Abstract
BACKGROUND:
Immune cell infiltration associated with tumor capsule disruption and tumor budding has been shown to reflect invasiveness, metastasis, and unfavorable prognosis in colorectal cancer. We investigated the influence of tumor budding on prognosis and its association with the immune microenvironment in lung adenocarcinoma.
METHODS:
Tumor slides from resected stage I lung adenocarcinomas were reviewed (n = 524 and n = 514, for training and validation cohorts, respectively) for assessment of tumor budding. CD3+ and forkhead box P3+ (FoxP3+) lymphocytes, CD68+ macrophages, IL-7 receptor, and IL-12 receptor β2 were analyzed using tissue microarrays constructed from tumor and stroma. Probability of recurrence was calculated using the competing risks method.
RESULTS:
In the training cohort, risk of recurrence for high-grade tumor budding was higher than it was for low-grade tumor budding (32% vs 12%, P < .001), which was confirmed in the validation cohort (P = .005). Tumor budding stratified the risk of recurrence for acinar-predominant (22% vs 9%, P < .001), papillary-predominant (22% vs 13%, P = .045), and solid-predominant (39% vs 19%, P = .022) tumors. Tumor budding was associated with higher stromal FoxP3+ lymphocyte infiltration, higher stromal FoxP3/CD3 risk index, higher tumoral and stromal CD68+ macrophage infiltration, and IL-7 receptor overexpression (P < .001, all associations). Tumor budding remained independently associated with recurrence on multivariate analysis (hazard ratio, 1.61; P = .008).
CONCLUSIONS:
Tumor budding is an independent prognostic factor of stage I lung adenocarcinoma and correlates with the protumor immune microenvironment. Our findings advocate investigating tumor-immune cell interactions at the invading edge as a biologic driver of tumor aggressiveness.
Adenocarcinoma is the most common histologic type of lung cancer, and the rate of adenocarcinoma has increased during the last decade.1,2 Following results of previous randomized trials assessing low-dose CT screening for lung cancers,3‐5 it is anticipated that there will be an increase in the number of patients diagnosed with early-stage lung adenocarcinoma. The present TNM staging system is the most reliable prognostic tool for lung cancers.6 Additionally, prognostic significance of histologic subtypes—based on the 2011 International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) lung adenocarcinoma classification system7—has been validated in large, independent cohorts spanning multiple countries.8‐12 A limitation of the IASLC/ATS/ERS classification is that the majority (> 40%) of adenocarcinoma cases are classified as intermediate-grade, acinar or papillary subtype, and these patients also displayed a wide spectrum of clinical outcomes.8‐12 Thus, it would be helpful to identify significant prognostic factors that can further stratify patients within the intermediate-grade group. In this study, we investigated whether tumor budding can predict patient disease recurrence and can be used to further stratify patients with histologically intermediate-grade tumors into prognostic subgroups.
Tumor budding, which is defined as the presence of isolated small tumor nests (composed of < 5 tumor cells) in the stroma at the outer edge of the tumor, has been shown to reflect tumor invasive behavior and is an unfavorable prognostic factor of colorectal cancers.13,14 In their attempts to unravel the biologic significance of tumor budding, investigators have noted that tumor budding may be associated with epithelial mesenchymal transition, thereby increasing cancer cell migration and invasion.15‐18 In breast cancer, immune-induced responses have been shown to promote epithelial mesenchymal transition.19‐21 More importantly, studies have demonstrated that tumor-associated macrophages—especially those of the tumor-promoting M2 phenotype—are frequently found within regions of tumor budding22 and that they have contributed to induction of cancer cell epithelial mesenchymal transition at the tumor invasive front.23‐25
Prognostic significance of tumor budding and its correlation with immune factors have yet to be investigated in early-stage lung adenocarcinoma. We demonstrated that stromal forkhead box P3 (FoxP3)/CD3 lymphocyte risk index, tumoral IL-7 receptor (IL-7R) overexpression, and loss of tumoral IL-12 receptor β2 (IL-12Rβ2) expression were independent prognostic factors of stage I lung adenocarcinoma.26 In this study, we investigated whether tumor budding correlated with tumor-infiltrating immune cells (CD3+ or FoxP3+ lymphocytes and CD68+ macrophages) and immune markers (IL-7R and IL-12Rβ2).
