Abstract
BACKGROUND:
Malignant pleural effusion is a common complication of advanced malignancies. Indwelling tunneled pleural catheter (IPC) placement provides effective palliation but can be associated with complications, including infection. In particular, hematologic malignancy and the associated immunosuppressive treatment regimens may increase infectious complications. This study aimed to review outcomes in patients with hematologic malignancy undergoing IPC placement.
METHODS:
A retrospective multicenter study of IPCs placed in patients with hematologic malignancy from January 2009 to December 2013 was performed. Inclusion criteria were recurrent, symptomatic pleural effusion and an underlying diagnosis of hematologic malignancy. Records were reviewed for patient demographics, operative reports, and pathology, cytology, and microbiology reports.
RESULTS:
Ninety-one patients (mean ± SD age, 65.4 ± 15.4 years) were identified from eight institutions. The mean × SD in situ dwell time of all catheters was 89.9 ± 127.1 days (total, 8,160 catheter-days). Seven infectious complications were identified, all of the pleural space. All patients were admitted to the hospital for treatment, with four requiring additional pleural procedures. Two patients died of septic shock related to pleural infection.
CONCLUSIONS:
We present, to our knowledge, the largest study examining clinical outcomes related to IPC placement in patients with hematologic malignancy. An overall 7.7% infection risk and 2.2% mortality were identified, similar to previously reported studies, despite the significant immunosuppression and pancytopenia often present in this population. IPC placement appears to remain a reasonable clinical option for patients with recurrent pleural effusions related to hematologic malignancy.
Malignant pleural effusion (MPE) is a common complication of advanced malignancies and often associated with decreased quality of life and morbidity.1,2 Advances in pleural palliation have led to the rather widespread adoption of the indwelling tunneled pleural catheter (IPC) as a tool for managing symptomatic, recurrent MPEs. This includes current British Thoracic Society guidelines, which offer a grade B recommendation for IPC placement as “effective in controlling recurrent and symptomatic malignant effusions in select patients.”3 IPC use has gained additional momentum after a randomized controlled trial demonstrated no significant difference in dyspnea scores at 6 weeks when comparing IPC with standard talc slurry.4 Although only a small number of patients experienced significant adverse events, the overall incidence of infectious complications was approximately 25% in the IPC arm compared with 4% in the talc slurry arm.4 Reports have identified IPC infection risks ranging from 4% to 10% in varying cohorts, including a large, international multicenter review5; in patients actively receiving chemotherapy6,7; and in an original single-center large series reporting on IPC placement.8
All these studies included rather heterogeneous etiologies of MPE, with most including some patients with hematologic malignancies. Patients with underlying hematologic malignancies may be at increased risk for complications related to common chemotherapeutic agents and underlying tumor biology.9‐11 To our knowledge, however, no study to date has focused specifically on this patient population, especially regarding the role of IPC placement for pleural palliation. In one large study, the incidence of MPEs related to hematologic malignancy was 20%12; however, little detail regarding their specific management or outcomes were offered. The aim of the current study was to review the clinical characteristics and outcomes of patients with hematologic malignancies undergoing IPC placement with a focus on pleurodesis and infectious complication rates.
Materials and Methods
A multiinstitution retrospective study of all pleural procedures resulting in IPC placement was performed at The Johns Hopkins Medical Institution, the Mayo Clinic, Duke University Medical Center, Swedish Cancer Institute, Hospital of the University of Pennsylvania, Virginia Commonwealth University Medical Center, Emory University, and Penn State-Milton S. Hershey Medical Center from January 2009 to December 2013. The institutional review boards of all centers approved this study (The Johns Hopkins Medical Institution, NA_00074178; Mayo Clinic, 13-009160; Duke University Medical Center, 00021763; Swedish Cancer Institute, 5566S-14; Hospital of the University of Pennsylvania, 819939; Virginia Commonwealth University Medical Center, HM20001126; Emory University Hospital, IRB00072194; Penn State Hershey-Milton S. Medical Center, 44826EM), and the requirement for informed consent for data collection and analysis was waived. Each patient underwent standard procedural consent for IPC placement per institutional practices and guidelines.
