Abstract
Background
We reviewed the effectiveness and safety of cardiac resynchronization therapy (CRT) for patients with New York Heart Association (NYHA) class IV non-ambulatory heart failure (NAHF).
Methods
From 2006 to 2011, 310 patients underwent CRT at Kobe University Hospital and Himeji Cardiovascular Center because of heart failure. Of these, 29 NAHF patients were retrospectively analyzed. The control group comprised 21 age- and ejection fraction-matched patients with NAHF who did not undergo CRT from the ICU database of Kobe University Hospital. The primary endpoint was all-cause death and hospitalization for heart failure. Response was defined as a >15% reduction in left ventricular end-systolic volume (LVESV).
Results
CRT was performed successfully without serious complications in all patients. Twenty-three patients (79%) were discharged 19±15 days after CRT implantation, while 6 (21%) died during their hospital stay due to progressive heart failure. Compared with the control group, patients in the CRT group showed significant improvements in the primary endpoint (log-rank p=0.04). Six patients (21%) were defined as responders and the Kaplan–Meier curve showed that responders experienced a better outcome than non-responders (log-rank p=0.029). LV dyssynchrony before implantation was significantly related to the occurrence of the primary endpoint (p=0.02).
Conclusions
CRT can be safely used in patients with NAHF and can improve long-term patient outcomes, especially in treatment responders.
Keywords: Cardiac resynchronization therapy, NYHA IV, Heart failure, Radial strain dyssynchrony
1. Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for patients with New York Heart Association (NYHA) class III–IV heart failure with impaired systolic failure. CRT improves exercise capacity and quality of life in addition to reducing heart failure hospitalizations and overall mortality. However, large CRT trials have not included patients with severe heart failure such as those dependent on intravenous inotropic drugs [1–3]. Furthermore, the 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy includes no mention of CRT for patients with non-ambulatory NYHA class IV (NAHF) [4]. As previously reported, the long-term survival of patients with NAHF varies according to patient population and the application of devices and medical therapy [5–7]. In the REMATCH trial [7], the six-month mortality rate in the optimal medical therapy cohort was 61%. While occasionally described as beneficial in patients with NAHF, CRT is not generally used as a “rescue therapy” for such patients. Furthermore, cardiac transplantation and left ventricular assist devices (LVADs) are not common therapies, especially in elderly patients. Therefore, we retrospectively assessed the effectiveness and safety of CRT for patients with NAHF.
2. Material and methods
2.1. Study design
This was a retrospective database study of patients who underwent CRT from two centers, Kobe University Hospital (Kobe, Japan) and Himeji Cardiovascular Center (Himeji, Japan). The database included 310 patients who received CRT between 2003 and 2011. Of these, we identified 44 patients with end-stage heart failure who required intravenous diuretics and/or inotropes and/or intra-aortic balloon pump (IABP) support for life maintenance at the point of CRT implantation. Weaning of intravenous drugs was attempted in all patients, but discharge from the intensive care unit (ICU) was not achieved. We excluded patients who required hospitalization due to reversible factors leading to heart failure decompensation such as an afterload mismatch, infection, acute myocardial infarction, or medically refractory arrhythmias.
In accordance with the above criteria, 29 patients were enrolled in this study. All patients gave their written informed consent and agreed to undergo CRT implantation with an awareness of the increased risk due to their critical condition. The study conformed to the principles of the Declaration of Helsinki.
Patients with NAHF with no indication for CRT served as a comparison group. The ICU database of Kobe University Hospital followed a total of 322 patients who were admitted to the ICU for heart failure decompensation between 2007 and 2012. Of these, 21 age- and ejection fraction (EF)-matched patients who required intravenous drugs for more than 2 weeks were enrolled in the control group. Same as in the CRT group, patients with heart failure due to reversible factors were excluded. Patient characteristics and clinical outcomes were reviewed.
