Abstract
Podoplanin (PDPN) is a transmembrane glycoprotein that promotes tumor cell migration, invasion, and cancer metastasis. In fact, PDPN expression is induced in many types of cancer. Thus, PDPN has emerged as a functionally relevant cancer biomarker and chemotherapeutic target. PDPN contains 2 intracellular serine residues that are conserved between species ranging from mouse to humans. Recent studies indicate that protein kinase A (PKA) can phosphorylate PDPN in order to inhibit cell migration. However, the number and identification of specific residues phosphorylated by PKA have not been defined. In addition, roles of other kinases that may phosphorylate PDPN to control cell migration have not been investigated. We report here that cyclin dependent kinase 5 (CDK5) can phosphorylate PDPN in addition to PKA. Moreover, results from this study indicate that PKA and CDK5 cooperate to phosphorylate PDPN on both intracellular serine residues to decrease cell motility. These results provide new insight into PDPN phosphorylation dynamics and the role of PDPN in cell motility. Understanding novel mechanisms of PDPN intracellular signaling could assist with designing novel targeted chemotherapeutic agents and procedures.
Keywords: Podoplanin, serine phosphorylation, Protein Kinase A, Cyclin dependent Kinase 5, cell migration
Introduction
Podoplanin (PDPN) is a unique transmembrane receptor glycoprotein. Tumor promoters including TPA [1], RAS [2], TGF-β1, IFN-γ [3], EGFR [4], and Src [5] induce PDPN expression. PDPN regulates the activities of Rho, ezrin, and other proteins linked to the actin cytoskeleton to mediate filopodia formation, cell motility, invasion, and metastasis [6, 7].
PDPN expression is critical for embryonic development, and is upregulated in many types of cancer [6, 8–10]. For example, PDPN promotes the motility and invasion of many transformed cell types including mammary carcinoma [6, 11], glioma [12], and oral squamous carcinoma cells [13–15]. PDPN is also expressed by lymphatic endothelial cells [16, 17] and cancer associated fibroblasts [18–20] which can augment tumor invasion and metastasis [19, 21, 22].
PDPN can serve as a functionally relevant tumor biomarker, and is an enticing chemotherapeutic target. Indeed, the extracellular domain of PDPN can be targeted by antibodies and specific lectins to suppress tumor cell growth and migration [15, 23–28]. However, signaling events mediated by the intracellular domain of PDPN may also illuminate mechanisms leading to cell motility and pathways to chemotherapy that have not been explored.
The PDPN intracellular domain consists of about 10 amino acids that direct interactions with ERM proteins to affect the actin cytoskeleton [29]. This domain contains 2 conserved serine residues that can be phosphorylated to decrease PDPN mediated cell migration [18]. However, specific kinases that phosphorylate these individual serine residues, and how these phosphorylation events affect cell migration, have not been clearly elucidated. Here, we present data indicating that PKA and CDK5 kinases cooperate to phosphorylate both serines in the intracellular tail of PDPN in order to decrease cell motility.
Materials and Methods
Immunoprecipitation and Western blot analysis
Cells were lysed in RIPA buffer (50mM Tris-HCl pH 7.4, 1% NP-40, 2.5mM sodium pyrophosphate, 150mM sodium chloride, 1mm EDTA, 1mM EGTA, 1mM sodium betaglycerophosphate, 1mM sodium orthovanadate, 50mM sodium fluoride, 1mM PMSF, 10µg/mL protease inhibitor cocktail) and clarified by centrifugation at 20000 g for 5 minutes. Lysates containing 5mg of protein were incubated with phosphoserine antibody 16B4 (Enzo ALX-804-167-C100) [30, 31] for 3h at 4°C followed by protein-L beads (Santa Cruz 2336) for 1.5h at 4°C. Immune complexes were washed four times with ice-cold PBS supplemented with 1mM sodium orthovanadate, 50mM sodium fluoride, 1mM PMSF, and 10µg/mL protease inhibitor cocktail, eluted in sample buffer, resolved on 12% SDS-PAGE gels, and transferred to Immobilon-P membranes (Millipore IH1079562, Billerica, MA, USA). Western blotting was performed with antisera specific for PDPN [18], GAPDH (Santa Cruz Biotechnology A1978, Santa Cruz, CA, USA), or β-actin (Sigma A1978, St. Louis, MO, USA), and recognized by appropriate secondary antiserum conjugated to horseradish peroxidase and detected using enhanced chemiluminescence (Thermoscientific 32106, Philadelphia, PA, USA) as described previously [5, 18, 25]. Membranes were stained with India ink to verify equal loading and transfer after blotting.
