Abstract
Background
Studies in childhood suggest that both body composition and early postnatal growth are associated with bone mineral density (BMD). However, little is known of the relationships between longitudinal changes in fat (FM) and lean mass (LM), and bone development in pre-pubertal children. We therefore investigated these associations in a population-based mother-offspring cohort, the Southampton Women’s Survey.
Methods
Total FM and LM were assessed at birth and 6-7 years of age by Dual-Energy X-ray Absorptiometry (DXA). At 6-7y, total cross-sectional area (CSA) and trabecular volumetric BMD (vBMD) at the 4% site (metaphysis) of the tibia was assessed using peripheral quantitative computed tomography [pQCT (Stratec XCT-2000)]. Total CSA, cortical CSA, cortical vBMD and strength-strain index (SSI) were measured at the 38% site (diaphysis). FM, LM and bone parameters were adjusted for age and sex and standardised to create within-cohort z-scores. Change in LM (ΔLM) or FM (ΔFM) was represented by change in z-score from birth to 7y and conditioned on the birth measurement. Linear regression was used to explore the associations between ΔLM or ΔFM and standardised pQCT outcomes, before and after mutual adjustment and for linear growth. The β-coefficient represents SD change in outcome per unit SD change in predictor.
Results
DXA at birth, in addition to both DXA and pQCT scans at 6-7y, were available for 200 children (48.5% male). ΔLM adjusted for ΔFM was positively associated with tibial total CSA at both the 4% (β=0.57SD/SD, p<0.001) and 38% sites (β=0.53SD/SD, p<0.001), cortical CSA (β=0.48SD/SD, p<0.001) and trabecular vBMD (β=0.30SD/SD, p<0.001), but not with cortical vBMD. These relationships persisted after adjustment for linear growth. In contrast, ΔFM adjusted for ΔLM was only associated with 38% total and cortical CSA, which became non-significant after adjustment for linear growth.
Conclusion
In this study, gain in childhood LM was positively associated with bone size and trabecular vBMD at 6-7 years of age. In contrast, no relationships between change in FM and bone were observed, suggesting that muscle growth, rather than accrual of fat mass, may be a more important determinant of childhood bone development.
Keywords: Osteoporosis, epidemiology, body composition, pQCT, growth, childhood
1. Introduction
Bone mineral accrual and bone geometry are important determinants of long term osteoporosis and fracture risk. Mathematical modelling has suggested that a 10% increase in peak bone mass will delay the onset of osteoporosis by 13 years [1]. Thus, understanding factors that influence bone mineral accrual during childhood may inform novel approaches to fracture prevention. It is well recognised that genotype, physical activity, nutrition and chronic disease in childhood and adolescence all contribute to bone development. These factors may have a direct influence on bone, or act indirectly through effects on linear growth and body composition. Indeed, we have previously demonstrated that growth in height in early childhood is associated with skeletal size, mineralisation [2, 3] and geometry [4]. Furthermore, these associations appear to persist into adulthood: in a UK cohort, pre-pubertal height gain velocity was positively associated with bone cross-sectional area and strength-strain index of the radius, assessed by peripheral quantitative computed tomography (pQCT) at 60-64 years [5]. How such relationships between bone development and overall growth relate to changes in body compartments (lean and fat mass) remains to be elucidated. Cross-sectional studies which have assessed associations between fat mass (FM) and BMC or aBMD measured by DXA have found conflicting results: positive [6-10], negative [8, 11] and non-significant [9] relationships have been reported, whilst some suggest that the relationships differ by sex [8, 9, 12] and stage of pubertal development [8, 13]. Associations described between FM and bone geometry assessed by pQCT in children have also been inconsistent [13-16]. Similar to the findings of the cross-sectional studies, many of the observed associations between longitudinal changes in body composition and bone development varied by sex, age and pubertal status [13], suggesting that the timing, rate and extent of changes in body composition might be important for bone mineral accrual and geometric development. In this study we therefore aimed to evaluate relationships between changes in body composition in infancy and early childhood, and bone geometry and vBMD assessed by pQCT in a cohort of pre-pubertal children participating in the Southampton Women’s Survey (SWS).
2. Methods
2.1 The Southampton Women’s Survey
Details of the Southampton Women’s Survey (SWS) have been published previously [17], but briefly, the SWS is a study of 12583, initially non-pregnant, women aged 20-34 years, resident in the city of Southampton, UK. Assessments of lifestyle, sociodemographic factors and anthropometry were performed at study entry (April 1998 – December 2002) and women who subsequently became pregnant were followed in detail throughout their pregnancy.
The SWS was conducted according to the guidelines laid down in the Declaration of Helsinki, and the Southampton and South West Hampshire Research Ethics Committee approved all procedures. Written informed consent was obtained from all participating women and by a parent or guardian with parental responsibility on behalf of their children.