Data Collection
IPC placement was identified from patient databases using Current Procedural Terminology coding records and cross-referenced with operative reports. Inclusion criteria were placement of an IPC for recurrent pleural effusion and an underlying diagnosis of a hematologic malignancy (lymphoma, leukemia, multiple myeloma, or other). Exclusion criteria were incomplete medical records for analysis. Records were reviewed for patient demographics, operative reports, and pathology, cytology, and microbiology reports. Records of underlying hematologic malignancy, treatments, and hematologic laboratory values at the time of IPC insertion and infection were identified. Patient records were subsequently reviewed for evidence of pleural infection by queries regarding post-IPC placement pleural fluid cultures, clinic visits, hospital admissions, and death records. Radiologic and operative records were queried regarding pleural space physiology at the time of IPC insertion to document evidence of expandable or nonexpandable lung. IPC infectious complications were defined by the presence of pleural pus, positive pleural fluid Gram stain or culture requiring subsequent intervention (antibiotics, IPC removal, etc), or cellulitis requiring systemic antibiotics. Data regarding IPC removal, specifically the timing and reason for removal, were collected. In patients dying with the IPC in situ, the date of removal was calculated as the date of death. To calculate days of IPC use in patients without records of death or IPC removal, data were censored on December 31, 2013, the last date of data collection. Study data were collected and managed using REDCap electronic data capture tools hosted at the Penn State-Milton S. Hershey Medical Center.
Eight centers participated in data collection, with individual audit times depending on local record availability. All centers offer multidisciplinary care in thoracic oncology and are considered to have expertise in the management of MPEs and placement of IPCs. Some patient data were reported in previously published studies examining pleural space infections.5,6
Statistical Analysis
Statistical analysis was conducted using Microsoft Excel, version 14.4.2 (Microsoft Corporation) and Stata 13.1 (StataCorp LP) software. Simple descriptive statistics, including mean, range, and percentage, were used to describe patient demographics and outcomes. We also analyzed the time to IPC removal and potential clinical predictors using a time-to-event regression model. Removal secondary to pleurodesis was considered an outcome of interest, whereas removal due to other reasons (infection, death, patient request, catheter malfunction, or other) were considered competing events because they had the potential to alter the probability of removal due to pleurodesis.13 Predictors of time to IPC removal were age, sex, underlying malignancy, chemotherapy activity, pleural space physiology, and pleural effusion etiology. We plotted cumulative incidence for each outcome and applied a competing-risk time-to-event regression model, providing subhazard ratios. Univariate models for each predictor were fitted to identify those that have an effect on the incidence of IPC removal with a P < .2 to be considered in a multivariate model.
Results
Ninety-one patients were identified from eight institutions between January 2009 and December 2013 (Table 1). No patients were excluded from subsequent analyses. The mean ± SD age within the cohort was 65.4 ± 15.4 years (range, 22-93 years). Malignancy was present in 96% (87 of 91 patients), with lymphoma being the most commonly identified malignancy (62%) followed by leukemia (21%) and multiple myeloma (13%). Four patients were classified as other (two with amyloidosis and two with myelofibrosis); however, all were undergoing active chemotherapy for their underlying disorder. The majority of patients had not undergone previous hematopoietic stem cell transplantation (HSCT), but 16 (18%) had undergone HSCT, with a range of 161 to 2,984 days between HSCT and IPC placement.
TABLE 1 ] .