Kinase assays
A phosphocellulose paper binding assay was used to measure phosphorylation of the peptide VVMKKISGRFSP (>95% purity, synthesized by GenScript USA Inc., Piscataway, NJ, USA), containing the entire intracellular region of PDPN (residues 161–172) as described [18]. Reaction mixtures contained 700 µM peptide in 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mg/ml bovine serum albumin, 0.25 mM ATP, and [γ-32P]-ATP (100 cpm/pmol). Reactions were initiated by adding 80 µg/ml PKA (Promega V5161, Madison, WI, USA), 22 µg/ml CDK5/p25 kinase enzyme (Promega V3231, Madison, WI, USA), or both PKA and CDK5/p25. Reactions were quenched after 10 minutes by the addition of 10% trichloroacetic acid, spotted onto P81 phosphocellulose filters, washed 3 times with 0.5% phosphoric acid, and analyzed by scintillation counting to determine picomoles of phosphate transferred. For mass spectrometry analysis, the kinase assay reactions contained 700 µM PDPN peptide in 30mM Tris-HCl (pH 7.5), 20 mM MgCl2, 1 mg/ml bovine serum albumin and 0.40 mM ATP.
Mass spectrometry
Peptide was purified (Millipore UFC501096 Carrigtwohill, Ireland) from kinase reactions and desalted (Millipore ZTC18M960, Billerica, MA, USA) according to manufacturer’s instructions. Peptide was then subjected to LC-MS/MS analysis on an Ultimate 3000 liquid chromatography (LC) system coupled with an Orbitrap Velos tandem mass spectrometry (MS/MS) instrument (Thermo Fisher Scientific, Philadelphia, PA, USA). Resulting MS/MS spectra were searched against a database containing PDPN peptide sequence using a local Mascot search engine (V.2.3). Methionine oxidation and serine/threonine phosphorylation were set as variable modifications in search parameters. Phosphopeptide sequence and phosphorylation sites were manually confirmed based on y- and b-ion series fragments.
Generation of mutant Pdpn cell lines
Pdpn single mutant constructs were produced with the QuikChange II XL Site-Directed Mutagenesis Kit according to the manufacturer’s protocol (Stratagene 200521, La Jolla, CA, USA). The vector pEF4Pdpn, encoding full length wild type mouse PDPN (PdpnWT) [18], was used as a template. PdpnSer167Ala, encoding PDPN with non-phosphorylatable serine at position 167 (PdpnS167A) was generated with the complementary primer pairs 5’GTTGTTATGAAGAAGATTGCTGGAAGGTTCTCGCC3’, 5’GGCGAGAACCTTCCAGCAATCTTCTTCATAACAAC3’ followed by 5’GGTTCTCGCCCTAAAGAGGGCCCTTCGAAGG3’, 5’CCTTCGAAGGGCCCTCTTTAGGGCGAGAACC3’. PdpnSer167Asp, encoding PDPN with serine 167 mutated to phosphomimetic aspartate (PdpnS167D), was generated with the complementary primers 5’GTTATGAAGAAGATTGATGGAAGGTTCTCGCCCTAAAGAGC3’, 5’GCTCTTTAGGGCGAGAACCTTCCATCAATCTTCTTCATAAC3’. PdpnSer171Ala, encoding PDPN with non-phosphorylatable serine at position 171 (PdpnS171A), was generated with the complementary primers 5’TGGAAGGTTCGCGCCCTAAAGAGGGCCCTTCGAAGG3’, 5’CCTTCGAAGGGCCCTCTTTAGGGCGCGAACCTTCCA3’. PdpnSer171Asp, encoding PDPN with serine 171 mutated to phosphomimetic aspartate (PdpnS171D), was generated with the complementary primers 5’TGGAAGGTTCGACCCCTAAAGAGGGCCCTTCGAAGG3’, 5’CCTTCGAAGGGCCCTCTTTAGGGGTCGAACCTTCCA3’. All constructs were verified by sequencing. PdpnKo cells were stably transfected with PdpnS167A, PdpnS167D, PdpnS171A or PdpnS171D constructs and maintained in DMEM supplemented with 10% FBS as described [18]. Cells were cotransfected with PDPN constructs subcloned into pEF4Zeo, as well as pBabePuro, and selected for growth in both zeocin and puromycin, to achieve very high transfection efficiencies without taking clones as previously described [5, 18, 32–34]. Clones were not taken to minimize potential effects of clonal variation, and experiments were done in parallel to control for differences in serum effects and other environmental factors between experiments.