Patient data were analyzed to determine the primary endpoint of all-cause mortality and post-implantation hospitalization for heart failure. After 6 months of follow-up, patients were classified as responders to CRT if they showed a more than 15% relative decrease in LVESV (left ventricular end-systolic volume) from baseline. Non-response was defined as an LVESV improvement of less than 15% or death within 6 months of implantation. Blood pressure and heart rate were recorded just before CRT implantation. Brain natriuretic peptide (BNP), creatinine, and sodium levels were measured at the time of admission (pre-implantation). Hemoglobin and albumin levels measured during patients׳ hospitalization before implantation were selected as the minimum levels. The QRS width and configuration were measured using a 12-lead ECG at the time of admission.
2.2. Implantation techniques
All patients were implanted with Medtronic (Minnesota, USA), St. Jude Medical (California, USA), or Boston Scientific (Massachusetts, USA) CRT devices. The LV lead was implanted in a branch of the coronary sinus, with the exception of one case in which it was implanted in the epicardial ventricular wall. This patient׳s coronary sinus could not be approached endovascularly because of an anomalous coronary vein. Right atrial (RA) and right ventricular (RV) leads were implanted in the RA appendage and RV apex, respectively. Lead positioning was assessed from post-operative chest X-rays in the anterior and lateral views.
If patients were in sinus rhythm, the atrio-ventricular delay was optimized individually based on an echocardiographic assessment of transmitral flow. The V-V delay was programmed to achieve the greatest degree of biventricular fusion by measurement of the echocardiographic time velocity index.
In patients with atrial fibrillation (AF), if biventricular pacing could not predominate over their intrinsic rhythm, a radiofrequency atrio-ventricular junctional ablation was performed after CRT implantation. The decision to implant a CRT-D or CRT-P device was left to the discretion of each physician.
2.3. Follow-up
Follow-up data were collected retrospectively after CRT implantation. All study patients were followed-up regularly in our hospitals. Patients visited the CRT clinic at one month, three months, and every three months thereafter. In addition to a medical interview, an electrocardiogram, chest X-ray, NYHA functional class assessment, biomarker level measurements (including BNP and creatinine), and CRT interrogations were performed at each follow-up visit. A standard echocardiography was performed at six months of follow-up in all patients. If patients died outside our hospitals, we contacted their families by phone and checked the cause of death.
2.4. Echocardiographic study and analysis
All echocardiographic studies were recorded by commercially available echocardiography systems. LV volumes and EF were assessed using the biplane Simpson׳s method. The severity of mitral regurgitation (MR) was assessed from color-flow Doppler images in the apical four-chamber view. MR was classified as grade 1 (jet area/left atrium area <20%), grade 2 (jet area/left atrium area of 20–40%), or grade 3 (jet area/left atrium area >40%). Digital data were retrospectively analyzed by dedicated software (Ultra Extend, Toshiba Medical System, Tochigi, Japan) for an off-line analysis. Baseline LV dyssynchrony was evaluated by speckle-tracking radial strain from the mid-LV short-axis images. LV dyssynchrony was defined according to the time interval between the anteroseptal and posterior wall segmental peak strains [8,9]. Four patients who depended on ventricular pacing due to AV block were analyzed during RV pacing.
2.5. Statistical analysis
Continuous data are presented as the mean±SD. Differences between means were compared using the Student׳s t-test for Gaussian variables. For non-Gaussian variables, data were compared using the Mann–Whitney test for independent samples or the Wilcoxon nonparametric test for paired samples. Categorical data are presented as percentages, with differences compared using a chi-square analysis. Cumulative event proportions were calculated with a Kaplan–Meier analysis, and differences in patient outcomes were assessed by the log-rank test. P-values of less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS version 20.0 software (Chicago, IL, USA).