Patient Demographics
| Demographic | Value |
| Age, y | |
| Mean | 65.4 |
| Range | 22-93 |
| Male (female) sex | 65 (26) |
| Primary malignancy | |
| Lymphoma | 56 (62) |
| Leukemia | 19 (21) |
| Multiple myeloma | 12 (13) |
| Other | 4 (4) |
| Previous HSCT | |
| Yes | 16 (18) |
| No | 72 (79) |
| Unknown | 3 (3) |
Data are presented as No. (%) unless otherwise indicated. HSCT = hematopoietic stem cell transplantation.
IPC placement (Table 2) occurred with relative equal distribution of insertion side (right, 44; left, 41; bilateral, 6). Pleural space physiology assessment (expandable vs nonexpandable lung) was documented in 33 patients (36%). Of those patients assessed, 15 (16%) were identified as having nonexpandable lung. Confirmation of MPE through a combination of pleural fluid cytology (n = 42) or pleural biopsy specimen (n = 8) occurred in 55% of the cohort. An additional 24 patients were suspected of having evidence of MPEs (cytology-negative exudative effusion), but no further diagnostic evaluation was performed. Six patients underwent IPC placement for recurrent transudative pleural effusions.
TABLE 2 ] .
IPC Placement and Removal Characteristics
| Characteristic | Value |
| IPC placement location | |
| Right | 44 (48) |
| Left | 41 (45) |
| Bilateral | 6 (7) |
| Hematologic parameters | |
| WBC count | 9.9 (0.1-83.3) |
| Hemoglobin level | 10.4 (7.5-14.3) |
| Platelet count | 164 (14-732) |
| Pleural physiology | |
| Nonexpandable lung | 15 (16) |
| Expandable lung | 18 (20) |
| Not assessed | 58 (64) |
| Patient status at placement | |
| Inpatient | 65 (71) |
| Outpatient | 26 (29) |
| Duration of IPC, d | |
| Mean ± SD | 89.9 ± 127.8 |
| Range | 2-867 |
| Indication for removal | |
| Death | 53 (58) |
| Autopleurodesis | 21 (23) |
| Infection | 4 (4) |
| Patient request | 6 (7) |
Data are presented as No. (%) or mean (range) unless otherwise indicated. IPC = indwelling tunneled pleural catheter.
The majority of IPC placements occurred within the inpatient population (71%). Of those undergoing IPC placement as an inpatient, in-hospital mortality was 9%. No patients undergoing IPC placement as an outpatient required admission to the hospital. No patient deaths occurred as a direct result of IPC placement.
IPC Removal
The mean in situ dwell time of all catheters within the cohort was 89.9 ± 127.1 days (range, 2-867 days), with a total of 8,160 IPC-days. At the time of data collection, IPCs remained in seven patients, with those catheters in situ for a mean of 110.9 days (range, 18-287 days). Twenty-eight IPCs were removed within 30 days of placement, with the majority being removed secondary to death (79%).
The most common documented reason for IPC removal was patient death (n = 53). The mean time to removal (or mean survival time) was 93.4 ± 154.6 days (range, 2-867 days). Spontaneous pleurodesis occurred in 21 patients and was the second most common indication for IPC removal. In patients with spontaneous pleurodesis, mean removal occurred at 63.2 ± 47.9 days (range 16-187 days). An additional six IPCs were removed secondary to patient request: Three patients underwent thoracoscopic pleurodesis with a sclerosing agent and three requested removal secondary to pain.