Materials and Methods
Patients
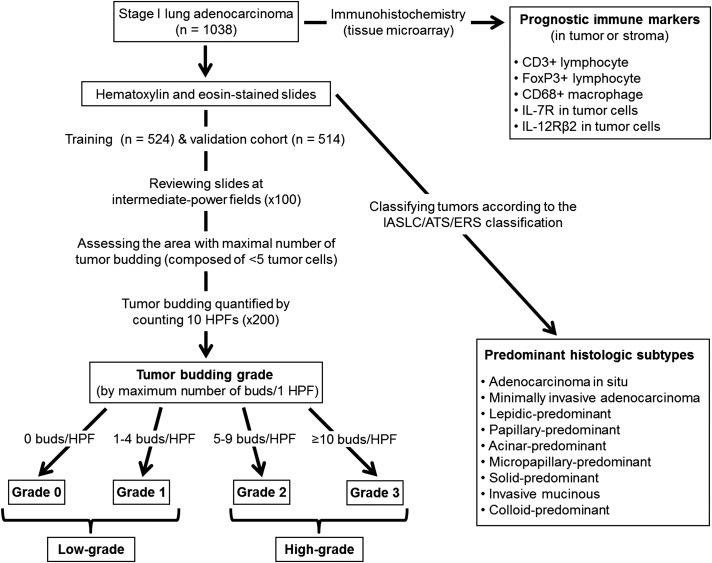
This retrospective study (WA0269-08) was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (MSK) and was designed in accordance with REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) guidelines (Fig 1).27 We reviewed patients with pathologic stage I solitary lung adenocarcinoma who had undergone surgical resection at MSK between 1995 and 2009. Tumor slides from 1,038 patients were available for histologic evaluation and were included in this study. Clinical data were collected from our prospectively maintained lung adenocarcinoma database. Of the 1,038 patients with available tumor slides, 585 were tested for EGFR and KRAS mutation status. Disease stage was assigned based of the seventh edition of the American Joint Committee on Cancer Staging Manual.28
Figure 1 –
Flowchart of the study design. We reviewed patients with pathologic stage I lung adenocarcinoma who had undergone surgical resection (N = 1,038). Tumors were classified according to the IASLC/ATS/ERS classification. Prognostic immune scores (CD3, FoxP3, CD68, IL-7R, and IL12-Rβ2), based on tissue microarray analysis, were dichotomized as low or high. To dichotomize tumor budding as low or high, the entire cohort was split into a training cohort (n = 524) and a validation cohort (n = 514). After reviewing tumor slides at intermediate-power fields at ×100 magnification, tumor budding was assessed at the area with the maximal number of smallest tumor nests composed of fewer than five tumor cells and was quantified by counting 10 HPFs at ×200 magnification. During evaluation using 10 HPFs, maximum number of tumor buds per one HPF was considered the number of buds for each tumor. Tumor budding was classified as grade 0 (zero buds per HPF), grade 1 (one to four), grade 2 (five to nine), or grade 3 (≥ 10). On the basis of our results, tumors were classified as having high-grade (grade 2-3) or low-grade (grade 0-1) tumor budding. FoxP3 = forkhead box P3; HPF = high-power field; IASLC/ATS/ERS = International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society; IL-7R = IL-7 receptor; IL-12Rβ2 = IL-12 receptor β2.
Histologic Evaluation
All available hematoxylin and eosin (H&E)-stained tumor slides were reviewed by two pathologists (K. K. and W. D. T.), both of whom were unaware of patients’ clinical outcomes, using an Olympus BX51 microscope (Olympus Corporation) with a standard 22-mm diameter eyepiece. Discrepancies in histologic evaluation between the pathologists were later resolved by consensus using a multihead microscope. Tumors were classified according to the IASLC/ATS/ERS classification7 and were grouped into three architectural grades on the basis of histologic subtype: (a) low-grade (adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant); (b) intermediate-grade (papillary-predominant and acinar-predominant); and (c) high-grade (micropapillary-predominant, solid-predominant, invasive mucinous, and colloid-predominant).29‐32
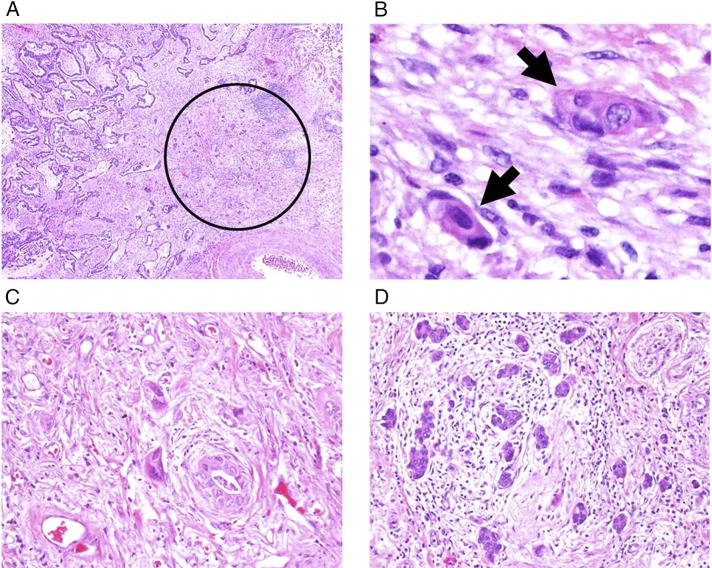
After reviewing the entire set of tumor slides at intermediate-power fields of ×100 magnification, we assessed tumor budding at the most invasive area with the maximal number of the smallest tumor nest (Fig 2A). Tumor budding was defined as small tumor nests composed of fewer than five tumor cells (Fig 2B) and was quantified by counting 10 high-power fields (HPFs) at ×200 magnification.13 During evaluation using 10 HPFs, the maximum number of tumor buds per one HPF was considered the number of buds for each tumor. We then classified tumor budding as grade 0 (zero buds per HPF), grade 1 (one to four) (Fig 2C), grade 2 (five to nine), or grade 3 (≥ 10) (Fig 2D).14,16,17
Figure 2 –
A-D, Histologic findings of tumor budding (original magnifications: A, ×40; B, ×400; C, D, ×200). A, Tumor budding identified in stroma of the invasive tumor edge (circle). B, Tumor budding defined as isolated small tumor nests composed of fewer than five tumor cells (arrows). C, Low-grade tumor budding. D, High-grade tumor budding.