Evaluation of cell migration
500,000 cells were seeded in each well of 12-well cluster plates (CytoOne CC7682-7512, Ocala, FL), allowed to form a monolayer, scratched at the center to create a wound, and migration was quantitated as the number of cells that entered a field of 400 × 400 micron in the center of the wound at 18 hours as previously described [5, 18, 25]. Data were taken from three representative experiments (n=3) with 5 fields per experiment. For some experiments, cells were treated with 20 µM PKA inhibitor H-89 (Calbiochem 371963) or 0.1 µM CDK5 inhibitor Roscovitine (Calbiochem 557360) immediately after wounding.
Statistical analysis
Statistical analyses were performed using Prism (version 5, GraphPad Software).
Results
PKA and CDK5 phosphorylate the intracellular domain of PDPN
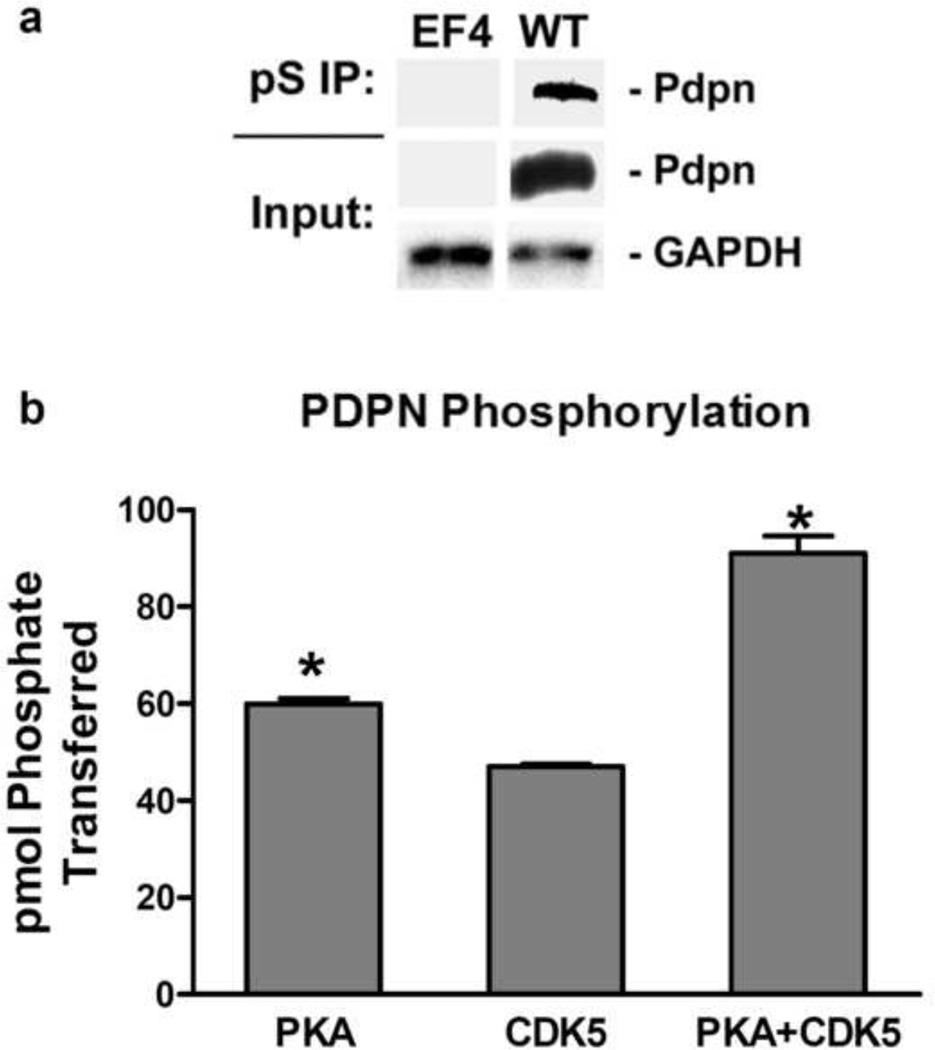
We generated cells from homozygous null PDPN knockout mice, called PdpnKo cells [18]. These cells serve as a clear foundation on which to examine the effects of specific PDPN isoforms and modifications on cell behavior. For example, as shown in Figure 1a, PDPN can be immunoprecipitated from these cells with phosphoserine antiserum, and then detected by Western blotting to demonstrate that it is phosphorylated by serine kinases in vivo.