2.2 Childhood assessments of body composition and bone development
There were 3158 singleton live births to mothers participating in the SWS. The children were followed up at birth and during infancy, and consecutive subsets of children were invited to participate in detailed assessments of anthropometry, body composition and bone mineralisation at birth and 6-7 years of age. The presence of a condition known to affect growth (eg chromosomal abnormalities, endocrinopathy) or bone structure (eg metabolic bone disease, osteogenesis imperfecta) was an exclusion criterion for follow-up. However, owing to the population based cohort, and the rarity of such conditions, no children were actually excluded from the current analysis for this reason.
2.2.1 Anthropometry and Dual Energy Xray Absorptiometry
At birth and 6-7 years, weight was measured using calibrated digital scales (SECA Ltd, Birmingam, UK). At birth crown-heel length was measured using a neonatometer (CMS Ltd, UK) and at 6-7 years, standing height was measured using a Leicester stadiometer (SECA Ltd, Birmingham UK). Within 2 weeks after birth and at 6-7 years whole body composition was assessed by DXA. A Lunar DPX-L instrument (GE Corporation, Madison, Wisconsin, USA) was used in infancy and a Hologic Discovery instrument (Hologic Inc., Bedford, MA, USA) in childhood. Neonatal or paediatric software, as appropriate, was used to derive BMC, FM and fat-free (lean) mass (LM) from a whole body scan using a three-compartment model. All scans were assessed by 2 reviewers for movement artefacts and those with excess movement (duplication or deletion of body parts) were excluded from analysis. The coefficients of variation for body composition analysis using the Lunar and Hologic DXA instrument were 1.4% and 1.4 –1.9%, respectively. The reliability of DXA in small subjects has been demonstrated previously [18].
2.2.2 Peripheral Quantitative Computed Tomography (pQCT)
At 6-7 years of age, bone geometry and volumetric bone mineral density (vBMD) of the tibia were assessed by pQCT using a Stratec XCT-2000 instrument (Stratec Inc, Pforzhein, Germany) in a subset of children. Two sites were scanned: a distal site, which largely reflects trabecular bone, and a diaphyseal site, comprised largely of cortical bone, corresponding to 4% and 38% of the distance from the medial malleolus to the tibial tuberosity, respectively. A scout view was obtained to place the reference line at the proximal border of the distal tibial growth plate. At each site, a single 2mm thick tomographic slice was sampled at a voxel size of 0.5mm. At the 4% distal site, total bone cross-sectional area (CSA) and trabecular BMC and vBMD were calculated using the manufacturer’s software version 5.4. A threshold of 280 mg/cm3 was used to separate bone from soft tissue, and subsequently, the default peeling algorithm was applied to the distal 4% scans to separate trabecular bone. With this peeling, 55% of the outer bone area was concentrically separated and defined as cortical and subcortical; the remaining 45% was defined as trabecular bone. At the 38% diaphyseal site, measurements of total CSA, cortical CSA and cortical vBMD were made using a threshold for cortical bone of 710mg/cm3. Torsional resistance was estimated by strength-strain index (SSI) at the 38% site. Scans with excess movement artefact were excluded from the analysis. The coefficient of variation for this pQCT instrument has previously been demonstrated to range from 0.88% (tibial total 4% vBMD) to 8.8% (total radial diaphyseal CSA), but typically 1-3% [19].
2.3 Statistical analysis
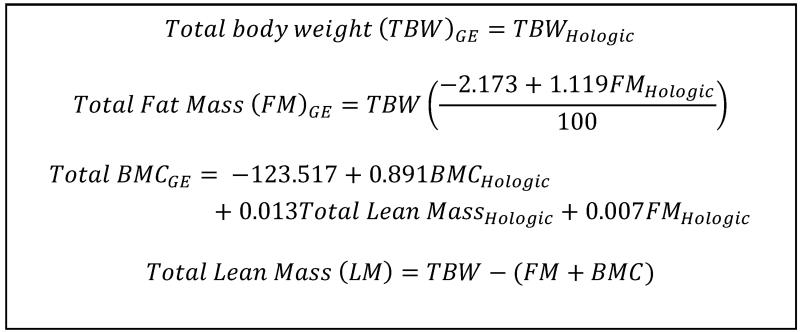
Differences in body composition, bone geometry and vBMD of boys and girls were assessed using t-tests and Mann-Whitney U tests for normally and non-normally distributed variables, respectively. Associations with age were assessed using Pearson’s and Spearman’s correlation coefficients. Owing to differences between boys and girls, the body composition variables were adjusted for the sex of the child. Additionally, DXA indices at both 6-7 years and birth were adjusted for age, and those at birth were also adjusted for gestational age, which was determined from last menstrual period and early ultrasound assessment. Previous work has demonstrated that body composition assessment by DXA may differ depending on the instrument used, due to differing measurement and calibration methods. We therefore used published cross-calibration equations, which were generated from a population including children aged >5 years, to calibrate measures from the Hologic Discovery instrument (at age 6-7 years) to measures obtained on the Lunar DPX-L (at birth) [20] (Figure 1).