Nuclear atypia was identified in the area with the highest degree of atypia and was graded as mild, moderate, or severe.29,33 Mitoses were evaluated using 50 HPFs at ×400 magnification (0.237 mm2 field) in areas with the highest mitotic activity and were counted as the average number of mitotic figures per 10 HPFs; they were classified as either low (zero to one mitotic figure per 10 HPFs), intermediate (two to four), or high (five or more).29,33 Visceral pleural invasion, lymphovascular invasion, and tumor necrosis were also investigated.
Immunohistochemical Analysis Using Tissue Microarrays
Prognostic immune scores via tissue microarray were obtained from our previous publication.26 Each tissue microarray core was scored for degree of immune cell infiltration in tumor and tumor-associated stroma: CD3+ lymphocyte (score 1 [positive cells/core < 50]; 2 [51-150]; 3 [> 150]), FoxP3+ lymphocyte (score 1 [< 20]; 2 [21-50]; 3 [> 50]), and CD68+ macrophage (score 1 [< 50]; 2 [51-100]; 3 [> 100]). Scores were averaged per patient and were defined by score 1 (average score, 1.0-1.67), 2 (1.67-2.33), 3 (> 2.33); scores 2 to 3 were regarded as high. Tumoral IL-7R was scored by intensity (0, no expression; 1, mild; 2, intermediate; 3, strong) and distribution (0, 0%; 1, < 50%; 2, ≥ 50%); scores ≥ 1 were regarded as positive. Tumoral IL-12Rβ2 was scored by intensity and scores ≥ 1 were regarded as positive.
Statistical Analysis
The cohort was split into training and validation sets (random 1:1 split, stratified by temporal interval of surgery as follows: 1995-2000, 2001-2003, and every 2-year intervals thereafter). Our primary objective was to investigate tumor budding as a predictor of recurrence. This was conducted in the training cohort and confirmed in the validation cohort. Next, we conducted secondary analysis, which was stratified by pathologic stage, surgical procedure, and histologic subtype, and analyzed the association between tumor budding and immune factors using the entire cohort.
Associations between variables were analyzed using the Pearson χ2 test (categorical variables) and the Wilcoxon signed-rank test (continuous variables). Cumulative incidence of recurrence (CIR) was estimated using a cumulative incidence function that accounted for death without recurrence as a competing event.34,35 Follow-up was calculated from date of surgery to date of any first recurrence, death from any cause, or last follow-up. Differences in CIR between groups were assessed using the Gray method (univariate nonparametric analysis) and the Fine-Gray competing risk model (multivariate analysis).35,36 Overall survival (OS) was estimated using the Kaplan-Meier method and nonparametric group comparisons were performed using the log-rank test.
The clinicopathologic factors significantly associated with either tumor budding or the outcome on univariate analysis were considered for inclusion on multivariate analysis. If two or more factors were strongly correlated with each other (ie, lymphatic, vascular and pleural invasion, nuclear atypia, and mitotic count) only one of them was included in the model.
All P values were based on two-tailed statistical analysis, and P < .05 was considered to indicate statistical significance. Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc) and R (version 3.0.1; R Development Core Team, The R Foundation), including the “survival” and “cmprsk” packages.
Results
Patient Clinicopathologic Characteristics
The median age of the 1,038 patients was 69 years (range, 23-96 years). Most patients were women (n = 646), and most had stage IA disease (n = 731). Regarding surgical procedures, 796 patients had undergone lobectomy, and 242 had undergone limited resection (segmentectomy [n = 76] and wedge resection [n = 166]). Only 14 patients (1%) received adjuvant chemotherapy.
During the study period, 14% of patients (n = 144) experienced recurrence (locoregional [n = 44] and distant [n = 100]) and 15% (n = 151) died of any cause without documented recurrence. The median follow-up period for patients who did not experience recurrence was 37.4 months (range, 0.3-160.0 months). Associations between clinicopathologic factors and disease recurrence on univariate analysis are summarized in Table 1.
TABLE 1 ] .
