Synopsis
Recently, a new research agenda emphasizing interactions between social factors and health has emerged. The term social determinant of health often refers to any nonmedical factor directly influencing health, including: values, attitudes, knowledge, and behaviors. Health across the life span is strongly and adversely affected by social disadvantage. Research in epigenetics indicates that alterations in DNA methylation may provide a causal link between social adversity and health disparity. Likewise, accelerated loss of telomeres (the protective ends of chromosomes) is highly correlated with chronic stress including social stress and aging, and may provide a link between adversity and some of the physiological stigmata associated with health disparities. Considerable research is still required to develop a sound mechanistic understanding of the role of epigenetics and perturbed telomere function in linking social adversity with health outcome.
Keywords: Epigenetics, methylation, health disparity, social disparity, telomere, DNA
Social Determinants of Child Health
The health consequences of material deficiency (e.g. extreme malnutrition or lack of water, inadequate clothing and shelter, etc) have been long known 1. However, recently, a new, more broadly applicable, research agenda emphasizing social factors and health has emerged 2. The term social determinant of health often refers to any nonmedical factor directly influencing health, including: values, attitudes, knowledge, and behaviors. However, it can also refer to more external sources of influence such as family, neighborhood and social network context. A large and convincing literature over the last several decades shows that health across the life span is strongly linked to social disadvantage 1-4.
For example, neighborhoods can influence health through their physical and geographic characteristics, such as air and water quality, lead paint exposure, proximity to both health promoting and suppressing features (i.e. hospitals and nutritious food stores vs. toxic factories and fast food), access to green space, and so on 2,5,6. Additionally, more social aspects of neighborhoods such as strong social cohesion show far better health and safety 7,8.
Recent evidence demonstrates that the chronic stress of social disadvantage, socioeconomic inequality, and racial discrimination act through a variety of biological pathways to influence health, including: neuroendocrine, developmental, immunologic, and vascular mechanisms2,9. In response to stressful events cortisol, cytokines, and other intermediates are released, and if there is long-term, repetitive or chronic exposure, these substances may damage key physiologic systems9,10. It is thought this mechanism of physiological strain results in more rapid onset or progression of chronic illnesses 11.
One of the largest and most consistently replicated literatures demonstrates the negative effects of social disadvantage in childhood on later child and adult health, socio-emotional wellbeing, and cognitive ability12-16. This literature shows that childhood social disadvantage works through a variety of complex mechanisms to result in dramatically different developmental outcomes, which are often apparent even in childhood, but which are typically more fully manifest in adulthood. Indeed, there is evidence that early childhood disadvantage appears to leave a “biological residue” which in turn has effects on development, health and wellbeing 16,17.
Social Determinants of Child Mental Health
There is strong evidence that the mental health of children, adolescents, and young adults is affected by social factors at personal, family, community, and national levels 11,18. In particular, the evidence is good that paired with safe and supportive social environment, such as family and schools, children need positive peer networks in order to have healthy mental health development. Even national level social determinants of health such as national wealth, income inequality, and access to education were associated with a range of mental health outcomes in young people 18.
Social Determinants of Asthma
Lung function, allergy, and asthma appear to have a strong links to early life stress and social disadvantage 19. Due to the large health inequalities in this area social stressors have been used extensively to explain racial disparities in childhood asthma 20. Indeed recent research suggests that the social context children are raised in may be equal to the natural environmental effects in asthma disease risk 21.
Biological Underpinnings of Social Determinants
Early life experience “gets under the skin” in ways that affect the health, wellbeing and child development. Although the most extensive research shows strong biological effects of physical and emotional abuse (and other similarly extreme childhood events) on health and developmental consequences, more recent research shows that less obvious but more regular adversities of early childhood also have a lasting influence on later health and development 22,23. Recent work has begun to focus on epigenetics as a key biological mechanism linking early life experience and health.
Description of Epigenetics
Despite having the same DNA, different cell types have distinct gene expression (mRNA) patterns in order to perform different functions 24. One mechanism of this differential gene expression is through epigenetic changes, which some have argued may also explain some of the variation in behavioral phenotypes of humans25. One key aspect of the epigenome is that, unlike the DNA sequence, it may be modified by environmental and pharmaceutical interventions. This provides the potential for reversing the effect of adverse life events on later health and wellbeing 17. Epigenetic changes or marks refer to alterations in DNA or histone structure that do not affect the sequence of DNA but may affect gene expression and therefore cellular function. The effect on cellular function may be sustained, and under many circumstances, it can be transmitted to subsequent generations of cells.