Figure 1. Equations used to convert measurements obtained at 6-7 years of age using a Hologic Discovery DXA instrument to data from a GE Lunar instrument. Taken from Shepherd et al JBMR 2012[20].
Fat mass was not normally distributed. Therefore, for consistency, both FM and LM were standardized using a Fisher-Yates transformation to a normally distributed variable with a mean of 0 and a standard deviation (SD) of 1, producing a within-cohort z-score for each child based on the subset of children who participated in the pQCT scan at 6-7 years (n=516). Longitudinal changes in body composition were calculated as the difference in FM or LM z-scores between two time points. To account for regression to the mean, these were conditioned on the measurement at birth.
Total CSA at the 4 and 38% sites and cortical CSA were also not normally distributed and so all pQCT outcomes were also transformed using Fisher-Yates transformations to provide within cohort z-scores. All pQCT measurements were positively associated with age, except trabecular vBMD which was negatively associated with age. Therefore the associations between longitudinal changes in body composition and whole body BMC and pQCT assessment of tibial bone geometry and vBMD were analysed using linear regression adjusting for age at pQCT and sex in all models.
As LM tends to increase with FM, the independent effect was assessed by adjusting change in LM (ΔLM) for change in FM (ΔFM) and vice versa using regression. Finally, in order to determine whether the relationships between change in body composition and tibial geometry and vBMD were independent of linear growth, we adjusted the models for the change in height z-score over the same time frame.
All analysis was performed using Stata v13.0 (Statacorp, College Station, Texas, USA). A p-value of <0.05 was accepted as statistically significant. Given the observational nature of the study, together with the substantial collinearity amongst both predictors and outcomes, testing for multiple comparisons was judged to be inappropriate [21].
3. Results
227 children had DXA at birth and 6-7 years in addition to pQCT of the tibia at 6-7 years, but 27 were not included in the analysis due to movement artefact on the 6-7 year DXA scan. Six (3.0%) scans at the 4% tibia and 41 (20.5%) at the 38% tibia had to be excluded from the analysis due to movement artefact. The children with and without scans at the 4% and 38% sites did not differ in sex, age, height, weight, LM or FM at 6-7 years (p>0.05 for all).
Children included in this analysis were of similar sex distribution (p=0.34) to those born to mothers in the remaining SWS cohort, but did have a higher birth weight (adjusted for gestation and sex) (3505±453g vs 3423±434g, p=0.01). Maternal age at delivery (p=0.19), parity (p=0.89), pre-pregnancy BMI (p=0.31), educational attainment (p=0.31) and social class (p=0.09) did not differ between children included and not included in this analysis. An additional 803 and 824 children participating in SWS had body composition analysis by DXA at birth and 6 years of age, respectively. FM, LM and percentage FM did not differ in these children to those included at either time point (p>0.05 for all). 316 children participated in the pQCT scan but had not had DXA at both birth and 6-7 years of age. These children were of similar age, sex, height, weight, tibial geometry and vBMD to those included in the analysis (p>0.05 for all).
At both birth and 6-7 years, the boys and girls included in this analysis were of similar age and weight (Table 1), but FM was significantly greater in the girls than the boys at both ages (p=0.001 & p<0.0001, respectively), and at 6-7 years of age, LM was significantly greater in the boys (p=0.002). Boys were also longer than girls at birth (p=0.01), but this difference was not present at 6-7 years (p=0.74). There were no significant differences in tibial geometric properties or vBMD between boys and girls at 6-7 years (Table 1). However, bone size and strength of the tibia were positively associated with age (4% CSA: r=0.14, p=0.05; 38% CSA: r=0.28, p<0.001; cortical area: r=0.42, p<0.001; SSI: r=0.19, p=0.02), whereas trabecular vBMD (r=−0.20, p=0.006) and cortical vBMD (r=−0.18, p=0.03) were inversely associated with age.