Clinicopathologic Associations Between Recurrence and Tumor Budding
| Characteristic | No. | 5-y CIR (%) | P Value | Tumor Budding, No. (%) | P Value | |
| Low-Grade | High-Grade | |||||
| All patients | 1,038 | 19 | … | 719 (69) | 319 (31) | … |
| Age, y | .94 | .075 | ||||
| ≤ 65 | 378 | 18 | 69a | 69a | ||
| > 65 | 660 | 16 | (23-96)b | (42-88)b | ||
| Sex | .003c | .026c | ||||
| Female | 646 | 13 | 464 (72) | 182 (28) | ||
| Male | 392 | 22 | 255 (65) | 137 (35) | ||
| Smoking status | .18 | .086 | ||||
| Never | 176 | 14 | 132 (75) | 44 (25) | ||
| Former/current | 862 | 17 | 587 (68) | 275 (32) | ||
| Surgery | < .001c | .059 | ||||
| Lobectomy | 796 | 14 | 539 (68) | 257 (32) | ||
| Limited resection | 242 | 25 | 180 (74) | 62 (26) | ||
| Pathologic stage | < .001c | < .001c | ||||
| IA | 731 | 13 | 563 (77) | 168 (23) | ||
| IB | 307 | 25 | 156 (51) | 151 (49) | ||
| Architectural grade | < .001c | < .001c | ||||
| Low | 139 | 5 | 125 (90) | 14 (10) | ||
| Intermediate | 650 | 14 | 449 (69) | 201 (31) | ||
| High | 249 | 28 | 145 (58) | 104 (42) | ||
| Visceral pleural invasion | < .001c | < .001c | ||||
| Absent | 866 | 14 | 652 (75) | 214 (25) | ||
| Present | 172 | 27 | 67 (39) | 105 (61) | ||
| Lymphatic invasion | < .001c | < .001c | ||||
| Absent | 707 | 12 | 566 (80) | 141 (20) | ||
| Present | 331 | 26 | 153 (46) | 178 (54) | ||
| Vascular invasion | < .001c | < .001c | ||||
| Absent | 778 | 12 | 630 (81) | 148 (19) | ||
| Present | 260 | 28 | 89 (34) | 171 (66) | ||
| Necrosis | < .001c | < .001c | ||||
| Absence | 869 | 13 | 645 (74) | 224 (26) | ||
| Presence | 169 | 33 | 74 (44) | 95 (56) | ||
| Nuclear atypia | < .001c | < .001c | ||||
| Mild | 451 | 12 | 391 (87) | 60 (13) | ||
| Moderate | 360 | 15 | 240 (67) | 120 (33) | ||
| Severe | 227 | 26 | 88 (39) | 139 (61) | ||
| Mitotic count | < .001c | < .001c | ||||
| Low | 522 | 9 | 1/10 HPFsa | 4/10 HPFsa | ||
| Intermediate | 216 | 19 | (0-76)b | (0-43)b | ||
| High | 300 | 26 | ||||
CIR = cumulative incidence of recurrence; HPF = high-power field.
Median.
Range.
Significant P values.
Tumor Budding and Risk of Recurrence/OS
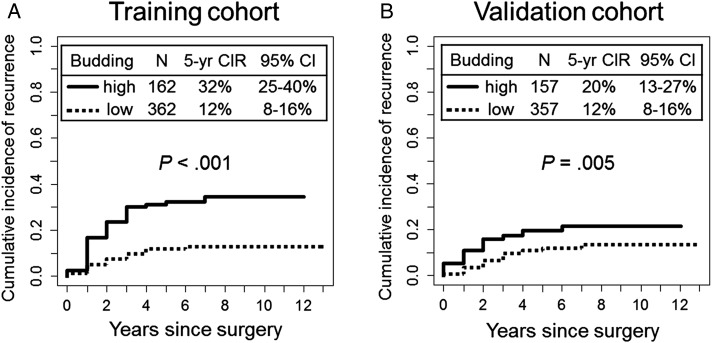
In the training cohort, 5-year CIR for patients with grade 2 and grade 3 tumor budding (5-year CIR, 32% and 33%, respectively) was higher than it was for patients with grade 0 and grade 1 tumor budding (5-year CIR, 9% and 19%, respectively). On the basis of this result, tumors were classified as having high-grade (grade 2-3 [five or more buds per HPF]) or low-grade (grade 0-1 [fewer than five buds per HPF]) tumor budding. The 5-year CIR for patients with high-grade tumor budding was significantly higher (32%) than it was for patients with low-grade budding (12%, P < .001) (Fig 3A). This finding was confirmed in the validation cohort; 5-year CIR for patients with high-grade tumor budding was significantly higher (20%) than it was for patients with low-grade budding (12%, P = .005) (Fig 3B).
Figure 3 –
A, B, CIR analysis of tumor budding. A, In the training cohort, 5-y CIR for patients with high-grade tumor budding was significantly higher (32%) than it was for patients with low-grade budding (12%; P < .001). B, In the validation cohort, 5-y CIR for patients with high-grade tumor budding was significantly higher (20%) than it was for patients with low-grade budding (12%; P = .005). CIR = cumulative incidence of recurrence.
