillips-Bute B, Stafford-Smith M, Podgoreanu MV, Grocott HP, Hill SE, Smith PK, Blumenthal JA, Reves JG, Newman MF. Neurologic Outcome Research Group of the Duke Heart C. Effects of extreme hemodilution during cardiac surgery on cognitive function in the elderly. Anesthesiology. 2007;107:577–584. doi: 10.1097/01.anes.0000281896.07256.71. [DOI] [PubMed] [Google Scholar]
- 156.Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84:1467–1473. doi: 10.1016/j.athoracsur.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 157.Grigore AM, Grocott HP, Mathew JP, Phillips-Bute B, Stanley TO, Butler A, Landolfo KP, Reves JG, Blumenthal JA, Newman MF. Neurologic Outcome Research Group of the Duke Heart C. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94:4–10. doi: 10.1097/00000539-200201000-00002. table of contents. [DOI] [PubMed] [Google Scholar]
- 158.Djaiani G, Fedorko L, Borger MA, Green R, Carroll J, Marcon M, Karski J. Continuous-flow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–1895. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- 159.Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ, Cardiotomy I. The cardiotomy trial: a randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116:I89–I97. doi: 10.1161/CIRCULATIONAHA.106.678987. [DOI] [PubMed] [Google Scholar]
- 160.Murkin JM, Martzke JS, Buchan AM, Bentley C, Wong CJ. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery II. Neurologic and cognitive outcomes. J Thorac Cardiovasc Surg. 1995;110:349–362. doi: 10.1016/S0022-5223(95)70230-X. [DOI] [PubMed] [Google Scholar]
- 161.Boodhwani M, Rubens FD, Wozny D, Rodriguez R, Alsefaou A, Hendry PJ, Nathan HJ. Predictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgery. Circulation. 2006;114:I461–I466. doi: 10.1161/CIRCULATIONAHA.105.001354. [DOI] [PubMed] [Google Scholar]
- 162.Grigore AM, Mathew J, Grocott HP, Reves JG, Blumenthal JA, White WD, Smith PK, Jones RH, Kirchner JL, Mark DB, Newman MF. Neurological Outcome Research G, Endeavors CIotDHCCAR. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95:1110–1119. doi: 10.1097/00000542-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 163.Nathan HJ, Wells GA, Munson JL, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a randomized trial. Circulation. 2001;104:I85–I91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 164.Nathan HJ, Rodriguez R, Wozny D, Dupuis JY, Rubens FD, Bryson GL, Wells G. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: five-year follow-up of a randomized trial. J Thorac Cardiovasc Surg. 2007;133:1206–1211. doi: 10.1016/j.jtcvs.2006.09.112. [DOI] [PubMed] [Google Scholar]
- 165.Chen MH, Liao Y, Rong PF, Hu R, Lin GX, Ouyang W. Hippocampal volume reduction in elderly patients at risk for postoperative cognitive dysfunction. J Anesth. 2013;27:487–492. doi: 10.1007/s00540-012-1548-6. [DOI] [PubMed] [Google Scholar]
- 166.Messerotti Benvenuti S, Zanatta P, Valfre C, Polesel E, Palomba D. Preliminary evidence for reduced preoperative cerebral blood flow velocity as a risk factor for cognitive decline three months after cardiac surgery: an extension study. Perfusion. 2012;27:486–492. doi: 10.1177/0267659112453475. [DOI] [PubMed] [Google Scholar]
- 167.Steinmetz J, Siersma V, Kessing LV, Rasmussen LS, Group I. Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. Br J Anaesth. 2013;110(Suppl 1):92–97. doi: 10.1093/bja/aes466. [DOI] [PubMed] [Google Scholar]