Abstract
The creatine kinase (CK) system is thought to play an integral role in maintaining levels of chemical energy in the form of adenosine triphosphate (ATP), which is essential for normal cardiac function. In the failing heart, it has long been established that multiple components of CK energy metabolism are commonly impaired and that these correlate with disease severity. A recent study published in Science Translational Medicine adds significantly to this body of evidence by demonstrating that the rate of ATP transfer via CK, measured non-invasively by Magnetic Resonance Spectroscopy, is an independent predictor of adverse clinical outcome in patients with non-ischemic cardiomyopathy. This finding invites speculation on the future role of metabolic imaging for risk stratification in patients with heart failure. The authors further assert an implied causal role for energetics in disease progression. While this is not supported by recent findings in loss-of-function mouse models, there is, nonetheless, a strong argument for the development of novel metabolic therapies for the failing heart.
The only established role for creatine kinase is to catalyse the reversible transfer of a high-energy phosphate moiety between ATP and creatine: Cr + ATP ↔ PCr +ADP + H+. This initial reaction takes place at the mitochondria to create phosphocreatine (PCr), which accumulates to high levels and is readily diffusible to sites of utilization. At times of increased energy demand PCr is available for rapid regeneration of ATP under the control of CK. Thus, the CK system is recognised to have both energy storage and energy transfer roles, while compartmentalisation via specific CK isoforms enables buffering of local reactant concentrations in a thermodynamically favourable way.1
Evidence for an impaired CK system in the human failing heart dates as far back as 1939 with the observation that total creatine levels are reduced by 30%.2 Since then, numerous animal and human studies have firmly established that a reduction in both creatine and CK enzymatic activity are a hallmark of the failing heart, regardless of aetiology.1, 3 During the 1980’s, advances in Phosphorus-31 Magnetic Resonance Spectroscopy (31P-MRS) enabled non-invasive measurement of high-energy phosphate metabolites in the living human heart for the first time.4 See Figure for stylised cardiac 31P-spectra illustrating the typically large PCr peak and three smaller peaks representing the phosphate groups of ATP. Absolute concentrations are difficult to obtain and for this reason it is common to report the PCr/ATP ratio as a measure of relative abundance (value ~1.8 in the normal heart). This ratio is a relatively robust marker of energetic status since it reflects loss of total creatine and the equilibrium constant for the CK reaction favours ATP synthesis by 100-fold, such that ATP levels are maintained near normal in all but the most advanced stages of heart failure.3 A fall in PCr/ATP therefore mostly reflects a fall in PCr levels. Clearly, however, there may be a pseudo-normalization of PCr/ATP under circumstances where ATP becomes depleted. A series of studies in the 1990’s showed that PCr/ATP was significantly reduced in patients with dilated cardiomyopathy and correlated with traditional measures of heart failure severity such as New York Heart Association (NYHA) class and ejection fraction.5, 6 Furthermore, patients that responded to medical treatment also showed an improvement in PCr/ATP,5 and in a prospective study PCr/ATP was shown to be an excellent prognostic indicator of mortality.7
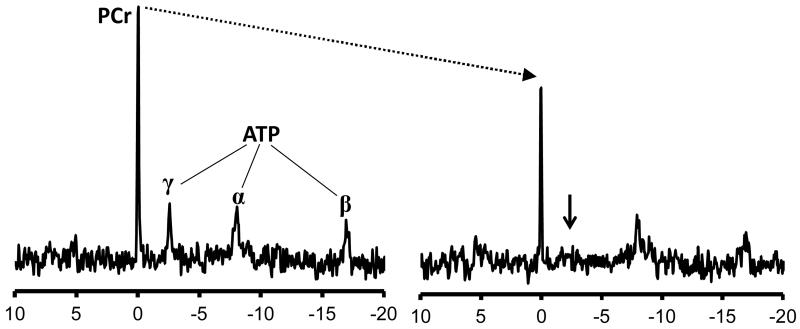
Figure.
31P spectra from a normal ex-vivo rat heart during a saturation transfer experiment (reproduced from 16). Selective irradiation at the frequency corresponding to the γ-ATP peak (small arrow) effectively renders this phosphate group NMR-invisible. Under the action of creatine kinase (CK), this invisible phosphate group is interconverted to phosphocreatine (PCr), but remains NMR-invisible. The observed result is a reduction in the visible PCr peak. The extent of this reduction reflects the rate of phosphotransfer via CK, i.e. CK flux.