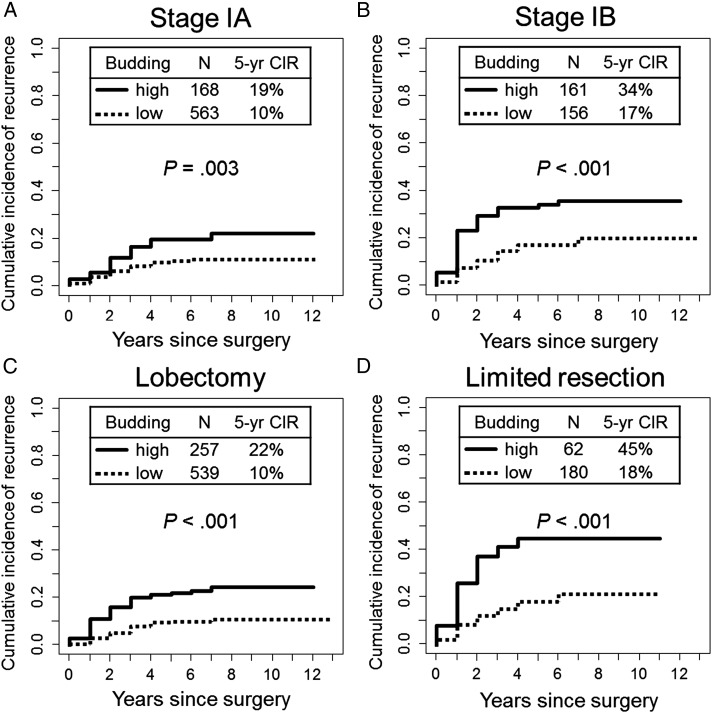
Next, subgroup analysis of CIR was performed using the entire cohort. In subgroup analysis by stage, 5-year CIR for patients with high-grade tumor budding was significantly higher than it was for patients with low-grade tumor budding among patients with stage IA (5-year CIR, 19% vs 10%; P = .003) (Fig 4A) and stage IB (5-year CIR, 34% vs 17%; P < .001) (Fig 4B) disease. In subgroup analysis by surgical procedure, 5-year CIR for patients with high-grade tumor budding was significantly higher than it was for patients with low-grade tumor budding among patients who had undergone lobectomy (5-year CIR, 22% vs 10%; P < .001) (Fig 4C) and limited resection (5-year CIR, 45% vs 18%; P < .001) (Fig 4D). Furthermore, in subgroup analysis by predominant subtype, 5-year CIR for patients with high-grade tumor budding was significantly higher than it was for patients with low-grade budding among patients with acinar-predominant tumors (5-year CIR, 22% vs 9%; P < .001), papillary-predominant tumors (5-year CIR, 22% vs 13%; P = .045), and solid-predominant tumors (5-year CIR, 39% vs 19%; P = .022) (Table 2). On multivariate analysis, high-grade tumor budding remained independently associated with risk of recurrence (hazard ratio, 1.61; 95% CI, 1.13-2.29; P = .008) (Table 3).
Figure 4 –
A-D, Subgroup analysis of CIR analysis of tumor budding by stage and surgical procedure. A, In patients with stage IA disease, 5-y CIR for patients with high-grade tumor budding was significantly higher (19%) than it was for patients with low-grade budding (10%; P = .003). B, In patients with stage IB disease, 5-y CIR for patients with high-grade tumor budding was significantly higher (34%) than it was for patients with low-grade budding (17%; P < .001). C, In patients undergoing lobectomy, 5-y CIR for patients with high-grade tumor budding was significantly higher (22%) than it was for patients with low-grade budding (10%; P < .001). (D) In patients undergoing limited resection, 5-y CIR for patients with high-grade tumor budding was significantly higher (45%) than it was for patients with low-grade budding (18%; P < .001). See Figure 3 legend for expansion of abbreviation.
TABLE 2 ] .
Association Between Tumor Budding and Recurrence in Each Histologic Subtype
| Histologic Subtype | Tumor Budding Grade | No. | 5-y CIR, % | P Value |
| Lepidic subtype | Low | 89 | 4 | .70 |
| High | 14 | 13 | … | |
| Acinar subtype | Low | 271 | 9 | < .001a |
| High | 140 | 22 | … | |
| Papillary subtype | Low | 178 | 13 | .045a |
| High | 61 | 22 | … | |
| Micropapillary subtype | Low | 35 | 36 | .86 |
| High | 25 | 30 | … | |
| Solid subtype | Low | 64 | 19 | .022a |
| High | 72 | 39 | … | |
| Invasive mucinous | Low | 38 | 14 | .073 |
| High | 6 | 50 | … |
See Table 1 legend for expansion of abbreviation.
Significant P values.
TABLE 3 ] .
Multivariate Analysis for CIR
| Variables | Hazard Ratio | 95% CI | P Value |
| Sex | |||
| Male vs female | 1.48 | 1.06-2.06 | .022 |
| Surgery | |||
| Limited resection vs lobectomy | 3.00 | 2.11-4.27 | < .001 |
| Pathologic stage | |||
| IB vs IA | 1.80 | 1.27-2.55 | .001 |
| Architectural grade | |||
| Intermediate vs high | 0.50 | 0.35-0.70 | < .001 |
| Low vs high | 0.23 | 0.08-0.64 | .005 |
| Lymphatic invasion | |||
| Positive vs negative | 1.34 | 0.92-1.95 | .13 |
| Necrosis | |||
| Positive vs negative | 1.13 | 1.04-1.22 | .004 |
| Mitotic count | |||
| Intermediate vs low | 1.16 | 0.70-1.92 | .57 |
| High vs low | 1.44 | 0.92-2.25 | .11 |
| Tumor budding | |||
| High vs low | 1.61 | 1.13-2.29 | .008 |
See Table 1 legend for expansion of abbreviation.
Tumor budding also correlated with mortality; 5-year OS of patients with high-grade tumor budding was significantly lower (64%) than it was for patients with low-grade tumor budding (76%) in the entire cohort (P < .001).
Tumor Budding and Clinicopathologic Characteristics
Tumor budding was positively associated with male sex (P = .026), pathologic stage (P < .001), architectural grade (P < .001), visceral pleural invasion (P < .001), lymphatic invasion (P < .001), vascular invasion (P < .001), tumor necrosis (P < .001), nuclear atypia (P < .001), and mitotic count (P < .001) (Table 1). High-grade tumor budding was more frequently identified in KRAS wild-type tumors (29%) than in KRAS mutated tumors (20%; P = .038). However, tumor budding was not associated with EGFR mutation.