3. Results
3.1. Baseline characteristics
The study population included 18 men (62%). The mean patient age was 67±12 years. The cause of heart failure was ischemic in 9 (31%) patients and non-ischemic in 20 (69%) patients. Twenty-five (86%) patients were implanted with a CRT-D device. The mean QRS width was 164±38 ms, and a left bundle branch block (LBBB) was noted in 14 patients (48%). Six (20%) patients were upgraded to CRT from a pacemaker or implantable cardioverter-defibrillator (ICD). Only one patient had a QRS<120 ms, but a decision to implant a CRT device was made because echocardiography revealed LV dyssynchrony. Five (17%) patients presented with chronic AF and 10 (35%) presented with paroxysmal AF. Of these, seven (24%) patients were referred for AV node ablation to achieve control of the ventricular rate. Six (13%) patients required IABP to control their heart failure before implantation. At the time of implantation, all patients required some type of intravenous medication: 19 (65%) patients required inotropes, 18 (62%) required diuretics, and 13 (45%) required vasodilators. On the echocardiographic data, severe LV dilatation was observed in most patients [mean LVEDV of 195±77 mL and mean LVESV of 151±164 mL], with a mean LVEF of 23±6%. LVEF was below 35% in all patients. The MR grade was 1.5±1.1. Radial strain demonstrated substantial LV dyssynchrony in this population (240±94 ms). At the time of implantation, 48% were using beta-blockers and 58% were using angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. The baseline characteristics are summarized in Table 1. Table 2 shows a comparison of characteristics between the CRT and control groups. Age, blood pressure, and LVEF were comparable between the two groups. However, QRS width was significantly greater in the CRT group than in controls (164±38 ms vs. 103±23 ms; p<0.05).
Table 1.
Baseline characteristics.
| Total | 29 |
|---|---|
| Age, y | 67±12 |
| Male, n | 18(62%) |
| CRTD, n | 25(86%) |
| Upgrade, n | 6(20%) |
| Ischemic cardiomyopathy, n | 9(31%) |
| Systolic blood pressure, mmHg | 97±16 |
| Resting heart rate, bpm | 82±15 |
| Intravenous inotropic agents, n | 19(65%) |
| Intravenous vasodilator agents, n | 13(45%) |
| Prior IABP, n | 6(20%) |
| Chronic atrial fibrillation, n | 5(17%) |
| Paroxysmal atrial fibrillation, n | 10(35%) |
| AV node ablation, n | 7(24%) |
| LVEF, % | 23±6 |
| LVEDV, mL | 195±77 |
| LVESV, mL | 151±64 |
| LV radial dyssynchrony, ms | 240±94 |
| MR, degree | 1.8±1.1 |
| LBBB | 13(45%) |
| QRS width, ms | 164±33 |
| Medication, n | |
| β-blocker | 14(48%) |
| ACE-I/ARB | 17(58%) |
| Diuretics | 25(86%) |
| Spironolactone | 19(65%) |
| Digitalis | 5(17%) |
| Hemoglobin, g/dL | 11.0±2.0 |
| Creatinine >1.5 mg/dL, n | 12(42%) |
| Hemodialysis | 2(6%) |
| Sodium, mmol/L | 135±4 |
| Albumin, g/dL | 3.2±0.4 |
| BNP, pg/mL | 1299±808 |
CRTD, cardiac resynchronization therapy combined with an implantable defibrillator; IABP, intra-aortic balloon pumping; AV node ablation, atrio-ventricular node ablation; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MR, mitral regurgiation; LBBB, left bundle branch block; ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BNP, brain natriuretic peptide.
Table 2.
Baseline parameters of CRT patients and controls.
| CRT (n=29) | Control (n=21) | p-Value | |
|---|---|---|---|
| Age, y | 67±12 | 69±8 | 0.39 |
| Systolic BP, mmHg | 97±16 | 96±15 | 0.14 |
| Ischemic cardiomyopathy, n | 9 (31%) | 9 (43%) | 0.39 |
| QRS width, ms | 164±38 | 103±23 | <0.01 |
| LVEF, % | 23±6 | 25±5 | 0.12 |
BP, blood pressure; LVEF, left ventricular ejection fraction.
3.2. Outcomes
CRT implantation was performed successfully without any serious complications in all patients, with the exception of one patient who could not be implanted due to a venous anomaly, as mentioned above. After implantation, all patients showed improvements of both systolic and diastolic blood pressures (with a mean systolic blood pressure increase of 8±6 mmHg). It was possible to wean all patients from intravenous medications after implantation, although six (21%) patients died of heart failure in the hospital without being discharged.