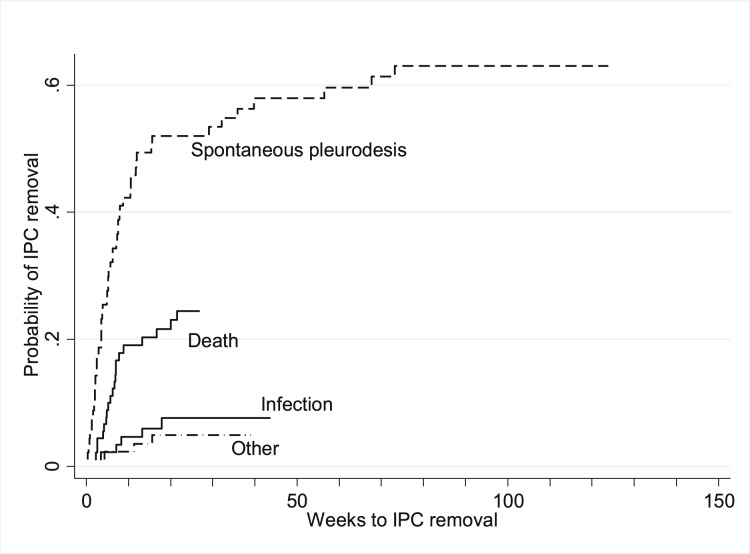
For the purpose of performing a time-to-event analysis, days to IPC removal were converted to weeks, and cumulative incidence plots of the various reasons for removal are displayed in Figure 1. These plots show that removal due to spontaneous pleurodesis occurred with a higher probability than removal due to other reasons. Furthermore, infections, death, and other reasons occurred earlier (within the first 50 weeks) than spontaneous pleurodesis. Univariate regression analysis (Table 3) indicated that only age > 70 years demonstrated significance at P < .2 (P = .09). However, compared with the youngest age group (< 60 years), a nonsignificant subhazard ratio of 0.37 was present; therefore, no multivariate model was considered.
Figure 1 –
Cumulative incidence plot of IPC removal. The probability of IPC removal within the cohort was analyzed in reference to the underlying reason for removal. This plot demonstrates that spontaneous pleurodesis occurred with a higher probability compared with removal for other reasons. IPC = indwelling tunneled pleural catheter.
TABLE 3 ] .
Regression Model Results of Time to IPC Removal
| Univariate Regression | ||||
| Variable | No. Patients (N = 91) | % | Subhazard Ratio | P Value |
| Age category | ||||
| < 60 y | 33 | 36.26 | Reference | … |
| 60-70 y | 27 | 29.67 | 0.79 | .63 |
| > 70 y | 31 | 34.07 | 0.37 | .09 |
| Sex | ||||
| Male | 65 | 71.43 | Reference | … |
| Female | 26 | 28.57 | 1.21 | .67 |
| Underlying malignancy | ||||
| Other | 35 | 38.46 | Reference | … |
| Lymphoma | 56 | 61.54 | 1.06 | .89 |
| Chemotherapy activity | ||||
| Active | 39 | 42.86 | Reference | … |
| Within the past 6 wk | 17 | 18.68 | 1.28 | .65 |
| > 6 wk ago | 23 | 25.27 | 0.48 | .27 |
| Unknown | 12 | 13.19 | 0.99 | .98 |
| Pleural space physiology at IPC placement | ||||
| Nonexpandable lung | 15 | 16.48 | Reference | … |
| Reexpandable lung | 18 | 19.78 | 2.19 | .35 |
| Not assessed | 58 | 63.74 | 1.89 | .39 |
| Pleural effusion etiology | ||||
| MPE (cytology or histology positive) | 50 | 54.95 | Reference | … |
| MPE suspected | 24 | 26.37 | 0.99 | .99 |
| Recurrent exudative effusion | 11 | 12.09 | 0.81 | .79 |
| Recurrent transudative effusion | 6 | 6.59 | 0.64 | .67 |
MPE = malignant pleural effusion. See Table 2 legend for expansion of other abbreviation.
Infection
All infections documented within the cohort (n = 7) were related to pleural space infections (Table 4). The overall infection rate was 7.7%. All patients had positive fluid culture results, with only one patient having frank pus. The majority of infections involved Staphylococcus aureus (five of seven). Most patients (85%) were admitted to the hospital for IV broad-spectrum antimicrobial treatment.
TABLE 4 ] .