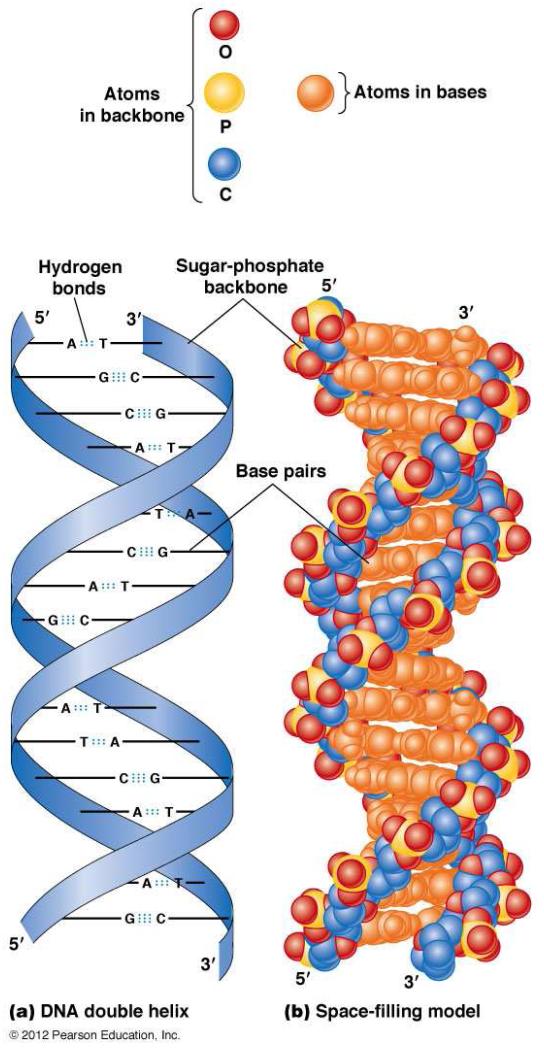
Recall that DNA is organized as a linear molecule, in which the four nucleotides (adenine, A; thymine, T; guanine, G; cytosine, C) form the core of the DNA molecule, and sugar-phosphates the backbone of the DNA (Figure 1). In humans, nuclear DNA is organized into 46 chromosomes: twenty-two autosomes and 1 sex chromosome from each parent. The flow of information in a cell has been termed the Central Dogma (Figure 2), in which information flows from DNA to messenger RNA (mRNA), to protein. Genes are arrayed along the chromosomes, and a gene can be viewed as consisting of the arrangement of base sequences that specifies a complementary mRNA, and, therefore, a specific protein, together with those nearby DNA sequences that determine when and to what extent the gene is transcribed into RNA.
Figure 1.
(a) A schematic representation of the double helical structure of DNA. A = adenosine; T = thymidine; C = cytosine; G = guanine. The strips represent the helical structure formed by the phosphodiester bonds (the “double helix,”) and the horizontal bars represent paired bases. (b) A space-filling model of the DNA double helix. The color-coded atoms are shown at the top of the figure.
From Hardin J, Bertoni, G, Kleinsmith, LJ. Becker’s World of the Cell – 8th ed. San Francisco: Benjamin Cummings; 2006; with permission.
Figure 2.
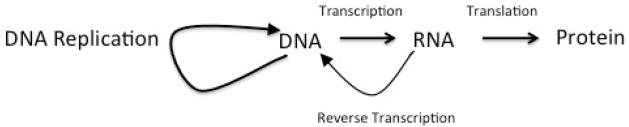
The Central Dogma of Molecular Biology, modified to include reverse transcription.
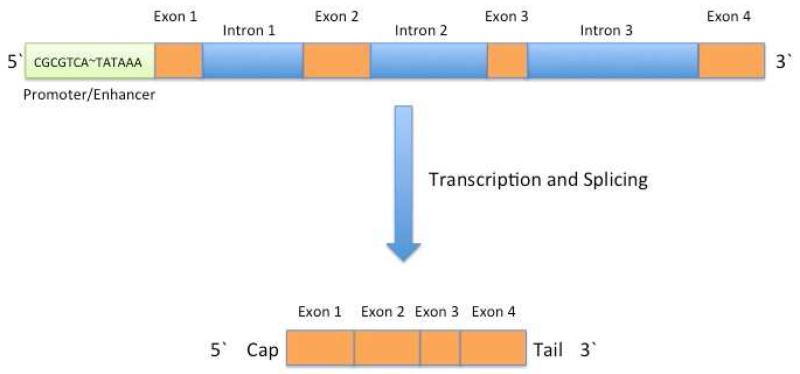
Figure 3 provides a schematic of a typical gene as it appears in DNA. Bases that code specific amino acids are organized in blocks termed, exons. Between the exons are the introns, which are composed of bases that do not specify specific amino acids but may contain control regions. Due to the orientation of the DNA strands, one side of the gene is termed the 5′ end, and the other, the 3′ end. At the 5′ end of the gene is a sequence of bases termed the promoter/enhancer, which is enriched for cytosine and guanine bases. Binding of the promotor by a series of transcription factors activates transcription, the process by which RNA polymerase syntheses a complementary strand of mRNA. Soon after the new primary RNA copy of the gene is synthesized, the introns are removed and the exons are stitched together. After several more steps, the mRNA is used by the ribosome as a template for synthesis of a polypeptide chain—the basic structure of all proteins.