Table 1. Body composition of boys and girls at birth and 6-7 years of age. Data shown as mean±SD or median (IQR), unless otherwise stated.
| Neonate | 6-7 years | |||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| N | 97 | 103 | 97 | 103 |
| Age (days for neonatal/years for childhood), median (range) | 8 (1-16) | 8 (1-15) | 7.10 (6.41-7.65) | 7.08 (6.36-7.69) |
| Height / Length at birth (cm) | 50.7 (48.6-52.1) | 49.7 (48.6-50.8)a | 122.9±5.9 | 122.6±5.6 |
| Weight (kg) | 3.51±0.54 | 3.52±0.49 | 23.5 (20.9-26.0) | 23.8 (20.6-27.0) |
| Lean mass (kg) | 3.00±0.42 | 2.91±0.35 | 17.11±2.21 | 16.10±2.24b |
| Fat mass (kg) | 0.46 (0.33-0.61) | 0.55 (0.41-0.72)b | 5.44 (4.23-6.79) | 7.04 (5.18-8.58)c |
| Tibial pQCT | ||||
| 4% Total Area (mm2) | 695 (567-753) | 669 (592-758) | ||
| 4% Trabecular vBMD (mg/cm3) | 303±55 | 308±54 | ||
| 38% Total Area (mm2) | 221 (200-251) | 228 (199-256) | ||
| 38% Cortical Area (mm2) | 124 (115-139) | 120 (111-136) | ||
| 38% Cortical vBMD (mg/cm3) | 1036±33 | 1030±37 | ||
| Strength Strain Index | 484±96 | 453±105 | ||
p<0.05
p<0.01
p<0.001 compared to boys of same age.
3.1 Change in body composition and tibial geometry and vBMD
Weight gain from birth to 6-7 years was positively associated with bone size (4%: β=0.60SD/SD 95%CI 0.46, 0.74, p<0.001; 38% β=0.64 95%CI 0.51, 0.77, p<0.001) and trabecular vBMD (β=0.16SD/SD 95%CI 0.02, 0.30, p=0.02), but not cortical vBMD (at 6-7 years. Similar associations were also observed with growth in height (4% CSA: β=0.64SD/SD 95%CI 0.48, 0.79, p<0.001; 38% CSA:β=0.60, 95%CI 0.45, 0.75, p<0.001; trabecular vBMD: β=0.23, 95%CI 0.08, 0.38, p=0.003). In univariate analysis, both ΔLM and ΔFM were positively associated with tibial geometry and whole body BMC at 6-7 years (Table 2). However, only ΔLM was positively associated with trabecular vBMD (β=0.26 SD/SD, 95%CI: 0.13, 0.40, p<0.001), and neither ΔLM nor ΔFM were significantly associated with cortical vBMD.
Table 2. Associations between change in body composition from birth to 6-7 years and tibial size and volumetric BMD assessed at age 6-7 years assessed by pQCT, and whole body BMC assessed by DXA. Data shown are standardised beta coefficient (SD per SD) and 95% confidence interval, with and without mutual adjustment, and for change in height.
| Change in lean mass | Change in fat mass | ||||||
|---|---|---|---|---|---|---|---|
| n | Univariate | Adjusted for ΔFM | Adjusted for ΔFM & height growth | Univariate | Adjusted for ΔLM | Adjusted for ΔLM & height growth | |
| Tibia (pQCT) | |||||||
| 4% CSA | 192 | 0.63 (0.49, 0.76) c | 0.57 (0.42, 0.73) c | 0.29 (0.10, 0.49) b | 0.35 (0.20, 0.50) c | 0.09 (−0.10, 0.27) | 0.00 (−0.15, 0.16) |
| 38% CSA | 157 | 0.63 (0.50, 0.76) c | 0.53 (0.37, 0.69) c | 0.26 (0.08. 0.44) b | 0.41 (0.27, 0.55) c | 0.18 (0.01, 0.36) a | 0.07 (−0.08, 0.22) |
| Cortical CSA | 157 | 0.57 (0.45, 0.70) c | 0.48 (0.32, 0.63) c | 0.23 (0.05, 0.40) a | 0.39 (0.25, 0.52) c | 0.18 (0.02, 0.34) a | 0.07 (−0.07, 0.21) |
| SSI | 154 | 0.70 (0.56, 0.84) c | 0.62 (0.45, 0.79) c | 0.36 (0.18, 0.55) c | 0.38 (0.22, 0.53)c | 0.13 (−0.06, 0.31) | −0.01 (−0.16, 0.14) |
| Trabecular vBMD | 192 | 0.26 (0.13, 0.40) c | 0.30 (0.16, 0.45) c | 0.24 (0.05, 0.43) a | 0.04 (−0.10, 0.17) | −0.10 (−0.25, 0.05) | −0.13 (−0.27, 0.02) |
| Cortical vBMD | 157 | 0.02 (−0.14, 0.18) | 0.08 (−0.10, 0.25) | 0.17 (−0.04, 0.38) | −0.10 (−0.25, 0.06) | −0.13 (−0.30, 0.04) | −0.12 (−0.29, 0.06) |
| Whole body (DXA) | |||||||
| BMC | 198 | 0,81 (0.72, 0.90) a | 0.71 (0.58, 0.83) c | 0.31 (0.18, 0.45) c | 0.54 (0.42, 0.66) c | 0.23 (0.08,0.39) b | 0.12 (0.02, 0.23) a |
| Anthropometry | |||||||
| Height | 198 | 0.76 (0.66, 0.86) c | 0.68 (0.56, 0.82) c | 0.06 (−0.02,0.15) | 0.47 (0.34, 0.59) c | 0.17 (0.01, 0.32) a | 0.03 (−0.04, 0.09) |
p<0.05;
p<0.01;
p<0.001;
CSA: Cross sectional area; vBMD: volumetric Bone Mineral Density; SSI: Strength-strain index; BMC: Bone mineral content; ΔFM: change in fat mass; ΔLM: change in lean mass
ΔLM was moderately associated with ΔFM (r=0.44, p<0.001). Therefore, to assess the independent effects of ΔLM and ΔFM on bone development, ΔLM was adjusted for ΔFM and vice versa. After adjustment, the previously observed relationships between ΔLM and tibial geometry and trabecular vBMD remained significant (Table 2). Conversely, the relationships between ΔFM and tibial geometry were no longer significant, apart from weak associations with 38% total and cortical CSA (Table 2).