The latest report by Bottomley et al.8 is a welcome and logical progression of this approach and builds on earlier studies from the same group to establish 31P-MRS methods to measure CK flux in the human heart.9-11 Acquiring these measurements is a major technical challenge, but the basic concept is simple enough – see Figure and 12 for more details. There are several reasons why flux might be superior to measuring metabolite levels. Firstly, it avoids the problem of PCr/ATP pseudo-normalization described above. Secondly, flux through CK is arguably more sensitive with a wider dynamic range, since it reflects both changes in metabolites and in CK activity. Thirdly, it has been argued that CK is part of a near-equilibrium enzymatic network, where changes in flux may occur in the absence of altered metabolite levels.13 However, it should be noted that there is a certain ambiguity to what exactly CK-flux represents since it is not uni-directional, but rather an average flux for all NMR-visible CK reactions within the volume of interest, and in common with metabolite quantification there is no distinction between different cellular compartments.12
Having previously established that CK flux is impaired in patients with heart failure,9 Bottomley et al. report on a prospective non-randomised study in 58 patients with non-ischemic cardiomyopathy and 17 healthy volunteers.8 All were confirmed free of coronary artery disease and had been clinically stable for at least 2 weeks prior to a single 31P-MRS examination to obtain a 1-D dataset from the anterior myocardium. Follow-up was for a median 4.7 years (up to 8.2 years) with death and/or hospitalization as end-points. The take home finding is that CK flux, but not metabolite levels or PCr/ATP, was an independent predictor of all cause and cardiovascular mortality.
Clearly this is a promising initial finding, which needs to be repeated in a larger and more diverse cohort. For example, it is important to know whether reduced CK flux is selective for heart failure. The authors have previously shown that CK flux is reduced in ischemic myocardium commensurate with the extent of infarct transmurality,10 hence the exclusion of coronary artery disease in this study. What other co-morbidities may affect CK flux? The PCr/ATP ratio has been shown to be reduced in hypertension, diabetes, obesity, valve disease and inherited heart muscle diseases such as hypertrophic cardiomyopathy (reviewed in 14), and, while not studied so far, it is likely that CK flux will also be decreased. Furthermore, are these findings true for all ages and ethnicities? It is a limitation that this study examines a single time-point, when change in flux over time may be an even better prognostic indicator. This would also answer the question of whether CK flux is a marker for therapeutic efficacy, i.e. does CK flux increase when NYHA class improves as previously shown for PCr/ATP ratio.5 Comparison with established prognostic indicators would be advantageous, for example, does CK flux correlate with EF% or NYHA class in these patients? How does it compare with plasma BNP levels, which are, after all, much quicker and simpler to measure as a supplementary prognostic indicator.
The current technology has methodological limitations relying as it does on a single 1-D slice from the anterior myocardium. This risks signal contamination from the intercostal muscles and necessarily assumes that energetic changes are homogeneous throughout the myocardium. As discussed, this is not true for ischemic heart disease, and patients with anterior MI would not be suitable for assessing heart failure prognosis using this approach. However, it is surely only a matter of time before 2D- and 3D-CSI approaches become available for CK flux measurement and circumvent this problem. As with PCr/ATP ratio in the 1990’s there remain major practical barriers to adoption of CK flux as a routine prognostic marker, not least is access to an MR system with specialised phosphorus coils and technical expertise. This makes it expensive and time consuming, with the average scan in this study taking 70 minutes, which may be too long for many patients to endure. Again, we can confidently expect these barriers to diminish as the technology matures. For example, we have recently shown that, compared to 3T as used by Bottomley et al, the ultra-high field strength of 7 Tesla improves the signal-to-noise (SNR) of 31P-MRS by a factor of 2.8, thus theoretically shortening examination time by a factor of >7 for the same SNR.15 To our knowledge, the Bottomley group is currently the only one worldwide that has established cardiac CK flux measurements. It remains to be seen whether such measurements are reproducible between laboratories, i.e. can we compare results between different centres. Given the inherent measurement errors and biological noise, a key question is whether variability is sufficiently low to detect small but physiologically significant differences. For example, if, as has been suggested, reduced CK flux occurs early in disease pathogenesis, then can it be used to predict patients who are about to develop heart failure? This kind of risk stratification is more likely to have a real impact on clinical practice than predicting poor prognosis in patients that are already diagnosed and receiving optimal therapy. A much larger prospective study in a general ageing population could address this question. In the meantime, it seems likely there will be a niche role for CK flux measurements in the assessment of new therapies specifically targeting cardiac energetics and metabolism.