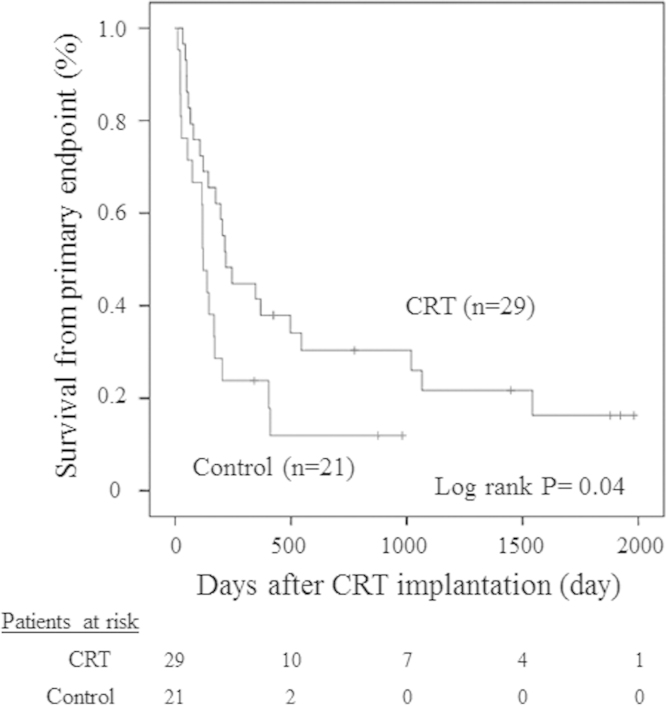
A post-implantation Kaplan–Meier curve is shown in Fig. 1. During follow-up, no patient received a cardiac transplantation or LVAD. Compared with controls, patients in the CRT group showed significant improvements in the primary endpoint (log-rank p=0.04). In the CRT group, 23 (79%) patients reached the primary endpoint and 17 (59%) patients died of cardiac causes. Two of these (7%) were identified as sudden deaths. One sudden death patient was implanted with a CRT-D device, and device interrogation revealed no life-threatening arrhythmias. The other patient was implanted with a CRT-P device, and the cause of his sudden death is unknown because we were unable to interrogate the device. Other causes of death were pneumonia, malignancy, systemic thrombosis, and pyogenic spondylitis.
Fig. 1.
Kaplan–Meier survival curves for all patients without death from worsening heart failure (HF) or hospitalization for deteriorating HF after cardiac resynchronization therapy (CRT). Compared with controls, patients in the CRT group showed significant improvements in the primary endpoint.
Device-related infections were observed in two (7%) patients. One case occurred 344 days after implantation. We removed the infected device and re-implanted a new one. Another case occurred 224 days after implantation. The infected device was removed but the patient was not re-implanted because he did not respond to bi-ventricular pacing. A dislodgement of the LV lead was observed in one (3%) patient who was re-implanted.
In the control group, 10 patients (48%) died of heart failure without discharge. Although the other 11 patients (52%) were discharged, five (24%) died of heart failure and two (10%) died of non-cardiac causes. There were significant fewer in-hospital deaths in the CRT group than in controls (20% vs. 48%, p<0.05).