Figure 3.
Schematic of a typical human gene. The 5′ end of the gene contains a promoter/enhancer region that is enriched for CpG sequence. The promoter also contains a special sequence, TATTAAA, which is a target for the transcription factors to bind. Several other sequences may intervene between the CpG island and the TATTAAA. Introns are shown in blue, and exons in orange. During transcription and splicing, an RNA copy of the gene is made, and the introns are excised. A 5′ cap and a 3′ tails are added to the final mRNA copy of the gene.
This arrangement provides for several points at which gene regulation can adjust the synthesis of proteins to meet the needs of the cell. Transcriptional control is a key form of regulation, through which the amount of mRNA synthesized from a particular gene is increased or decreased as necessary.
Upon receipt of an appropriate signal, the cell can deploy or withdraw specific transcription factors within minutes, thereby rapidly modulating the transcription of specific genes. This type of signaling response is rapid, and easily reversible. On the other hand, epigenetic changes to DNA generally take days to years to occur, and as mentioned, they tend to be stable.
DNA Methylation
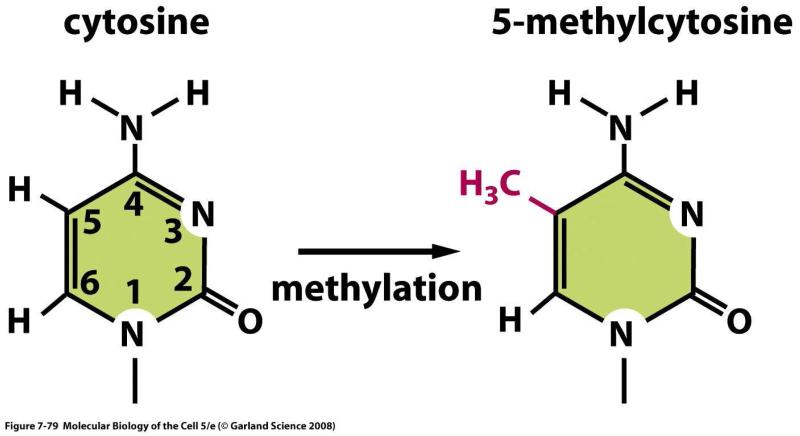
The promotor regions of genes are enriched for sequences containing cytosine alternating with guanine (5′-CG-3′ abbreviated CpG). Areas in which the proportion of CpG’s is greater than statistically predicted are termed CpG islands. Wherever a CpG occurs, the C is susceptible to being modified by the enzyme DNA methyltranferase through the addition of a methyl (CH3) group (forming 5-methylcytosine) (Figure 4). A promoter containing a group of CpG sequences that have been methylated is less able to bind relevant transcription factors, and this attenuates or halts transcription. Since the addition of a methyl group to cytosine is a covalent reaction, it may be an enduring change; furthermore, the DNA replication apparatus has mechanisms for ensuring that the corresponding CpG is methylated in newly synthesized DNA
Figure 4.
5-methylcytosine is formed by the addition of a methyl (--CH3) group to cytosine. From Alberts, Bruce, Johnson A, Lewis J, et al. Molecular Biology of the Cell, 5th ed New York: Garland Science, 2008; with permission.
Clusters of CpG residues are not only found in promoters, but also interspersed within genes, and along intergenic regions. The role played in cellular physiology by methylation of these other CpG sites is the subject of considerable research, and they may be more important in controlling transcription than the CpG islands.
The methylation of DNA is just one way in which a cell can create an epigenetic mark. DNA is tightly coiled around highly basic proteins called histones. One effect of this winding is to greatly compress the DNA, allowing it to be packaged into a cell nucleus. Fully extended, the DNA of a chromosome would extend about 75 mm but in its coiled state it is about 5 μm (compression of about 15,000 fold!). Often, when DNA is tightly wound on a nucleosome, the DNA regulatory sites (such as the promoter) become inaccessible to transcription signals, and the affected genes become silent. Histone proteins have several sites at which they can be covalently modified, principally by methylation or acetylation. The effect of these covalent changes may be to slightly relax the DNA, thereby freeing regulatory sites for interactions with various transcriptional activator proteins. These histone changes are also termed epigenetic marks. As is the case with DNA methylation, the cell is able to duplicate the histone marks on newly synthesized histones that are destined for daughter cells. Thus, histone-based epigenetic marks are heritable even though they are not coded in the DNA.