So what do these findings tell us about the pathophysiology of heart failure? The Bottomley study adds to a growing number of clinical studies over several decades that provide correlative evidence that energetic changes are closely intricated with the development of heart failure. This clearly implies, but does not prove causality, and the results from mouse models have been much more equivocal. Our laboratory has recently shown that mice completely deficient in creatine and PCr do not develop more severe heart failure and have normal survival following chronic myocardial infarction.16 We have previously demonstrated similar findings in rats with pharmacological depletion of creatine and in mice with genetic deletion of CK,17, 18 showing that CK deficiency is unlikely, in itself, to contribute to worsening heart failure. We would therefore argue that the issue of causality has yet to be settled, but perhaps this is a side-show that can await clarification at a later date. The more important issue is not whether CK deficiency is an underlying cause of heart failure, but more pertinently, whether augmenting the CK system (i.e. supra-physiological stimulation) has therapeutic promise. An important proof-of-principle study, also from the Johns Hopkins group, recently demonstrated that the answer to this question is yes. Transgenic mice over-expressing the muscle isoform of CK in the heart maintained CK flux at higher levels in a heart failure model of pressure-overload, and this was associated with higher ejection fraction and improved survival.19 This opens a whole new and exciting avenue for future study and the ability to make non-invasive measurements of CK flux is likely to play a pivotal role. Clearly this technology is still in its infancy but we can expect to hear a lot more from metabolic flux imaging in the years to come.
Acknowledgments
Sources of Funding
Work in our laboratory is funded by a grant from the British Heart Foundation (RG/13/8/30266).
References
- 1.Neubauer S. The failing heart--an engine out of fuel. New Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann G, Decherd GM. The chemical nature of heart failure. Ann Intern Med. 1939;12:1233–1244. [Google Scholar]
- 3.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 4.Bottomley PA. Noninvasive study of high-energy phosphate metabolism in human heart by depth-resolved 31P NMR spectroscopy. Science. 1985;229:769–772. doi: 10.1126/science.4023711. [DOI] [PubMed] [Google Scholar]
- 5.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer S, Horn M, Pabst T, Gödde M, Lübke D, Jilling B, Hahn D, Ertl G. Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J. 1995;16:115–118. doi: 10.1093/eurheartj/16.suppl_o.115. [DOI] [PubMed] [Google Scholar]
- 7.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 8.Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic Rates of ATP Transfer Through Creatine Kinase (CK Flux) Predict Clinical Heart Failure Events and Death. Science Translational Medicine. 2013;5:215re213. doi: 10.1126/scitranslmed.3007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottomley PA, Wu KC, Gerstenblith G, Schulman SP, Steinberg A, Weiss RG. Reduced myocardial creatine kinase flux in human myocardial infarction: an in vivo phosphorus magnetic resonance spectroscopy study. Circulation. 2009;119:1918–1924. doi: 10.1161/CIRCULATIONAHA.108.823187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol Acutely Increases Adenosine Triphospate Energy Delivery in Failing Human Hearts. J Am Coll Cardiol. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingwall JS. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol Heart Circ Physiol. 1982;242:H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- 13.Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 14.Hudsmith LE, Neubauer S. Magnetic Resonance Spectroscopy in Myocardial Disease. JACC: Cardiovascular Imaging. 2009;2:87–96. doi: 10.1016/j.jcmg.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers CT, Clarke WT, Snyder C, Vaughan JT, Neubauer S, Robson MD. Human cardiac 31P magnetic resonance spectroscopy at 7 tesla. Magn Reson Med. 2013 doi: 10.1002/mrm.24922. DOI:10.1002/mrm.24922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lygate CA, Aksentijevic D, Dawson D, Ten Hove M, Phillips D, de Bono JP, Medway DJ, Sebag-Montefiore L, Hunyor I, Channon KM, Clarke K, Zervou S, Watkins H, Balaban RS, Neubauer S. Living without creatine: unchanged exercise capacity and response to chronic myocardial infarction in creatine-deficient mice. Circ Res. 2013;112:945–955. doi: 10.1161/CIRCRESAHA.112.300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn M, Remkes H, Stromer H, Dienesch C, Neubauer S. Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation. 2001;104:1844–1849. doi: 10.1161/hc3901.095933. [DOI] [PubMed] [Google Scholar]
- 18.Nahrendorf M, Spindler M, Hu K, Bauer L, Ritter O, Nordbeck P, Quaschning T, Hiller KH, Wallis J, Ertl G, Bauer WR, Neubauer S. Creatine kinase knockout mice show left ventricular hypertrophy and dilatation, but unaltered remodeling post-myocardial infarction. Cardiovasc Res. 2005;65:419–427. doi: 10.1016/j.cardiores.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A, Akki A, Wang Y, Leppo MK, Chacko VP, Foster DB, Caceres V, Shi S, Kirk JA, Su J, Lai S, Paolocci N, Steenbergen C, Gerstenblith G, Weiss RG. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]