ΔLM and ΔFM were correlated with height at 6-7 years (r=0.75, p<0.001 and r=0.47, p<0.001, respectively) and conditional growth in height from birth to 6-7 years (r=0.76, p<0.001 and r=0.47, p<0.001, respectively). The relationships between ΔLM adjusted for ΔFM and tibial geometry and trabecular vBMD at 6-7 years remained statistically significant after adjustment for height growth, although the change in tibial parameter for each SD change in LM was attenuated (Table 2). No statistically significant associations were observed between ΔFM adjusted for ΔLM and tibial geometry and vBMD after adjustment for height change (Table 2).
When the relationships were analysed separately by sex, the associations between ΔLM adjusted for ΔFM and tibial geometry remained significant in both the boys and girls, but the relationship with trabecular vBMD was only present in the girls (Table 3). Furthermore, inclusion of linear growth between birth and 6-7 years attenuated the relationships to below statistical significance in the boys but not the girls (Table 3). However, tests for a formal interaction between ΔLM adjusted for FM by sex and pQCT were only statistically significant for cortical CSA (p=0.006).
Table 3. Associations between change in lean mass (ΔLM) adjusted for change in fat mass (ΔM) from birth to 6-7 years and tibial size and volumetric BMD assessed at age 6-7 years it boys and girls. Data shown are standardised beta coefficient (SD per SD) and 95% confidence interval, with and without adjustment for change in height.
| ΔLM adjusted for ΔFM | ΔLM adjusted for ΔFM and height growth | |||
|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |
| 4% CSA | 0.47 (0.20, 0.74) b | 0.70 (0.51, 0.89) c | 0.15 (−0.17, 0.48) | 0.45 (0.22, 0.69) c |
| 38% CSA | 0.46 (0.22, 0.71) c | 0.56 (0.33, 0.78) c | 0.16 (−0.10, 0.43) | 0.32 (0.06, 0.58) a |
| Cortical CSA | 0.24 (−0.00, 0.48) | 0.67 (−.47, 0.87) c | −0.00 (−0.28, 0.27) | 0.42 (0.20, 0.63) c |
| SSI | 0.50 (0.25, 0.75) c | 0.76 (0.51, 0.98) c | 0.23 (−0.05, 0.51) | 0.51 (0.26, 0.76) c |
| Trabecular vBMD | 0.12 (−0.12, 0.37) | 0.39 (0.21, 0.57) c | −0.01 (−0.31, 0.30) | 0.38 (0.14, 0.61) b |
| Cortical vBMD | 0.11 (−0.16, 0.39) | 0.03 (−0.21, 0.27) | 0.26 (−0.07, 0.58) | 0.07 (−0.22, 0.37) |
p<0.05;
p<0.01;
p<0.001;
CSA: Cross sectional area; vBMD: volumetric Bone Mineral Density; SSI: Strength-strain index; ΔFM: change in fat mass; ΔLM: change in lean mass
4. Discussion
To our knowledge, this is the first study to describe relationships between longitudinal changes in body composition from birth and bone development assessed by pQCT in pre-pubertal children. Our findings suggest that in infancy and early childhood, gains in lean mass are positively associated with tibial bone size, and trabecular vBMD at 6-7 years of age. Conversely, gains in fat mass, after adjustment for gain in LM, was not significantly associated with tibial geometry. There were no associations between either ΔLM or ΔFM and cortical vBMD. Furthermore, both ΔLM and ΔFM were positively associated with whole body BMC assessed by DXA, possibly reflecting the increased bone size observed with pQCT measurements rather than increased mineralisation.