IPC Infection Data
| IPC Data | No. Patients |
| Infection | |
| Empyema | 7 |
| Cellulitis | 0 |
| Death related to IPC infection | 2 |
| Hospital admission required | 6 |
| Additional pleural intervention performed | |
| IPC removal | 4 |
| Additional drainage | 1 |
| Surgery | 3 |
See Table 2 legend for expansion of abbreviation.
Additional pleural procedures were performed in four patients. Three underwent decortication procedures (one thoracotomy and two video-assisted thoracic surgeries), and one underwent tube thoracostomy for empyema drainage. All four patients underwent removal of their previously placed IPC. Three did not undergo additional pleural procedures or IPC removal. Of these three patients, two died of septic shock related to empyema.
Discussion
We present, to our knowledge, the largest study to examine clinical outcomes related to the placement of IPCs for recurrent pleural effusion in patients with underlying hematologic malignancies. The mean time to IPC removal of all catheters within the cohort was 89.9 days, but in patients undergoing spontaneous pleurodesis the mean time to removal was 63.2 days. Univariate analysis was unable to identify clinical predictors of spontaneous pleurodesis within this cohort.
We identified a 7.7% overall infection risk within the cohort, and a minimal number of patients underwent additional pleural procedures (4.4%). The overall mortality attributed to IPC infection was low at 2.2%, but it appears to be higher than previously reported studies.4,5 Compared with data from larger cohorts of mostly solid tumor-related MPEs, infection rates were not significantly different in patients with hematologic malignancy despite the common occurrence of neutropenia and active use of immunosuppressive chemotherapeutic regimens.
Previous studies of IPC placement generally involved all patients with MPE regardless of their underlying malignancy, potentially clouding interpretation of risk for certain patient populations based on the rather heterogeneous pool of subjects. Although the literature identifies no significant increased risk of infection in patients undergoing active chemotherapy with an IPC,6,7 infectious complications remain a common complication of IPC placement, with rates ranging from 4% to 25%.4‐6,8 Patients with hematologic malignancies generally are considered to be at increased risk for infectious complications, leading some oncologists to not pursue IPC placement and, therefore, offering limited options for pleural palliation and recurrent dyspnea. Patients with hematologic malignancy are inherently unique in their underlying tumor biology, immune system, and treatment regimens,9‐11,14,15 but of interest, this presumed increase in infection risk was not clearly evident within the current cohort. Although it is not possible to prove causality with a retrospective study design, these findings suggest that IPC placement in hematologic malignancy may not carry an increased infectious risk over and above the generalized accepted risk for IPC placement despite the significant immunosuppression these patients often experience.
Within the current cohort, the mortality associated with IPC empyema was higher than previously reported.5,8 In the seven patients identified with empyema, those undergoing further pleural procedures did not die of infection (n = 4). However, in those undergoing antibiotic treatment and continued pleural space drainage through their in situ IPC (n = 3), two died of septic shock. It was unclear whether more aggressive interventions would have altered the outcome, prompting us to review these deaths further. Both patients were denied further aggressive care (surgical intervention) because both appeared to have progressive malignancy despite multiple chemotherapeutic regimens and were not identified as appropriate surgical candidates. Both patients were managed conservatively with IPC drainage, antibiotics, and supportive care. It remains unknown whether the significant immunosuppression in this patient population required more-aggressive interventions compared with the patient with solid malignancy with IPC empyema. In the setting of each patient’s end-stage disease it remains unclear whether further interventions (surgery or additional tube thoracostomy drainage) would have provided a benefit.
Few data exist regarding overall pleural effusion management in patients with hematologic malignancies. Pleural effusions within this population can be malignant but may also represent significant lymphatic obstruction, chylothorax, or other etiologies. The underlying etiology may lead to varying treatment decisions for pleural disease management, but the paucity of data can make this decision difficult, often leading to anecdotal experience guiding treatment decisions.