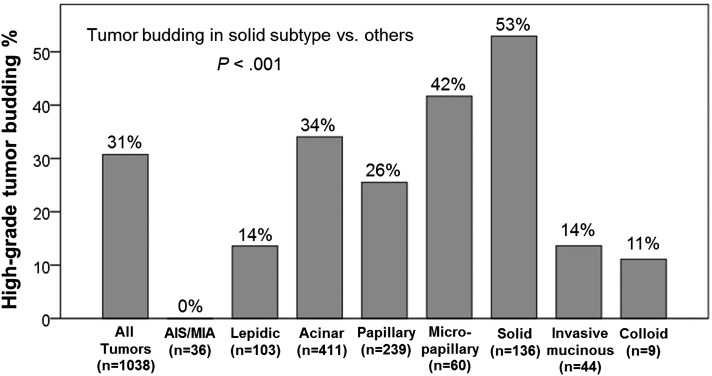
Figure 5 shows the percentage of tumors with high-grade tumor budding by histologic subtype. High-grade tumor budding was most frequently identified in solid-predominant tumors (53%), followed by micropapillary-predominant (42%) and acinar-predominant (34%) tumors. The rate of high-grade tumor budding was significantly higher in solid-predominant tumors than in tumors of other subtypes (P < .001). Micropapillary-predominant tumors also exhibited more frequent high-grade tumor budding than other tumors; however, this finding was not statistically significant (P = .062).
Figure 5 –
Association between tumor budding and histologic subtype. High-grade tumor budding was most frequently identified in solid-predominant tumors (53%), followed by micropapillary-predominant (42%), and acinar-predominant (34%) tumors. Rate of high-grade tumor budding was significantly higher in solid-predominant tumors than in tumors of other subtypes (P < .001). AIS = adenocarcinoma in situ; MIA = minimally invasive adenocarcinoma.
Tumor Budding and Immune Markers
High-grade tumor budding was significantly associated with high stromal CD3+ lymphocyte infiltration (P < .001), high stromal FoxP3+ lymphocyte infiltration (P < .001), high stromal FoxP3/CD3 risk index (P < .001), tumoral and stromal CD68+ macrophage infiltration (P < .001 and P < .001, respectively), and tumoral IL-7R overexpression (P < .001) (Table 4). However, there was no association between tumor budding and tumoral CD3+ lymphocyte infiltration (P = .66), tumoral FoxP3+ lymphocyte infiltration (P = .37), tumoral FoxP3/CD3 risk index (P = .79), and tumoral IL-12Rβ2 expression (P = 1.00).
TABLE 4 ] .
Association Between Tumor Budding and Immune Markers
| Immune Markers | Tumor Budding, No. (%) | P Value | |
| Low-Grade | High-Grade | ||
| Tumoral CD3+ lymphocyte | .66 | ||
| Low | 352 (70) | 154 (30) | |
| High | 274 (68) | 129 (32) | |
| Stromal CD3+ lymphocyte | < .001a | ||
| Low | 448 (73) | 167(27) | |
| High | 170 (59) | 118 (41) | |
| Tumoral FoxP3+ lymphocyte | .37 | ||
| Low | 309 (71) | 128 (29) | |
| High | 315 (68) | 150 (32) | |
| Stromal FoxP3+ lymphocyte | < .001a | ||
| Low | 426 (74) | 153 (26) | |
| High | 185 (59) | 127 (41) | |
| Tumoral FoxP3/CD3 risk index | .79 | ||
| Low | 362 (69) | 159 (31) | |
| High | 258 (68) | 119 (32) | |
| Stromal FoxP3/CD3 risk index | < .001a | ||
| Low | 459 (71) | 183 (29) | |
| High | 136 (59) | 96 (41) | |
| Tumoral CD68 macrophage | < .001a | ||
| Low | 194 (81) | 46 (19) | |
| High | 388 (64) | 222 (36) | |
| Stromal CD68 macrophage | < .001a | ||
| Low | 231 (80) | 58 (20) | |
| High | 305 (61) | 191 (39) | |
| Tumoral IL-7R | < .001a | ||
| Low | 411 (77) | 126 (23) | |
| High | 215 (58) | 157 (42) | |
| Tumoral IL-12Rβ2 | .99 | ||
| Low | 475 (69) | 214 (31) | |
| High | 151 (69) | 68 (31) | |
FoxP3 = forkhead box P3; IL-7R = IL-7 receptor; IL-12Rβ2 = IL-12 receptor β2.
Significant P values.
Discussion
We have demonstrated that, in patients with stage I lung adenocarcinoma, tumor budding was an independent prognostic factor that can be used to further stratify risk of recurrence in patients with stage IA, stage IB, acinar-predominant, papillary-predominant, and solid-predominant tumors. Furthermore, it positively correlated with protumor immune cell infiltration, including regulatory T cells and tumor-associated macrophages.