These findings further our understanding of the interrelationships between bone, fat and muscle development in pre-pubertal children. Few studies have reported on the relationships between lean mass and bone geometry assessed by pQCT in children. Two studies have found positive associations cross-sectionally [13, 14] and one study demonstrated a positive relationship between radial CSA and the increase in size of the lean tissue compartment over 3 years [13]. We similarly demonstrated positive longitudinal relationships between ΔLM and tibial size and trabecular vBMD in pre-pubertal children. In this observational cohort, we cannot conclude a causal relationship between gains in LM and larger bone size and density, and indeed the relationship between skeletal muscle mass and bone accrual is difficult to dissect. Possible mechanisms include direct mechanical or hormonal signalling between muscle and bone [22, 23]; alternatively this finding could represent collinear growth of both tissue types. The mechanostat theory suggests that bone has a homeostatic mechanism which enables mineralisation and geometric properties to adapt to loads imposed on it. Indeed, physical activity in childhood is associated with both bone mineral accrual and increases in LM [24], and elucidating whether physical activity is acting directly on the bone and/or via increased muscle mass is difficult. Nonetheless, it is possible that physical activity is a confounding factor in our findings, although some [25], but not all [26], studies have shown that peak LM precedes peak bone mass, further supporting the notion that skeletal muscle may influence bone acquisition. Furthermore, although obese children have greater bone CSA and BMD than healthy weight children [27], our findings would support those of Wetzsteon et al [27] that this most likely results from increased LM accrual in obesity rather than FM accrual or simply higher total body weight. Potential mechanisms for the association between muscle mass and bone development are still to be fully elucidated, but might involve direct mechanotransduction of strains via the osteocyte. However, this would assume that muscle size is an accurate proxy for muscle function, and future studies to elucidate the relationships between changes in muscle strength and bone development would further our understanding of the relationships between muscle and bone. There is also increasing evidence that skeletal muscle secretes myokines which might influence bone size and mineralisation [23]. However, it also remains possible that there is coupling of muscle and bone growth without direct interaction between the two tissues. Indeed, hormonal factors, for example, testosterone, glucocorticoids, GH/IGF-1 axis and vitamin D, can positively influence both skeletal muscle and bone [23], and we have previously identified positive associations between early gain in length and indices of bone geometry in childhood [3-5]. However, in the current analysis adjustment for linear growth attenuated the relationships but they remained statistically significant, so it is unlikely that our findings solely reflect collinear acquisition of height, lean mass and bone mass.
Even in these prepubertal children we found that the relationships between changes in LM and bone development were stronger in females than males. Hrafnkelsson et al similarly found using DXA, rather than pQCT, that for every kg increase in LM between ages 7 and 9 years, the increase in whole body BMC and BMD was greater in girls than boys, and the association between ΔLM and hip aBMD was only statistically significant in females [28]. However, pubertal maturation was not reported in that study, but might confound its findings, as a greater proportion of females compared to males would have been expected to commence puberty during their follow-up period. In our study, the numbers for analysis by sex were small, and formal interaction terms only achieved statistical significance for cortical CSA; further replication of this finding in other cohorts is therefore required.
There have been rather more studies investigating the potential influence of fat mass on bone development. Previous studies investigating the cross-sectional associations between fat mass, and bone geometry assessed by pQCT in children, report inconsistent findings, including positive [14, 15], negative [13] and U-shaped [16] relationships. Furthermore, these relationships differ by age, sex and pubertal stage. Wey et al. reported a negative cross-sectional association between FM and radial diaphyseal area in males, but in females there was a significant interaction with age such that the association changed from positive in younger girls to negative in adolescents [13]. Clark et al similarly observed contrasting associations between FM and total body less head bone area and BMC measured by DXA in girls at Tanner stage 1 compared with Tanner stage 3 [8]; we have previously documented stronger relationships between lean-adjusted fat mass and tibial trabecular vBMD at 6 years of age in males than females [14]. As such, there is a need for longitudinal studies over a narrow age range at recruitment and the need for caution in extrapolating findings from one stage of pubertal development to another. Indeed our study suggests that longitudinal changes in FM are not associated with tibial geometry or vBMD in pre-pubertal children. This is in contrast to two previous studies, though both of these included a more diverse range of ages and pubertal stages [13, 29].
Furthermore, Wey et al. explored the relationships with measurements at the radius, which might differ from those in the tibia, and the interpretation of their findings and comparison to our own is further complicated by interactions between ΔFM and both age and menarchal status [13]. For example, in young females, Wey et al found ΔFM was positively associated with radial metaphyseal vBMD, a relationship which became negative at older ages. However, paradoxically, there was a negative association between ΔFM and the same outcome in females after menarche, which was null before menarche. Increases in concentrations of sex steroids, size and location of fat depots and changes to circulating adipokines during puberty could confound and/or alter the bone-fat relationships and, thus, future longitudinal evaluation of our cohort through pubertal development, currently ongoing, might also reveal differing associations with age and sexual maturation.