It is important to note that while epigenetic marks are heritable from parent cell to daughter cell, this is often misunderstood to mean that in multicellular organisms, such as humans, epigenetic marks are transferred directly from parent to child. Rather, during the process of gamete formation most epigenetic marks are cleared, and each generation develops a new set of epigenetic marks. However, as indicated below, under appropriate circumstances environmental signals (including those supplied through maternal behavior) may result in patterns of epigenetic marks in the offspring that reflect those also found in the parent.
At the biochemical level, epigenetics affects transcription and ultimately the protein repertoire of a cell. The epigenetic mechanism serves four essential cellular roles: 1) X-chromosome inactivation; 2) differentiation; 3) imprinting; 4) medium and long-term transcriptional control. This review focuses on how social and environmental signals shape DNA methylation and thereby transcription. Aberrations in DNA methylation are frequently associated with cancer, but this phenomenon is not within the scope of this review.
Measurement of DNA Methylation
Although it is likely that marks based in both DNA and histones are important epigenetic signals of adversity and stress, for technical reasons, most of the social science research to date has involved detecting changes only in DNA methylation. Determining whether a specific CpG site is methylated is relatively simple and several approaches are in routine laboratory use. More recently, chip technology has been applied to determine the methylation state of 500,000 CpG sites per DNA sample. Although the technology is straightforward, early experience suggests that there are significant challenges to analyzing the data, ranging from batch effects (artifacts induced by day to day variation in lab procedures) to the statistical challenges implicit in very large numbers of repeated measures in a limited number of samples. Furthermore, while it is relatively straightforward to identify which CpG sites are hyper- or hypo- methylated under a certain condition, it is much more difficult to associate this observation with a specific functional significance. This is because the biological effect of a change in methylation status at a particular CpG or cluster of CpGs is often unknown or unpredictable. While work with both rodents and humans has demonstrated the value of methylation changes in explaining how environmental inputs are translated to durable behavioral effects, this work has, so far, depended upon measurement of the methylation state of specific sites with known or clearly predictable functions. How methylation profiles across hundreds of thousands of sites should be correlated with underlying social inputs and health or behavior states remains an important topic for research. Furthermore, since the methylation state of differentiated tissues is highly specific, it is not clear how methylation profiles developed in circulating blood cells or saliva cells will provide information about changes in DNA from less accessible tissue such as brain, cells of the autonomic nervous system, or specific immune cells. All of these questions require extensive additional research.
Description of Telomeres
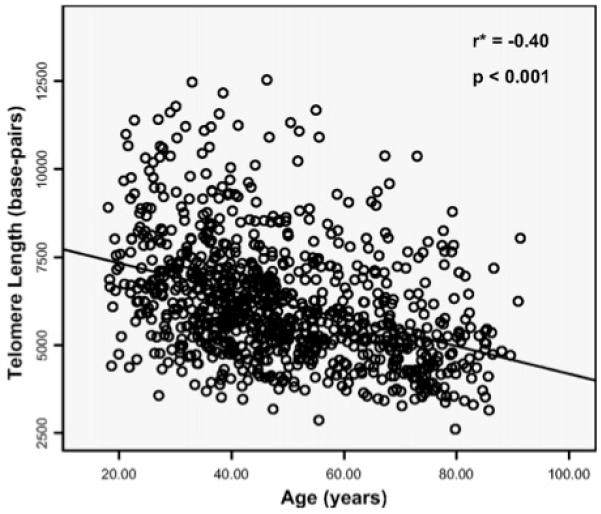
During DNA replication, the fact that DNA is replicated from the 5′ to 3′ direction means that the end of one strand of the chromosome shortens with each cycle of chromosomal replication and cellular division. To prevent loss of genetically important information, chromosomes are capped by repetitive DNA sequences (TTAGGG)n and associated proteins, termed telomeres26. In addition, the presence of the telomere prevents fusion of adjoining chromosomal ends. Over time, with each cell division, the telomere ends become shorter, and so the telomere has been referred to as a “mitotic clock.” Associated with progressive telomere shortening, the cell activates pathways that prevent further cell division (replicative senescence). Stem cells maintain telomere length by activating an enzyme, “telomerase.” Telomerase consists of both a protein catalytic unit (TERT) and a RNA template unit (TERC), used to specify the sequence of the newly synthesized telomeric bases. Most specialized cells do not express, or express very low levels of telomerase, so their telomeres progressively shorten with the age of the organism (Figure 5).
Figure 5.
Correlation between leukocyte telomere length and age. From Njajou OT, Cawthon RM, Damcott CM, Wu SH, et al. Telomere length is paternally inherited and is associated with parental lifespan PNAS 2007;104:12135-12139; with permission.