In an attempt to identify prognostic factors for intermediate-grade (acinar- or papillary-predominant) lung adenocarcinomas, our group previously demonstrated, using the largest cohort of stage I lung adenocarcinoma patients to date, that mitotic count,29 cribriform pattern,30 thyroid transcription factor-1,31 and 18F-fluorodeoxyglucose uptake on PET scan32 can be used to further stratify intermediate-grade tumors into two subsets with respect to disease recurrence. In the present study, we have shown that tumor budding can stratify both patients with intermediate-grade tumors and patients with solid-predominant tumors by risk of recurrence. Among patients with solid-predominant tumors, 5-year CIR for those with high-grade tumor budding (39%) was twice as high as it was for patients with low-grade tumor budding (19%). Although the solid-predominant subtype is considered a poor prognostic factor of lung adenocarcinoma, tumor budding may help identify a subgroup of patients whose risk of recurrence is similar to patients with intermediate-grade tumors. This observation will be useful in investigations that target the underlying biology of aggressiveness in solid-predominant tumors. We found that invasive mucinous and colloid-predominant tumors had tumor budding at a less frequent rate, despite the relatively aggressive behavior of these tumors, thereby suggesting that there may be a different mechanism that drives aggressiveness. Previous studies have demonstrated positive correlations between EGFR mutation and histologic subtypes (especially lepidic pattern).9,11,37 However, we found no association between EGFR mutation status and tumor budding.
With an expected increase in the number of diagnosed stage I lung adenocarcinomas, the role of limited resection vs lobectomy is debated. As limited resection for small lung tumors is associated with higher recurrences—25% in this series—factors that can predict higher rates of recurrence can help identify patients who may benefit from lobectomy. We showed that presence of a micropapillary pattern is a significant prognostic factor in patients who had undergone limited resection.38 Additionally, our current study has demonstrated that tumor budding can be used to select a group of patients who are, on average, at an increased risk of recurrence (5-year CIR, 45% vs 18%).
We found that high-grade tumor budding was positively associated with protumor immune markers previously identified by our group—stromal FoxP3+ lymphocyte infiltration, stromal FoxP3/CD3 lymphocyte index, and tumoral IL-7R expression.26 FoxP3 is a marker of regulatory T lymphocytes, a subset of lymphocytes known to suppress the host immune response, which may play a significant protumor role in the cancer immune microenvironment.39,40 FoxP3+ T cells were abundant in the tumor budding region and positively correlated with tumor invasiveness, including lymphovascular and perineural invasion, thereby suggesting that FoxP3+ regulatory T cells may promote tumor invasiveness.22,41,42 Additionally, we investigated associations between tumor budding and tumor-associated macrophages and found that high-grade tumor budding was positively associated with CD68+ macrophage infiltration in tumor nests and tumor-related stroma. Studies have suggested that tumor-infiltrating immune cells disrupt intercellular junctions and cell-surface adhesion molecules of cancer cells, thereby causing destruction of the tumor capsule and activation of tumor dissemination.43,44 In particular, it has been reported that tumor-associated macrophages were found at significant concentrations within the region of tumor budding at the tumor invasive front22 and that they promoted cancer cell invasiveness via epithelial mesenchymal transition.23‐25
We did not perform colocalization studies on the same slide using immunofluorescent techniques, which was a limitation of our study. However, our prospective investigation of the invasive tumor edge and associated immune cells may help us better understand biologic mechanisms that underlie this association. Interestingly, studies in colorectal cancers have suggested that cytokeratin immunohistochemistry can detect a higher percentage of positive tumor budding cases than using H&E-stained slides alone.45,46 Further studies are warranted to investigate whether cytokeratin immunohistochemistry can be a more reliable method than an H&E-stained section in detecting tumor budding in lung adenocarcinomas.
Conclusions
In conclusion, we have demonstrated that tumor budding was an independent, unfavorable prognostic factor for disease recurrence in patients with resected stage I lung adenocarcinoma. Moreover, tumor budding positively correlated with protumor immune markers—stromal FoxP3+ lymphocyte infiltration, stromal FoxP3/CD3 lymphocyte index, and tumoral IL-7R expression—and tumor-associated macrophage infiltration. These observations may warrant investigations into interactions between FoxP3+ regulatory T cells, the IL-7/IL-7R signaling axis, tumor-associated macrophages, and tumor invasiveness. Furthermore, these findings may carry potential implications for immunomodulatory therapies designed to control regulatory T cells and tumor-associated macrophages and to suppress aggressive patterns of tumor invasion, such as tumor budding.
Acknowledgments
Author contributions: W. D. T. and P. S. A. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. K. K., W. D. T., and P. S. A. contributed to study design, data acquisition, analysis, and interpretation; Y.-C. Y., J. V.-V., L. C., and D. R. J. contributed to data acquisition, analysis, and interpretation; E. N. D. and C. S. S. contributed to data analysis and interpretation; and K. K., Y.-C. Y., J. V.-V., L. C., E. N. D., C. S. S., D. R. J., W. D. T., and P. S. A. contributed to the drafting, revising, and review of the manuscript for important intellectual content and final approval of the version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Joe Dycoco, BA, of the MSK Thoracic Surgery Service for assisting with the MSK Thoracic Surgery Service’s Lung Cancer Database; Irina Linkov, PhD, of the MSK Pathology Core Facility for her technical assistance with immunohistochemistry; and David Sewell, MA, MFA, and Alex Torres, MS, of the MSK Thoracic Surgery Service for their editorial assistance.
