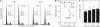Abstract
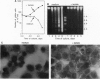
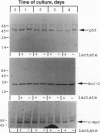
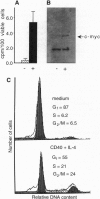
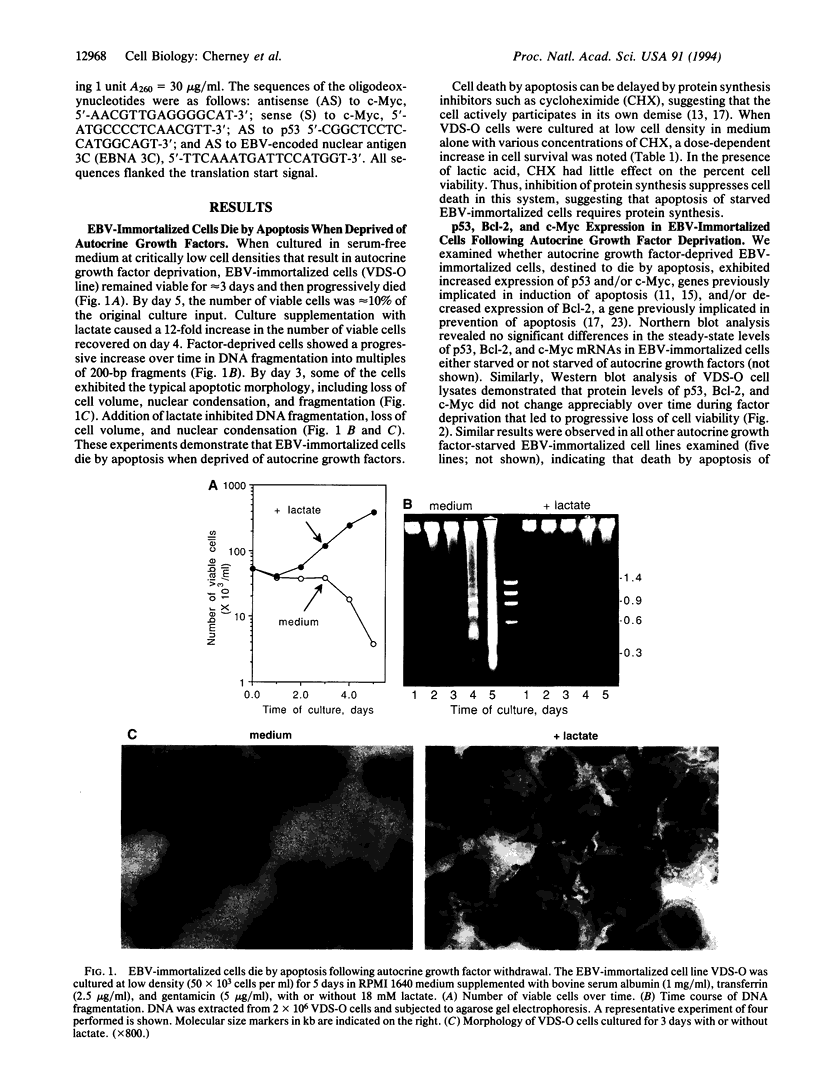
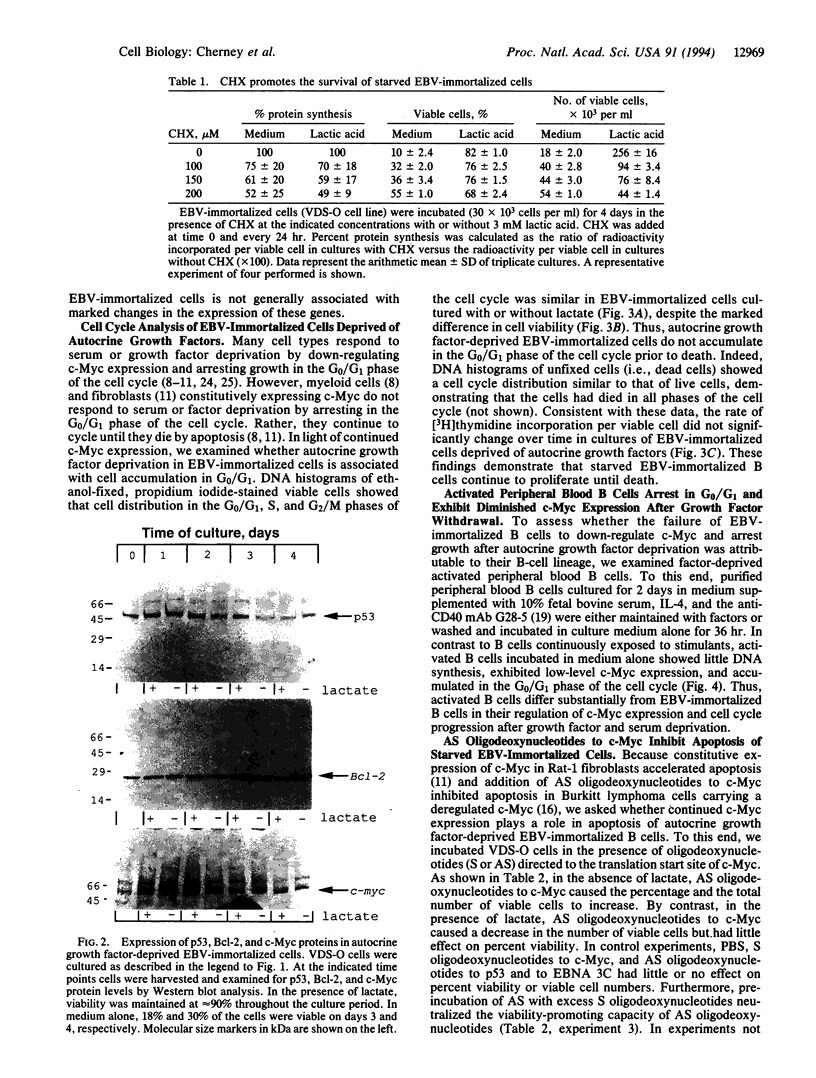
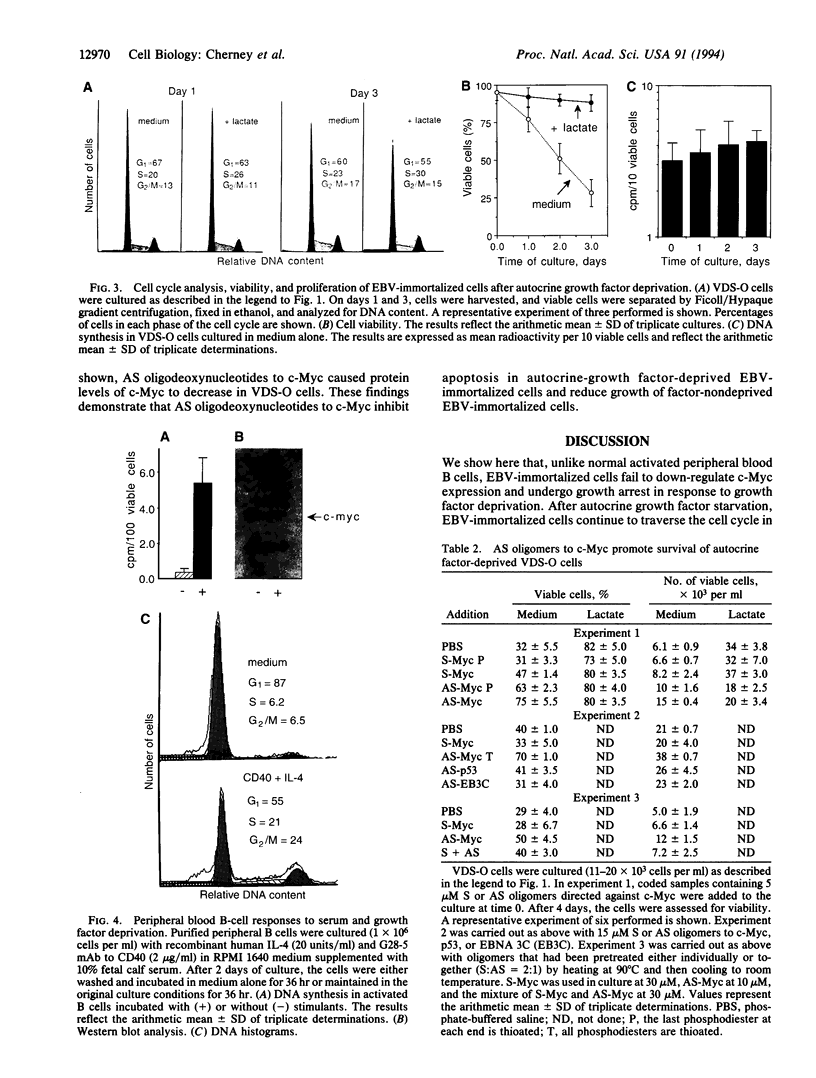
When deprived of autocrine growth factors, Epstein-Barr virus (EBV)-immortalized B cells stop growing and die. In this study, we show that death of EBV-immortalized cells deprived of autocrine growth factors occurred by apoptosis. Cycloheximide, a protein synthesis inhibitor, inhibited apoptosis, suggesting that de novo protein synthesis is required. Because p53, Bcl-2, and c-Myc were previously implicated in the induction or prevention of apoptosis in other systems, we assessed their possible involvement here. Unlike normal cells that respond to growth factor deprivation by down-regulating c-Myc expression, EBV-immortalized cells continued to express c-Myc, p53, and Bcl-2 at levels comparable to those measured prior to starvation. Consistent with data demonstrating that c-Myc expression is sufficient to drive quiescent cells into the cell cycle, autocrine growth factor-deprived EBV-immortalized cells did not undergo growth arrest but rather continued to proliferate until death, which occurred randomly throughout the cell cycle. In contrast to EBV-immortalized B cells, normal peripheral blood B cells activated in vitro with anti-CD40 monoclonal antibody and interleukin 4 rapidly down-regulated c-Myc expression and underwent growth arrest in response to growth factors and serum deprivation. These findings demonstrated that c-Myc expression is deregulated in EBV-immortalized cells. Addition of antisense oligonucleotides to c-Myc specifically promoted the survival of starved EBV-immortalized cells and suppressed growth of nonstarved EBV-immortalized cells. Thus, deregulated expression of c-Myc in EBV-immortalized cells promotes proliferation and apoptosis following autocrine growth factor deprivation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Macduff B. M., Spriggs M. K., Fanslow W. C. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol. 1993 May 1;150(9):3671–3680. [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Blazar B. A., Sutton L. M., Strome M. Self-stimulating growth factor production by B-cell lines derived from Burkitt's lymphomas and other lines transformed in vitro by Epstein-Barr virus. Cancer Res. 1983 Oct;43(10):4562–4568. [PubMed] [Google Scholar]
- Buja L. M., Eigenbrodt M. L., Eigenbrodt E. H. Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993 Dec;117(12):1208–1214. [PubMed] [Google Scholar]
- Colotta F., Polentarutti N., Sironi M., Mantovani A. Expression and involvement of c-fos and c-jun protooncogenes in programmed cell death induced by growth factor deprivation in lymphoid cell lines. J Biol Chem. 1992 Sep 15;267(26):18278–18283. [PubMed] [Google Scholar]
- Dean M., Levine R. A., Ran W., Kindy M. S., Sonenshein G. E., Campisi J. Regulation of c-myc transcription and mRNA abundance by serum growth factors and cell contact. J Biol Chem. 1986 Jul 15;261(20):9161–9166. [PubMed] [Google Scholar]
- Estrov Z., Kurzrock R., Pocsik E., Pathak S., Kantarjian H. M., Zipf T. F., Harris D., Talpaz M., Aggarwal B. B. Lymphotoxin is an autocrine growth factor for Epstein-Barr virus-infected B cell lines. J Exp Med. 1993 Mar 1;177(3):763–774. doi: 10.1084/jem.177.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Hotchin N. A., Allday M. J., Crawford D. H. Deregulated c-myc expression in Epstein-Barr-virus-immortalized B-cells induces altered growth properties and surface phenotype but not tumorigenicity. Int J Cancer. 1990 Mar 15;45(3):566–571. doi: 10.1002/ijc.2910450332. [DOI] [PubMed] [Google Scholar]
- Lacy J., Summers W. P., Summers W. C. Post-transcriptional mechanisms of deregulation of MYC following conversion of a human B cell line by Epstein-Barr virus. EMBO J. 1989 Jul;8(7):1973–1980. doi: 10.1002/j.1460-2075.1989.tb03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L., Newcomb E. W., Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987 Apr 24;49(2):161–170. doi: 10.1016/0092-8674(87)90556-3. [DOI] [PubMed] [Google Scholar]