3.3. Responder vs. non-responder
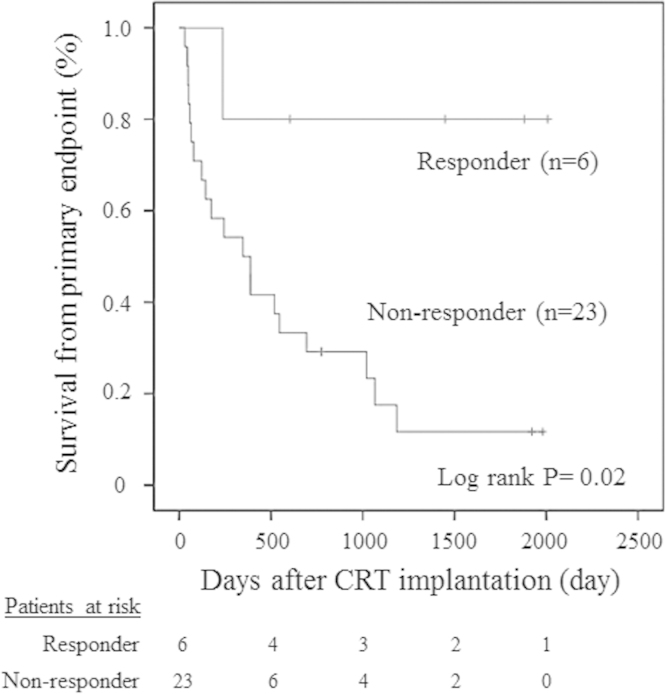
In accordance with the above criteria, six patients were classified as responders. The Kaplan–Meier curve showed a better outcome in responders than in non-responders (log-rank p=0.029; Fig. 2). A comparison of baseline clinical and echocardiographic characteristics between responders and non-responders is shown in Table 3. Responders had significantly greater LV dyssynchrony (341 ms vs. 218 ms, p=0.02) and higher albumin levels (3.6 vs. 3.2, p=0.03). The presence of LBBB and QRS width were not associated with response. In the responder group, two patients were implanted in the lateral vein and four were implanted in the posterolateral vein. No one was implanted in the anterolateral or posterior veins. As a result, there was no statistical relationship between the response to CRT and the LV lead position.
Fig. 2.
The Kaplan–Meier curve showed better outcomes in responders than in non-responders.
Table 3.
Baseline parameters of responders and non-responders.
| Responder (n=6) | Non-responder (n=23) | p-Value | |
|---|---|---|---|
| Age, y | 69±7 | 66±12 | 0.85 |
| Male, n | 4(67%) | 13(56%) | 0.55 |
| Ischemic cardiomyopathy, n | 3(50%) | 5(22%) | 0.14 |
| LBBB, n | 4(67%) | 9(39%) | 0.39 |
| QRS width, ms | 181±22 | 160±41 | 0.21 |
| Paroxysmal AF, n | 1(2%) | 8(35%) | 0.24 |
| Persistent AF, n | 0 | 5(22%) | – |
| Inotropes, n | 3(50%) | 14(61%) | 0.79 |
| Creatinine >1.5 mg/dL, n | 3(50%) | 7(30%) | 0.41 |
| Systolic BP, mmHg | 96±14 | 97±16 | 0.97 |
| LVEF, % | 22±5 | 23±6 | 0.44 |
| LVEDV, mL | 219±66 | 189±78 | 0.38 |
| LVESV, mL | 184±56 | 142±63 | 0.21 |
| MR, degree | 1.5±0.6 | 1.6±0.5 | 0.51 |
| LV dyssynchrony, ms | 341±48 | 206±80 | 0.02 |
| BNP, pg/mL | 714±358 | 1352±823 | 0.06 |
| Sodium, mmol/L | 135±3 | 135±5 | 0.98 |
| Hemoglobin, g/dL | 12.6±1.3 | 10.2±1.8 | 0.07 |
LBBB, left bundle branch block; AF, atrial fibrillation; BP, blood pressure; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MR, mitral regurgiation; BNP, brain natriuretic peptide.
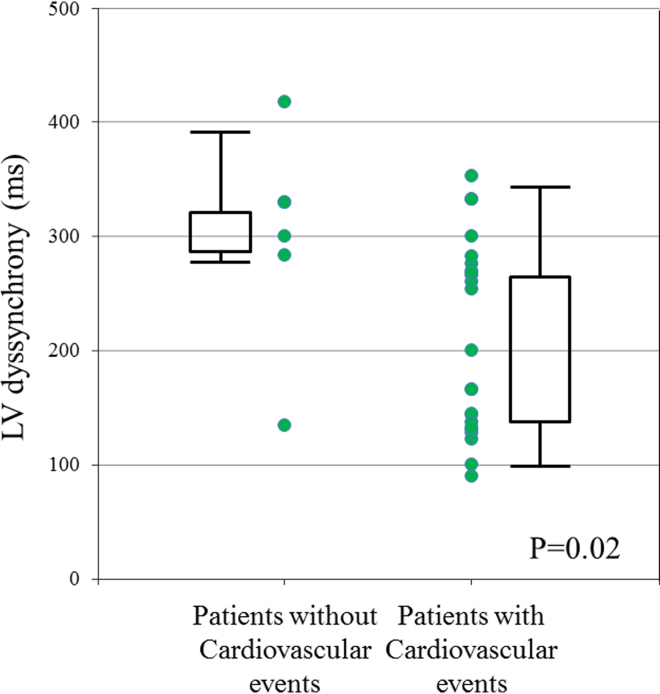
Fig. 3 shows the plot of LV dyssynchrony in patients with and without cardiovascular events. A total of 26 (89%) patients presented with LV dyssynchrony above 130 ms. All three patients with LV dyssynchrony below 130 ms reached the primary endpoint. Patients without cardiovascular events presented with significantly greater LV dyssynchrony than that in patients with events (p=0.02).