ABBREVIATIONS
- CIR
cumulative incidence of recurrence
- FoxP3
forkhead box P3
- H&E
hematoxylin and eosin
- HPF
high-power field
- IASLC/ATS/ERS
International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society
- IL-7R
IL-7 receptor
- IL-12Rβ2
IL-12 receptor β2
- MSK
Memorial Sloan Kettering Cancer Center
- OS
overall survival
Footnotes
Preliminary data from this study were presented at the World Conference on Lung Cancer, October 27-31, 2013, Sydney, NSW, Australia.
FUNDING/SUPPORT: This work was supported by grants from the National Institutes of Health [R21 CA164568-01A1, R21 CA164585-01A1, R01 CA136705-06, U54 CA137788, P50 CA086438-13, and P30 CA008748], the US Department of Defense [PR101053 and LC110202], Mr William H. Goodwin and Mrs Alice Goodwin, the Commonwealth Foundation for Cancer Research, and the Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117(2):294-299. [DOI] [PubMed] [Google Scholar]
- 2.Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team, Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer. 2007;120(4):868-874. [DOI] [PubMed] [Google Scholar]
- 6.Goldstraw P, Crowley J, Chansky K, et al. ; International Association for the Study of Lung Cancer International Staging Committee; Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706-714. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653-664. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8(1):52-61. [DOI] [PubMed] [Google Scholar]
- 10.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30(13):1438-1446. [DOI] [PubMed] [Google Scholar]
- 11.Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer. 2013;81(3):371-376. [DOI] [PubMed] [Google Scholar]
- 12.Mansuet-Lupo A, Bobbio A, Blons H, et al. The new histologic classification of lung primary adenocarcinoma subtypes is a reliable prognostic marker and identifies tumors with different mutation status: the experience of a French cohort. Chest. 2014;146(3):633-643. [DOI] [PubMed] [Google Scholar]
- 13.Karamitopoulou E, Zlobec I, Kölzer V, et al. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol. 2013;26(2):295-301. [DOI] [PubMed] [Google Scholar]
- 14.Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25(10):1315-1325. [DOI] [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taira T, Ishii G, Nagai K, et al. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer. 2012;76(3):423-430. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi Y, Ishii G, Kojima M, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol. 2010;5(9):1361-1368. [DOI] [PubMed] [Google Scholar]
- 18.Koga K, Hamasaki M, Kato F, et al. Association of c-Met phosphorylation with micropapillary pattern and small cluster invasion in pT1-size lung adenocarcinoma. Lung Cancer. 2013;82(3):413-419. [DOI] [PubMed] [Google Scholar]
- 19.Santisteban M, Reiman JM, Asiedu MK, et al. Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells. Cancer Res. 2009;69(7):2887-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011;71(13):4707-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiman JM, Knutson KL, Radisky DC. Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res. 2010;70(8):3005-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlobec I, Minoo P, Terracciano L, Baker K, Lugli A. Characterization of the immunological microenvironment of tumour buds and its impact on prognosis in mismatch repair-proficient and -deficient colorectal cancers. Histopathology. 2011;59(3):482-495. [DOI] [PubMed] [Google Scholar]
- 23.Su S, Liu Q, Chen J, et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell. 2014;25(5):605-620. [DOI] [PubMed] [Google Scholar]
- 24.Helm O, Held-Feindt J, Grage-Griebenow E, et al. Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer. 2014;135(4):843-861. [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Xu JY, Shi XY, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93(7):844-854. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K, Kadota K, Sima CS, et al. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor β2 (IL-12Rβ2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31(4):490-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol. 2005;2(8):416-422. [PubMed] [Google Scholar]
- 28.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009:253-270. [Google Scholar]
- 29.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27(5):690-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadota K, Nitadori J, Sarkaria IS, et al. Thyroid transcription factor-1 expression is an independent predictor of recurrence and correlates with the IASLC/ATS/ERS histologic classification in patients with stage I lung adenocarcinoma. Cancer. 2013;119(5):931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kadota K, Colovos C, Suzuki K, et al. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann Surg Oncol. 2012;19(11):3598-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol. 2012;25(2):260-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chappell R. Competing risk analyses: how are they different and why should you care? Clin Cancer Res. 2012;18(8):2127-2129. [DOI] [PubMed] [Google Scholar]
- 35.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141-1154. [Google Scholar]
- 37.Kadota K, Yeh YC, D’Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38(8):1118-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105(16):1212-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71(22):6915-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaput N, Louafi S, Bardier A, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58(4):520-529. [DOI] [PubMed] [Google Scholar]
- 42.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. Eur J Immunol. 2013;43(6):1518-1528. [DOI] [PubMed] [Google Scholar]
- 43.Jiang B, Mason J, Jewett A, et al. Tumor-infiltrating immune cells: triggers for tumor capsule disruption and tumor progression? Int J Med Sci. 2013;10(5):475-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Man YG, Mason J, Harley R, Kim YH, Zhu K, Gardner WA. Leukocyte-mediated cell dissemination and metastasis: findings from multiple types of human tumors. J Cell Biochem. 2011;112(4):1154-1167. [DOI] [PubMed] [Google Scholar]
- 45.Satoh K, Nimura S, Aoki M, et al. Tumor budding in colorectal carcinoma assessed by cytokeratin immunostaining and budding areas: possible involvement of c-Met. Cancer Sci. 2014;105(11):1487-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puppa G, Senore C, Sheahan K, et al. Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology. 2012;61(4):562-575. [DOI] [PubMed] [Google Scholar]