Mutations in telomere-associated proteins produce a number of serious hereditary diseases in humans. Dyskeratosis congenita (dystrophic nails, oral leukoplakia and skin depigmentation) in infancy is followed by bone marrow failure, occasionally, pulmonary fibrosis, cirrhosis, and increased susceptibility for cancer. First attributed to a mutation in a gene on the X-chromosome, dyskerin (DKC1), a number of other telomere-associated mutations have subsequently been associated with the disorder and related conditions. Patients with dyskeratosis congenita have abnormally short telomeres through life27.
Accelerated telomere shortening is also associated with a number of acquired disease processes, including cancer associated with chronic inflammation, such as esophageal cancer with Barrett’s esophagitis, colon cancer with ulcerative colitis, and lung cancer. Several population based and clinical studies have also correlated telomere length shortening with coronary artery disease. Since cancer and coronary disease are the major causes of death in older individuals, and telomere shortening is a consequence of normal aging (Figure 5), some have postulated that aging (or the diseases of aging) is related to telomere shortening. In this model, telomere shortening beyond a critical limit results in senesce of various cell populations, impeding for example, repair, and immune surveillance. Although it is clear that, on average, telomere length decreases as humans age, this association does not establish a cause and effect relationship, and many questions regarding the role of telomere shortening on the age-associated changes in cellularity and reparative ability remain to be elucidated.
As described subsequently, an emerging literature also links accelerated telomere shortening with stress, including both environmental stress such as malnutrition or violence, and social stress, such as perceived racism, depression, and absence of a father. Despite a large literature that replicates this basic observation, it is not clear whether telomere attrition contributes mechanistically to the health effects associated with chronic stress, or whether it is merely a biomarker that reflects these effects.
The mechanism by which stress modulates telomere shortening is not well understood. Some research suggests that the physiological correlates of stress (i.e., activation of the HPA axis, inflammation) impose an increased oxidative burden on the cell, which damages the telomere, resulting in accelerated telomere attrition28. Other studies point to depressed telomerase function, also associated with stress, as contributing to reduced average telomere length 29. The evidence for either mechanism is not strong. Understanding how stress affects telomere length is an urgent research priority, because without better knowledge of the biological mechanisms that link stress and other moderators of wellness and health to telomere length, it is not possible to articulate a convincing model to explain either the causes or the consequences of the observed disparities in telomere length.
Measurement of Telomere Length (TL)
Several approaches are used to determine the average telomere length in a sample of DNA. The classic approach is the terminal restriction fragment (TRF) length Southern blot assay. In many laboratories a rapid quantitative real time PCR (qRT-PCR) reaction is used to compare the amount of telomere DNA (TTAGGG) in a sample with the amount of another, control gene. Often results are expressed as a ratio of telomere to control DNA (T/S ratio)30, but more recently investigators have used internal control oligomers to report the length of the telomere in base pairs31. Using this method, our laboratory has observed that the average telomere length in a sample of adult females (average age 34.2 years) is 6.12 kb, and in a sample of 2,818 children (average age 9.28 years), it is 9.66 kb. As between boys and girls the telomere length was 9.70 and 9.88 kb, respectively (p = ns)32. Several other approaches are occasionally employed, and all produce roughly similar results. Recent reports have used DNA derived from saliva to measurement telomere length. Although saliva TL is significantly longer than TL derived from peripheral blood mononuclear cells (the usual source) across an individual, the two sample types produce highly correlated measurements 16.
Social determinants of epigenetic marks
Research on epigenetic regulation of gene activity related to behavior and the influence of the social environment on epigenetic regulation began about a decade ago25. Despite this relative novelty, there is now evidence that life experiences (and especially early life experiences) can directly influence genetic function by altering the epigenetic patterns in specific loci on the genome 33. It is important to note that the vast majority of work looking at environmental influences on DNA methylation or DNA methylation effects on health has been done on animals (especially mice, rats, and voles). Animals studies have shown that the strong effect of mother’s nurturing on rat pups’ ability to handle stress and form attachments is associated in part to increases in CpG methylation of the promoters regions of the glucocorticoid and estrogen receptor genes and the BDNF gene34. Another line of research shows that animals that have been stressed either through social isolation, nutritional deprivation, or contextual uncertainty also exhibit changes in methylation —typically decreasing methylation at the promoter regions of CNS genes 35.
In addition to parenting quality, other environmental or life experiences are related to methylation. For example, using buccal cells one study recently found that adversity (such as physical abuse) in infancy and preschool was related to methylation pattern differences in adolescents 22. Adverse early life experiences have also been tied to differences in epigenetic patterns for genes related to mental health, 36 drug addiction 37 and obesity 38.
Social determinants of telomere length
Telomere length appears to be a biomarker of social stress. Telomere shortening has been associated with depression, harsh parenting, paternal absence, and perceived racism. Stress related telomere shortening could evoke physiological weathering in a way similar to aging 39. Research suggests several possible behavioral mediators of the negative association between stress and TL, including smoking, mental illness (particularly depression), caregiver stress, and obesity40. Considering the strong association between social deprivation and these mediators, it is not surprising that some measures of social standing and social deprivation have also been found to be associated with TL 16,41.