Fig. 3.
A plot of left ventricular (LV) dyssynchrony in patients with and without cardiovascular events. All patients with LV dyssynchrony below 130 ms reached the primary endpoint. Patients without cardiovascular events presented with significantly greater LV dyssynchrony than that in patients with events (p=0.02).
4. Discussion
The major findings of our study are as follows. First, a CRT device can be safely implanted in NAHF patients without any serious complications. Second, CRT reduces the number of in-hospital deaths without discharge. Third, CRT reduces mortality and hospitalization for heart failure in this severely afflicted population, especially if they are responders.
Previously, various small studies reported that CRT is an effective therapy in patients with NAHF [7,10–15]. These studies demonstrated favorable outcomes in patients treated with CRT, including the ability to wean patients from intravenous inotropes to oral drugs. Milliez et al. demonstrated an acute hemodynamic and biological improvement in 20 dobutamine-dependent patients with NAHF following CRT implantation [14]. A marked blood pressure elevation with a mean systolic blood pressure increase of 15 mmHg was observed in all patients. This led to a rapid improvement of urine output, biological markers of renal function, and BNP levels. Theodorakis et al. reported on long-term prognosis in 18 patients with NAHF who received CRT [15]. Sustained improvements in NYHA classification and 6-min walk distance were documented 855 days after implantation. The cumulative proportion of deaths and heart transplantations at 18 months was 18%. Echocardiographic data at 12 months post-implantation documented a significant reduction in LVESV (248 vs. 269 mL, p=0.03). Herweg et al. studied 10 patients with inotrope-dependent NAHF and reported improved outcomes [10]. These patients underwent CRT-D implantation with inotropic support, and all were alive 1088±284 days post-implantation including three patients who underwent cardiac transplantation. Our study results are essentially consistent with those of prior reports with regard to safety and long-term outcomes.
To the best of our knowledge, this is the first study to analyze factors predicting response in this severely afflicted population of patients with heart failure undergoing CRT. Our study shows that similar to other reports on conventional CRT indications, LV dyssynchrony is significantly related to response [8,9]. The STAR study was the first to associate radial strain LV dyssynchrony (>130 ms) with long-term survival after CRT. Although the STAR study included NYHA IV patients, it did not describe whether they were ambulatory. No trials have reported on the usefulness of LV dyssynchrony prior to CRT implantation in patients with NAHF. One case report described a 73-year-old man with a pacemaker who was admitted for refractory heart failure [16]. A speckle tracking strain identified severe LV dysfunction and LV dyssynchrony following RV pacing. As the patient׳s clinical condition worsened despite optimal medical treatment, a CRT device was implanted as rescue therapy. After implantation, the patient׳s symptoms rapidly improved and an echocardiographic assessment showed dramatic improvement in his LV systolic function. This case report suggested that an echocardiographic assessment of dyssynchrony is useful for deciding on a CRT indication in patients with refractory heart failure due to RV pacing. Established predictors of CRT response such as the presence of LBBB and QRS width are not associated with reverse remodeling. These outcomes may be the result of a selection bias, because the indication for CRT implantation was at the discretion of each physician and involved consideration of various factors including both the electrographic and echocardiographic status.
In 2004, Auricchio et al. stated, “CRT may be contraindicated in patients in whom weaning from parenteral inotropic therapy has not been possible” [17]. Our study showed the reverse in a broader population of severely ill patients, if they had significant LV dyssynchrony.