Although commonly described, no large series confirms or denies that systemic chemotherapy allows for timely pleural effusion resolution in hematologic malignancy. A review article by Alexandrakis et al16 noted that pleural effusions in hematologic malignancy are common, yet no specific management direction was offered except to say that most will respond to conventional chemotherapy directed at the primary disease. A small pediatric study (six patients) offered the same conclusions but noted that four of the children had recurrent effusions treated with repetitive therapeutic thoracentesis.17 A large, single-center series of patients with acute leukemia and myelodysplastic syndromes undergoing mainly thoracentesis procedures noted that 56% underwent more than one pleural procedure but only 6% underwent IPC placement. No long-term follow-up data were available for these six patients.18
Within the current cohort, there appears to be a selection and referral bias potentially reflecting the lack of clear guidelines regarding pleural disease management within this population. Despite most institutions reporting similar audit times, the range of patients undergoing IPC placement was rather large (n = 6-27). Unfortunately, because of the aforementioned lack of published data, the management of pleural disease in this patient population most likely remains institution and provider dependent.
Several limitations of this study bear mentioning. This was a retrospective study with the inherent weaknesses related to the study design. Selection bias for IPC placement was unable to be accounted for because we did track patients who refused IPC placement or who were not referred for placement. Exclusion of these patients may have artificially lowered the complication rates, but anecdotally, this number is small because the majority of these patients received oncologic care at these tertiary care centers that routinely handle the severity of illness common to this patient population. Incomplete patient data were minimized secondary to the use of electronic medical systems available at each institution. Loss to follow-up may have affected our ability to correctly classify patients with pleural space infections or complications related to IPC. We believe that this rate of loss to follow-up is low for three reasons. First, inherent to the nature of IPC placement, the physician performing the intervention is responsible for managing the complications. In patients receiving routine care at an outside institution, most questions or concerns related to IPC management are referred back to the performing physician, allowing for appropriate follow-up and recognition of complications. Second, these patients, by definition, have complicated hematologic malignancies that often are not treated in the community setting and, thus, received most of their care at larger, tertiary centers that allow for advanced hematologic malignancy care (ie, HSCT). Third, we were able to obtain follow-up data confirming IPC removal in > 92% of patients, suggesting that patients maintained contact and care within the respective medical systems in which they received IPC placement. We were unable to identify specific clinical factors that may help to predict IPC removal within this cohort; however, the study may have been underpowered to detect such a signal.
IPC placement remains an appropriate option for patients experiencing dyspnea secondary to recurrent pleural effusions. However, patient selection for the procedure is important. The IPC was designed to be a long-term ambulatory drainage system, but the ability to appropriately select patients for IPC placement appears to be an unanswered question. Within the current cohort, > 30% of IPC placements were subsequently removed within 30 days, including a significant number of removals secondary to death. Although in certain clinical situations IPC placement may be appropriate in patients with very limited life spans, other procedures, such as intermittent therapeutic thoracentesis, may be more appropriate.
In conclusion, we present, to our knowledge, the largest study examining clinical outcomes related to IPC placement in patients with underlying hematologic malignancy. Spontaneous pleurodesis and timing appear similar to previously reported literature. The overall infection rate within the current cohort also appears similar to previously reported studies, despite the significant immunosuppression often present within this population. The IPC appears to remain a viable option for recurrent pleural effusion management in patients with an underlying hematologic malignancy. However, with the current lack of randomized trials regarding initial pleural effusion management, further study is warranted.
Acknowledgments
Author contributions: C. R. G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C. R. G. and L. Y. contributed to the data collection, data analysis, manuscript writing, and manuscript review; H. J. L. contributed to the data analysis, manuscript writing, and manuscript review; J. H. S., F. M., M. W., P. J. C., J. B., D. S., A. C. A., S. S., J. A. G., C. L. W., and D. F.-K. contributed to the data collection and manuscript review; R. O. contributed to the data collection, data analysis, and manuscript review; and B. A. S. N. contributed to the data analysis, manuscript writing, and manuscript review.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Feller-Kopman has received consulting fees from CareFusion Corporation. Dr Yarmus has received consulting fees from ROCKET, Inc. Drs Gilbert, Lee, Skalski, Maldonado, Wahidi, Choi, Bessich, Sterman, Argento, Shojaee, Gorden, Wilshire, and Nonyane and Mr Ortiz have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Center for Research Resources. All work and data analyses were performed and confirmed by each institution, and all authors had access to the final data and manuscript submission.