The TL literature has focused almost exclusively on adults, although several studies have used retrospective reports to measure childhood conditions, and one prospective study has examined the association between childhood conditions between ages 5 and 10 and TL, and a second study determined that the duration of exposure to institutional care between 22 and 54 months was negatively associated with telomere length 26,40. Recently, we reported that the association between children’s social environment and TL is moderated by specific variants or alleles in dopaminergic or serotonergic pathways. Involved genes associated with serotonergic transmission are HTT and TPH2 and with dopaminergic transmission are COMT, DAT1, DRD2 and DRD4.
Epigenetic Association with Child Mental Health
A growing literature suggests that DNA methylation plays an important role in mental health disorders as well 42. For example, exposure to third trimester depressed maternal mood is associated with methylation status of a CpG-island of NR3C1 in newborns and altered hypothalamic-pituitary-adrenal stress reactivity at age three months43. The association is indicative of a potential epigenetic mechanism linking maternal depression and newborn physiology. A second example suggests that depression is associated with higher methylation levels in the 5-hydroxytryptamine transporter (5-HTT, or SLC6A4) gene44. This work also ties back into the larger work on 5-HTTLPR and depression45 by showing both genetic (the S allele of 5-HTTLPR) and epigenetic factors (methylation of the 5-HTTLPR promoter) may interact with environmental states to moderate risk of depression. However, this research area topic is still nascent and therefore the mechanisms that lead to changes in methylation or how that methylation modifies biology to influence mental health are still unknown.
Epigenetic Association with Asthma
Like the rest of the epigenetic literature, the work on asthma and allergy exploits both candidate gene and genome-wide approaches46. The candidate gene approach was the first method and still most common, but epigenome-wide data are becoming more available. For example, the 17q12-21 locus is one of the most widely replicated genetic loci for asthma. Interestingly, the effect of polymorphisms at this locus seems to be suppressed in females by higher levels of methylation of this locus47, which would tend to reduce expression of the risk allele. Another interaction between a genetic variant, this time in Il-4R, and the extent of methylation of a related CpG site has recently been described. The Il-4R gene variant (rs rs3024685) is not independently associated with risk of asthma, but the combination of this variant with a high level of methylation increased asthma risk by approximately 47-fold48. The biochemical mechanism underlying this effect remains to be better defined, but interactions between genetic variants (‘the genome’) and the extent of methylation (‘the epigenome’) may underlie many examples in which conventional rules of genetics fail to account for observed phenotypes. These studies show how polymorphisms and epigenetic regulations are interrelated and how future studies should be structured to examine these interactions. Not surprisingly the majority of the environmental exposures in this literature implicate the natural environment rather than social 46. However, in many cases, the social and natural environments are highly correlated—and thus more research is needed to evaluate possible indirect and potentially spurious associations.
Current Recommendations
Substantial evidence indicates that pathways initiated by childhood social adversity can be interrupted. Studies show that high-quality early development interventions—including center-based programs to nurture and stimulate children and to support and educate parents—greatly ameliorate the effects of social disadvantage on children’s cognitive, emotional/behavioral, and physical development; the first five years of life appear to be most crucial, although opportunities for intervention continue throughout childhood and adolescence 49-51. However, the extent to which these improvements are based on or related to epigenetic changes has not yet been evaluated, although there is great interest in pursuing these mechanisms. Therefore, while the early epigenetic literature cannot support specific recommendations, we expect that in the next few years the mechanistic link between early social adversity, early childhood development, and interventions to enhance development will begin to come into focus. Ideally, this research will be longitudinal, collaborative and may involve very large data sets and new statistical methods based on bioinformatics.
Finally, although not explored in this paper, a major limitation of this area is the lack of integrated research in social and natural environmental effects on epigenetics. Social and natural environment are highly correlated and yet are rarely discussed together. To what extent one might explain the effects of the other environment type on methylation patterns has not fully been explored. This might be a particularly useful way to expand available data and research.
Key Points.
Epigenetic factors, especially DNA methylation, and telomere length are currently being examined as biological mechanisms linking social factors and health.
Social deprivation is associated with a wide range of epigenetic change in children and young adults.
Epigenetic markers are associated with obesity and eating disorders, mental health, and asthma.
Research is still too new to provide actionable evidence for a causal mechanism linking social experiences and child health through epigenetics and telomere length.
Research exploring the overlap between social and natural environmental links to epigenetics and health is desperately needed.