The present study had some limitations. First, it was not a randomized study and the sample size was small. Thus, additional studies are required to validate our findings in a large number of patients. Second, due to the retrospective nature of the study, many different echocardiographic recording systems were used to assess cardiac function. Although the same software was used to analyze speckle-tracking LV dyssynchrony, it may differ from previously reported data.
5. Conclusion
CRT can be safely used in patients with NAHF and can improve long-term patient outcomes, especially in treatment responders. LV dyssynchrony has the potential to predict better outcomes in this severely afflicted population.
Conflict of interest
The Section of Arrhythmia, Division of Cardiovascular Medicine, Kobe University Graduate school of Medicine is financially supported by Medtronic and St. Jude Medical.
References
- 1.Bristow M.R., Saxon L.A., Boehmer J. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J.G., Daubert J.C., Erdmann E. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 3.Lindenfeld J., Feldman A.M., Saxon L. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation. 2007;115:204–212. doi: 10.1161/CIRCULATIONAHA.106.629261. [DOI] [PubMed] [Google Scholar]
- 4.Epstein A.E., DiMarco J.P., Ellenbogen K.A. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F., Briancon S., Juilliere Y. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l׳Insuffisance Cardiaque Avancee en Lorraine. J Am Coll Cardiol. 1999;33:734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 6.Packer M., Coats A.J., Fowler M.B. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson L.W., Miller L.W., Desvigne-Nickens P. Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure) Circulation. 2004;110:975–981. doi: 10.1161/01.CIR.0000139862.48167.23. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H., Nesser H.J., Buck T. Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur Heart J. 2010;31:1690–1700. doi: 10.1093/eurheartj/ehq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mochizuki Y., Tanaka H., Tatsumi K. Easy-to-use comprehensive speckle-tracking approach for cardiac resynchronization therapy. Circ J. 2014;78:2250–2258. doi: 10.1253/circj.cj-14-0114. [DOI] [PubMed] [Google Scholar]
- 10.Herweg B., Ilercil A., Cutro R. Cardiac resynchronization therapy in patients with end-stage inotrope-dependent class IV heart failure. Am J Cardiol. 2007;100:90–93. doi: 10.1016/j.amjcard.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 11.Landolina M., Lunati M., Gasparini M. Comparison of the effects of cardiac resynchronization therapy in patients with class II versus class III and IV heart failure (from the InSync/InSync ICD Italian Registry) Am J Cardiol. 2007;100:1007–1012. doi: 10.1016/j.amjcard.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Zaeem F., Giedriemiene D., Coleman C. CRT-D therapy in patients with decompensated NYHA class-four CHF. Cardiol Res Pract. 2012:319205. doi: 10.1155/2012/319205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowburn P.J., Patel H., Jolliffe R.E. Cardiac resynchronization therapy: an option for inotrope-supported patients with end-stage heart failure? Eur J Heart Fail. 2005;7:215–217. doi: 10.1016/j.ejheart.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Milliez P., Thomas O., Haggui A. Cardiac resynchronisation as a rescue therapy in patients with catecholamine-dependent overt heart failure: results from a short and mid-term study. Eur J Heart Fail. 2008;10:291–297. doi: 10.1016/j.ejheart.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Theodorakis G., Katsikis A., Livanis E. Long term effects of cardiac resynchronization therapy in non-ambulatory NYHA IV heart failure patients. PACE. 2011;34:1553–1560. doi: 10.1111/j.1540-8159.2011.03205.x. [DOI] [PubMed] [Google Scholar]
- 16.Guiot A., Castel A.L., Guyomar Y. Cardiac resynchronization therapy as rescue therapy in end-stage heart failure: illustration by three-dimensional speckle tracking strain echocardiography. Echocardiography. 2012;29:264–266. doi: 10.1111/j.1540-8175.2012.01814.x. [DOI] [PubMed] [Google Scholar]
- 17.Auricchio A., Abraham W.T. Cardiac resynchronization therapy: current state of the art: cost versus benefit. Circulation. 2004;109:300–307. doi: 10.1161/01.CIR.0000115583.20268.E1. [DOI] [PubMed] [Google Scholar]