- Masutani H., Nakamura H., Ueda Y., Kitaoka Y., Kawabe T., Iwata S., Mitsui A., Yodoi J. ADF (adult T cell leukemia-derived factor)/human thioredoxin and viral infection: possible new therapeutic approach. Adv Exp Med Biol. 1992;319:265–274. doi: 10.1007/978-1-4615-3434-1_27. [DOI] [PubMed] [Google Scholar]
- Milner A. E., Grand R. J., Waters C. M., Gregory C. D. Apoptosis in Burkitt lymphoma cells is driven by c-myc. Oncogene. 1993 Dec;8(12):3385–3391. [PubMed] [Google Scholar]
- Pike S. E., Markey S. P., Ijames C., Jones K. D., Tosato G. The role of lactic acid in autocrine B-cell growth stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11081–11085. doi: 10.1073/pnas.88.24.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramqvist T., Magnusson K. P., Wang Y., Szekely L., Klein G., Wiman K. G. Wild-type p53 induces apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene. 1993 Jun;8(6):1495–1500. [PubMed] [Google Scholar]
- Rasmussen A. M., Smeland E. B., Erikstein B. K., Caignault L., Funderud S. A new method for detachment of Dynabeads from positively selected B lymphocytes. J Immunol Methods. 1992 Feb 5;146(2):195–202. doi: 10.1016/0022-1759(92)90228-l. [DOI] [PubMed] [Google Scholar]
- Sabourin L. A., Hawley R. G. Suppression of programmed death and G1 arrest in B-cell hybridomas by interleukin-6 is not accompanied by altered expression of immediate early response genes. J Cell Physiol. 1990 Dec;145(3):564–574. doi: 10.1002/jcp.1041450325. [DOI] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga K., Cherney B., Tosato G. IL-10 inhibits apoptotic cell death in human T cells starved of IL-2. Int Immunol. 1993 Dec;5(12):1599–1608. doi: 10.1093/intimm/5.12.1599. [DOI] [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Weissman I. L. Neither macromolecular synthesis nor myc is required for cell death via the mechanism that can be controlled by Bcl-2. Mol Cell Biol. 1993 Nov;13(11):7000–7005. doi: 10.1128/mcb.13.11.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]