We studied a population-based cohort of children, characterised with objective measures of body composition and bone indices. There are however a number of limitations to this study. Firstly, we included only a small proportion of children born into the SWS cohort. Restricting the analysis to only those with complete data was necessary to enable longitudinal analysis. However, the children had similar body composition at birth and 6-7 years, and bone geometry and vBMD at 6-7 years to children in SWS who had participated in the some, but not all, aspects of the follow-up studies. Secondly, DXA and pQCT are well validated in adults, but obtaining scans in children can be more difficult due to their tendency to move. We used specific neonatal/ paediatric software, and movement artefact was minimal across the cohort, though we removed scans that exhibited unacceptable artefact. Body composition assessment by DXA of small subjects has been previously validated using biochemical assessment of carcass nitrogen content and lipid extraction to determine LM and FM, respectively, in piglets which were sacrificed immediately after DXA scanning [18]. The DXA instruments used in the neonatal period and at 6-7 years did differ, but we used a published cross-calibration equation that was derived from paediatric data to convert data between Hologic and Lunar DXA instruments [20].. pQCT is exquisitely sensitive to movement artefact, and resulted in exclusion of a greater number of scans, particularly at the diaphyseal site. However, the children with movement artefact on pQCT scans did not differ from those included in the analysis. Furthermore, although partial volume effects may have influenced absolute pQCT measures, this should not have affected the ranking of individuals in the population. Finally, in this observational analysis, we cannot conclude a causal relationship between changes in body composition and bone development. In particular, although physical activity has been objectively measured in children born to the SWS, the number of children having undergone these assessments in this present cohort was too small to allow meaningful statistical analysis. Future work incorporating an assessment of physical activity might enable clarification of the mechanisms underpinning these observations.
In summary, our findings would support the notion that lean mass gain in childhood is positively associated with achieved bone size and mineralisation. Therefore interventions to increase lean mass during pre-pubertal growth, particularly in girls, might have positive benefits for bone strength, potentially leading to improved peak bone mass (together with muscle mass), with a reduced risk of fracture in older age.
Highlights.
Growth in lean mass from birth to 6-7 years was associated with tibial cross-sectional size and strength in childhood.
Growth in fat mass was not associated with bone size
Trabecular, but not cortical, volumetric BMD at 6-7 years was positively associated with gain in lean mass since birth.
Acknowledgments
We thank the mothers who gave us their time; and a team of dedicated research nurses and ancillary staff for their assistance. This work was supported by grants from the Medical Research Council, British Heart Foundation, Arthritis Research UK, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. Participants were drawn from a cohort study funded by the Medical Research Council and the Dunhill Medical Trust. The research leading to these results has also received funding from the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement n°289346. CLG is supported by Arthritis Research UK (Grant reference 20000). We thank Mrs G Strange and Mrs R Fifield for helping prepare the manuscript.
Footnotes
Disclosures
KMG has acted as a consultant to Abbott Nutrition and Nestle Nutrition, and has received reimbursement for speaking at an Abbott Nutrition Conference on Pregnancy Nutrition and Later Health Outcomes, at a Nestle Nutrition Institute Workshop and at a workshop funded by the International Life Sciences Institute (ILSI Europe). He is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone
References
- [1].Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- [2].Harvey NC, Mahon PA, Kim M, Cole ZA, Robinson SM, Javaid K, Inskip HM, Godfrey KM, Dennison EM, Cooper C. Intrauterine growth and postnatal skeletal development: findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2012;26:34–44. doi: 10.1111/j.1365-3016.2011.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harvey NC, Mahon PA, Robinson SM, Nisbet CE, Javaid MK, Crozier SR, Inskip HM, Godfrey KM, Arden NK, Dennison EM, Cooper C. Different indices of fetal growth predict bone size and volumetric density at 4 years of age. J.Bone Miner.Res. 2010;25:920–927. doi: 10.1359/jbmr.091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harvey NC, Cole ZA, Crozier SR, Ntani G, Mahon PA, Robinson SM, Inskip HM, Godfrey KM, Dennison EM, Cooper C. Fetal and infant growth predict hip geometry at 6 y old: findings from the Southampton Women’s Survey. Pediatr Res. 2013;74:450–6. doi: 10.1038/pr.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kuh D, Wills AK, Shah I, Prentice A, Hardy R, Adams JE, Ward K, Cooper C. Growth from birth to adulthood and bone phenotype in early old age: a british birth cohort study. J Bone Miner Res. 2014;29:123–33. doi: 10.1002/jbmr.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ka K, Rousseau MC, Lambert M, O’Loughlin J, Henderson M, Tremblay A, Alos N, Nicolau B. Association between lean and fat mass and indicators of bone health in prepubertal caucasian children. Horm Res Paediatr. 2013;80:154–62. doi: 10.1159/000354043. [DOI] [PubMed] [Google Scholar]