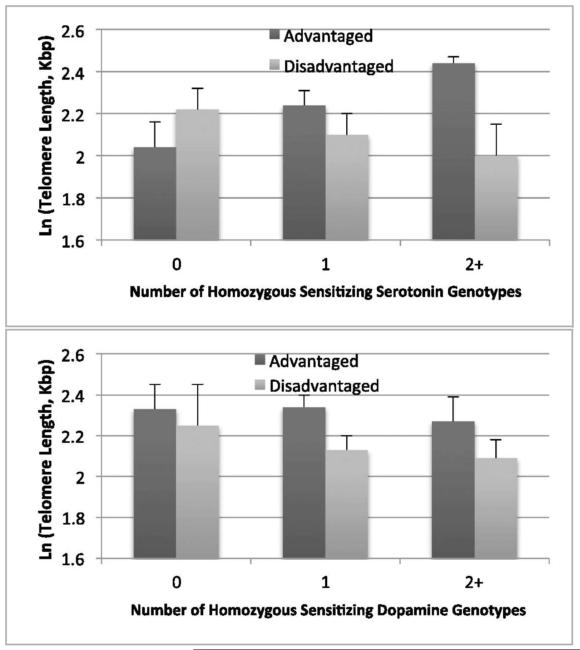
Figure 6.
ln(telomere length) by environment type (advantaged vs. disadvantaged) and serotonin pathway (Upper) and dopamine pathway (Lower) homozygous genotype counts. For the serotonin pathway genotypes, the environment effect is borderline for 0 genotypes (P = 0.09), not significant for 1 genotype (P = 0.32), and significant for 2+ genotypes (P = 0.02). For the dopamine pathway genotypes, the environment difference is not significant for 0 genotypes (P = 0.63), significant for 1 genotype (P = 0.05), and borderline for 2+ genotypes (P = 0.08). This indicates that specific alleles in neurotransmitter pathways moderate the effect of social stress on telomere length.
From Mitchell C, Hobcraft J, McLanahan, SS, et al. Social disadvantage, genetic sensitivity, and children’s telomere length PNAS 2014; 16: 5944-5949; with permission.
Acknowledgement
Funding for some of the research reported in this paper was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD076592). In addition, this work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Grants R01HD36916, R01HD39135, and R01HD40421 and by a consortium of private foundations of the Fragile Families and Child Wellbeing Study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Daniel A Notterman, Department of Molecular Biology, Princeton University, Princeton, New Jersey 08544.
Colter Mitchell, University of Michigan-Ann Arbor, Institute of Social Research, Ann Arbor, Michigan 48109.
References
- 1.Rosen G. The History of Public Health. Johns Hopkins Univ. Press; Baltimore, MD: 1993. [Google Scholar]
- 2.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annual Review of Public Health. 2011;32:381–398. doi: 10.1146/annurev-publhealth-031210-101218. [DOI] [PubMed] [Google Scholar]
- 3.Adler NE, Ostrove JM. Socioeconomic status and health: what we know and what we don’t. Annals of the New York Academy of Sciences. 1999;896(1):3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences. 2010;1186(1):69–101. doi: 10.1111/j.1749-6632.2009.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Reports. 2001;116(5):404. doi: 10.1093/phr/116.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallis JF, Glanz K. The role of built environments in physical activity, eating, and obesity in childhood. The Future of Children. 2006;16(1):89–108. doi: 10.1353/foc.2006.0009. [DOI] [PubMed] [Google Scholar]
- 7.Morenoff JD, Sampson RJ, Raudenbush SW. Neighborhood inequality, collective efficacy, and the spatial dynamics of urban violence. Criminology. 2001;39(3):517–558. [Google Scholar]
- 8.Ross CE. Neighborhood disadvantage and adult depression. Journal of Health and Social Behavior. 2000;41(2):177–187. [Google Scholar]
- 9.Wolfe B, Evans W. In: The Biological Consequences of Socioeconomic Inequalities. Seeman TE, editor. Russell Sage Foundation; 2012. [Google Scholar]
- 10.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 11.Shonkoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012 Jan;129(1):e232–246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 12.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010 Feb;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 14.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children’s intellectual development. Demography. 2000 Nov;37(4):431–447. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 15.Medicine Io . From Neurons to Neighborhoods: The Science of Early Childhood Development. Natl. Acad. Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 16.Mitchell C, Hobcraft J, McLanahan SS, et al. Social disadvantage, genetic sensitivity, and children’s telomere length. Proceedings of the National Academy of Sciences of the United States of America. 2014 Apr 22;111(16):5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009 Aug 25;106(34):14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viner RM, Ozer EM, Denny S, et al. Adolescence and the social determinants of health. Lancet. 2012 Apr 28;379(9826):1641–1652. doi: 10.1016/S0140-6736(12)60149-4. [DOI] [PubMed] [Google Scholar]
- 19.Wright RJ. Epidemiology of stress and asthma: from constricting communities and fragile families to epigenetics. Immunology and allergy clinics of North America. 2011 Feb;31(1):19–39. doi: 10.1016/j.iac.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gee GC, Payne-Sturges DC. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environmental health perspectives. 2004 Dec;112(17):1645–1653. doi: 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright RJ. Moving towards making social toxins mainstream in children’s environmental health. Current opinion in pediatrics. 2009 Apr;21(2):222–229. doi: 10.1097/MOP.0b013e3283292629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essex MJ, Boyce WT, Hertzman C, et al. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child development. 2013 Jan-Feb;84(1):58–75. doi: 10.1111/j.1467-8624.2011.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of cognitive neuroscience. 2009 Jun;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 24.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. The EMBO journal. 1998 Sep 1;17(17):4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szyf M. Early life, the epigenome and human health. Acta Paediatr. 2009 Jul;98(7):1082–1084. doi: 10.1111/j.1651-2227.2009.01382.x. [DOI] [PubMed] [Google Scholar]