ABBREVIATIONS
- HSCT
hematopoietic stem cell transplantation
- IPC
indwelling tunneled pleural catheter
- MPE
malignant pleural effusion
Footnotes
FUNDING/SUPPORT: There was no funding available for the performance of this study. The use of the REDCap database was supported by the Penn State Clinical and Translational Research Institute; a University of Pennsylvania Clinical and Translational Research Award; and a National Institutes of Health, National Center for Research Resources [Grant UL1RR033184].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Antunes G, Neville E, Duffy J, Ali N; Pleural Diseases Group, Standards of Care Committee, British Thoracic Society. BTS guidelines for the management of malignant pleural effusions. Thorax. 2003;58(suppl 2):ii29-ii38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbetakis N, Asteriou C, Papadopoulou F, et al. Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg. 2010;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ; BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(suppl 2):ii32-ii40. [DOI] [PubMed] [Google Scholar]
- 4.Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389. [DOI] [PubMed] [Google Scholar]
- 5.Fysh ETH, Tremblay A, Feller-Kopman D, et al. Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest. 2013;144(5):1597-1602. [DOI] [PubMed] [Google Scholar]
- 6.Mekhaiel E, Kashyap R, Mullon JJ, Maldonado F. Infections associated with tunnelled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchology Interv Pulmonol. 2013;20(4):299-303. [DOI] [PubMed] [Google Scholar]
- 7.Morel A, Mishra E, Medley L, et al. Chemotherapy should not be withheld from patients with an indwelling pleural catheter for malignant pleural effusion. Thorax. 2011;66(5):448-449. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362-368. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig H, Zojer N. Recent therapeutic advances in hematological malignancies: dealing with treatment-related complications. Ann Oncol. 2008;19(suppl 5):v71-v78. [DOI] [PubMed] [Google Scholar]
- 10.Maschmeyer G, Hiddemann W, Link H, et al. Management of infections during intensive treatment of hematologic malignancies. Ann Hematol. 1997;75(1-2):9-16. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien SN, Blijlevens NM, Mahfouz TH, Anaissie EJ. Infections in patients with hematological cancer: recent developments. Hematology Am Soc Hematol Educ Program. 2003;2003(1):438-472. [DOI] [PubMed] [Google Scholar]
- 12.Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56(4):905-909. [DOI] [PubMed] [Google Scholar]
- 13.Satagopan JM, Ben-Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91(7):1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wetzler M, Byrd JC, Bloomfield CD. Acute and chronic myeloid leukemia. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill Medical Publishing Division; 2005:631-641. [Google Scholar]
- 15.Armitage JO, Longo DL. Malignancies of lymphoid cells. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine. New York, NY: McGraw-Hill Medical Publishing Division; 2005:641-655. [Google Scholar]
- 16.Alexandrakis MG, Passam FH, Kyriakou DS, Bouros D. Pleural effusions in hematologic malignancies. Chest. 2004;125(4):1546-1555. [DOI] [PubMed] [Google Scholar]
- 17.Pietsch JB, Whitlock JA, Ford C, Kinney MC. Management of pleural effusions in children with malignant lymphoma. J Pediatr Surg. 1999;34(4):635-638. [DOI] [PubMed] [Google Scholar]
- 18.Faiz SA, Bashoura L, Lei X, et al. Pleural effusions in patients with acute leukemia and myelodysplastic syndrome. Leuk Lymphoma. 2013;54(2):329-335. [DOI] [PubMed] [Google Scholar]