- [7].Ackerman A, Thornton JC, Wang J, Pierson RN, Jr., Horlick M. Sex difference in the effect of puberty on the relationship between fat mass and bone mass in 926 healthy subjects, 6 to 18 years old. Obesity (Silver Spring) 2006;14:819–25. doi: 10.1038/oby.2006.95. [DOI] [PubMed] [Google Scholar]
- [8].Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–41. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–7. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- [10].Goulding A, Taylor RW, Grant AM, Murdoch L, Williams SM, Taylor BJ. Relationship of total body fat mass to bone area in New Zealand five-year-olds. Calcif Tissue Int. 2008;82:293–9. doi: 10.1007/s00223-008-9121-x. [DOI] [PubMed] [Google Scholar]
- [11].Wosje KS, Khoury PR, Claytor RP, Copeland KA, Kalkwarf HJ, Daniels SR. Adiposity and TV viewing are related to less bone accrual in young children. J Pediatr. 2009;154:79–85.e2. doi: 10.1016/j.jpeds.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44:752–7. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- [13].Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011;96:106–14. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cole ZA, Harvey NC, Kim M, Ntani G, Robinson SM, Inskip HM, Godfrey KM, Cooper C, Dennison EM. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone. 2012;50:562–7. doi: 10.1016/j.bone.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46:977–984. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Viljakainen HT, Pekkinen M, Saarnio E, Karp H, Lamberg-Allardt C, Makitie O. Dual effect of adipose tissue on bone health during growth. Bone. 2011;48:212–7. doi: 10.1016/j.bone.2010.09.022. [DOI] [PubMed] [Google Scholar]
- [17].Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int.J.Epidemiol. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Brunton JA, Weiler HA, Atkinson SA. Improvement in the accuracy of dual energy x-ray absorptiometry for whole body and regional analysis of body composition: validation using piglets and methodologic considerations in infants. Pediatr.Res. 1997;41:590–596. doi: 10.1203/00006450-199704000-00022. [DOI] [PubMed] [Google Scholar]
- [19].Oliver H, Jameson KA, Sayer AA, Cooper C, Dennison EM. Growth in early life predicts bone strength in late adulthood: the Hertfordshire Cohort Study. Bone. 2007;41:400–5. doi: 10.1016/j.bone.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, Levine MA. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res. 2012;27:2208–16. doi: 10.1002/jbmr.1654. [DOI] [PubMed] [Google Scholar]
- [21].Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365:1591–5. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- [22].Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep. 2014;12:135–41. doi: 10.1007/s11914-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cianferotti L, Brandi ML. Muscle-bone interactions: basic and clinical aspects. Endocrine. 2014;45:165–77. doi: 10.1007/s12020-013-0026-8. [DOI] [PubMed] [Google Scholar]
- [24].Morris FL, Naughton GA, Gibbs JL, Carlson JS, Wark JD. Prospective Ten-Month Exercise Intervention in Premenarcheal Girls: Positive Effects on Bone and Lean Mass. Journal of Bone and Mineral Research. 1997;12:1453–1462. doi: 10.1359/jbmr.1997.12.9.1453. [DOI] [PubMed] [Google Scholar]
- [25].Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–5. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- [26].Xu L, Nicholson P, Wang Q, Alen M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24:1693–8. doi: 10.1359/jbmr.090405. [DOI] [PubMed] [Google Scholar]
- [27].Wetzsteon RJ, Petit MA, Macdonald HM, Hughes JM, Beck TJ, McKay HA. Bone structure and volumetric BMD in overweight children: a longitudinal study. J Bone Miner Res. 2008;23:1946–53. doi: 10.1359/jbmr.080810. [DOI] [PubMed] [Google Scholar]
- [28].Hrafnkelsson H, Sigurdsson G, Magnusson KT, Sigurdsson EL, Johannsson E. Fat mass increase in 7-year-old children: more bone area but lower bone mineral density. J Bone Miner Metab. 2013;31:442–8. doi: 10.1007/s00774-013-0423-3. [DOI] [PubMed] [Google Scholar]
- [29].Laddu DR, Farr JN, Laudermilk MJ, Lee VR, Blew RM, Stump C, Houtkooper L, Lohman TG, Going SB. Longitudinal relationships between whole body and central adiposity on weight-bearing bone geometry, density, and bone strength: a pQCT study in young girls. Arch Osteoporos. 2013;8:156. doi: 10.1007/s11657-013-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]