- 26.Shalev I, Entringer S, Wadhwa PD, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013 Sep;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001 Sep 27;413(6854):432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 28.von Zglinicki T. Oxidative stress shortens telomeres. Trends in Biochemical Sciences. 2002 Jan 7;27(7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 29.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004 Dec 7;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic acids research. 2002 May 15;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biological procedures online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneper L. 2015 [Google Scholar]
- 33.Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental psychobiology. 2010 May;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 34.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006 Jun;147(6):2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 35.Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child development. 2010 Jan-Feb;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 36.Galea S, Uddin M, Koenen K. The urban environment and mental disorders: Epigenetic links. Epigenetics: official journal of the DNA Methylation Society. 2011 Apr;6(4):400–404. doi: 10.4161/epi.6.4.14944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends in molecular medicine. 2008 Aug;14(8):341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campion J, Milagro F, Martinez JA. Epigenetics and obesity. Progress in molecular biology and translational science. 2010;94:291–347. doi: 10.1016/B978-0-12-375003-7.00011-X. [DOI] [PubMed] [Google Scholar]
- 39.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & disease. 1992 Summer;2(3):207–221. [PubMed] [Google Scholar]
- 40.Shalev I, Moffitt TE, Sugden K, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Molecular psychiatry. 2013 May;18(5):576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll JE, Diez-Roux AV, Adler NE, Seeman TE. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA) Brain, behavior, and immunity. 2013 Feb;28:108–114. doi: 10.1016/j.bbi.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment ‘get into the mind’? Epigenetics at the intersection of social and psychiatric epidemiology. Social science & medicine (1982) 2012 Jan;74(1):67–74. doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics: official journal of the DNA Methylation Society. 2008 Mar-Apr;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 44.Olsson CA, Foley DL, Parkinson-Bates M, et al. Prospects for epigenetic research within cohort studies of psychological disorder: a pilot investigation of a peripheral cell marker of epigenetic risk for depression. Biological psychology. 2010 Feb;83(2):159–165. doi: 10.1016/j.biopsycho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell C, Notterman D, Brooks-Gunn J, et al. Role of mother’s genes and environment in postpartum depression. Proceedings of the National Academy of Sciences of the United States of America. 2011 May 17;108(20):8189–8193. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durham AL, Adcock IM. Epigenetic Aspects of Chronic Diseases. Springer; London: 2011. Epigenetic regulation of asthma and allergic diseases; pp. 147–161. [Google Scholar]
- 47.Naumova AK, Al Tuwaijri A, Morin A, et al. Sex- and age-dependent DNA methylation at the 17q12-q21 locus associated with childhood asthma. Human genetics. 2013 Jul;132(7):811–822. doi: 10.1007/s00439-013-1298-z. [DOI] [PubMed] [Google Scholar]
- 48.Soto-Ramirez N, Arshad SH, Holloway JW, et al. The interaction of genetic variants and DNA methylation of the interleukin-4 receptor gene increase the risk of asthma at age 18 years. Clinical epigenetics. 2013;5(1):1. doi: 10.1186/1868-7083-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell F, Conti G, Heckman JJ, et al. Early childhood investments substantially boost adult health. Science (New York, N.Y.) 2014 Mar 28;343(6178):1478–1485. doi: 10.1126/science.1248429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoagwood K, Burns BJ, Kiser L, Ringeisen H, Schoenwald SK. Evidence-based practice in child and adolescent mental health services. Psychiatric services (Washington, D.C.) 2001 Sep;52(9):1179–1189. doi: 10.1176/appi.ps.52.9.1179. [DOI] [PubMed] [Google Scholar]
- 51.Morrison J, Pikhart H, Ruiz M, Goldblatt P. Systematic review of early childhood interventions in European countries (1990-2013) that aimed to address health and development. European Journal of Public Health. 2014;24(suppl 2) doi: 10.1186/1471-2458-14-1040. 2014-10-01 00:00:00. [DOI] [PMC free article] [PubMed] [Google Scholar]