Figure 1. PKA and CDK5 phosphorylate serine residues in the PDPN intracellular domain.
(a) Total protein (input) and protein immunoprecipitated with phosphoserine antiserum (pS IP) from homozygous null PdpnKo cells transfected with empty parental vector (EF4) or wild type PDPN (WT) was analyzed for PDPN and GAPDH by Western blotting as indicated. (b) Peptide containing the entire intracellular region (VVMKKISGRFSP) of PDPN was incubated with PKA, CDK5, or both PKA and CDK5 along with [γ-32]ATP for 10 minutes. Data are shown as picomoles of phosphate incorporated into the PDPN peptide (mean+SD, n=2). Asterisks indicate p<0.05 compared to CDK5 treated cells by t-Test.
The intracellular domain of PDPN contains 2 conserved serine residues. These serines are located at residues 167 and 171 in mouse PDPN, corresponding to serines 157 and 161 in human PDPN. We thus sought to identify which of these serines is phosphorylated to affect PDPN signaling events.
Kinase specific phosphorylation sites on PDPN intracellular domain were predicted using the ExPASy NetPhosK server. These analyses identified PKA and CDK5 as ideal candidates for kinases that phosphorylate the intracellular serines of PDPN. Therefore, a peptide corresponding to the entire intracellular domain of PDPN was used as a substrate to evaluate the ability of PKA and CDK5 to phosphorylate these serine residues. As shown in Figure 1b, PKA transferred about 60 pmol of phosphates to the PDPN peptide in 10 minutes. These data are consistent with previous studies indicating that PKA can phosphorylate PDPN intracellular serines [18].
In addition to PKA, CDK5 also transferred about 40 pmol of phosphate to the PDPN peptide in 10 minutes (Figure 1b). Interestingly, simultaneous incubation with PKA and CDK5 resulted in approximately 100 pmol of phosphate to the PDPN peptide in 10 minutes (Figure 1b). Thus, PKA and CDK5 displayed an additive effect on PDPN phosphorylation. Although serine 167 of PDPN has been considered to be a putative PKC phosphorylation site [13], we did not detect PDPN peptide phosphorylation by PKC (data not shown).
PKA phosphorylates PDPN on S167 or S171, while CDK5 preferentially phosphorylates S171
We performed LC-MS/MS analysis of the PDPN peptide from in vitro kinase assays in order to determine which PDPN residues are phosphorylated by PKA and CDK5. As shown in Figure 2, treatment of the peptide with PKA or CDK5 resulted in singly phosphorylated species, in which either S167 or S171 was modified. MS spectra detected PDPN peptides with phosphorylation at serine 167 (S167) or serine 171 (S171) from PKA and CDK5 treated samples. We did not detect any PDPN peptides with phosphorylation at both S167 and S171 residues from these reactions (Figures 2 and 3). These data indicate that each kinase can phosphorylate one or the other serine in a single reaction, but not both.
Figure 2. PKA and CDK5 phosphorylate either PDPN S167 or S171.
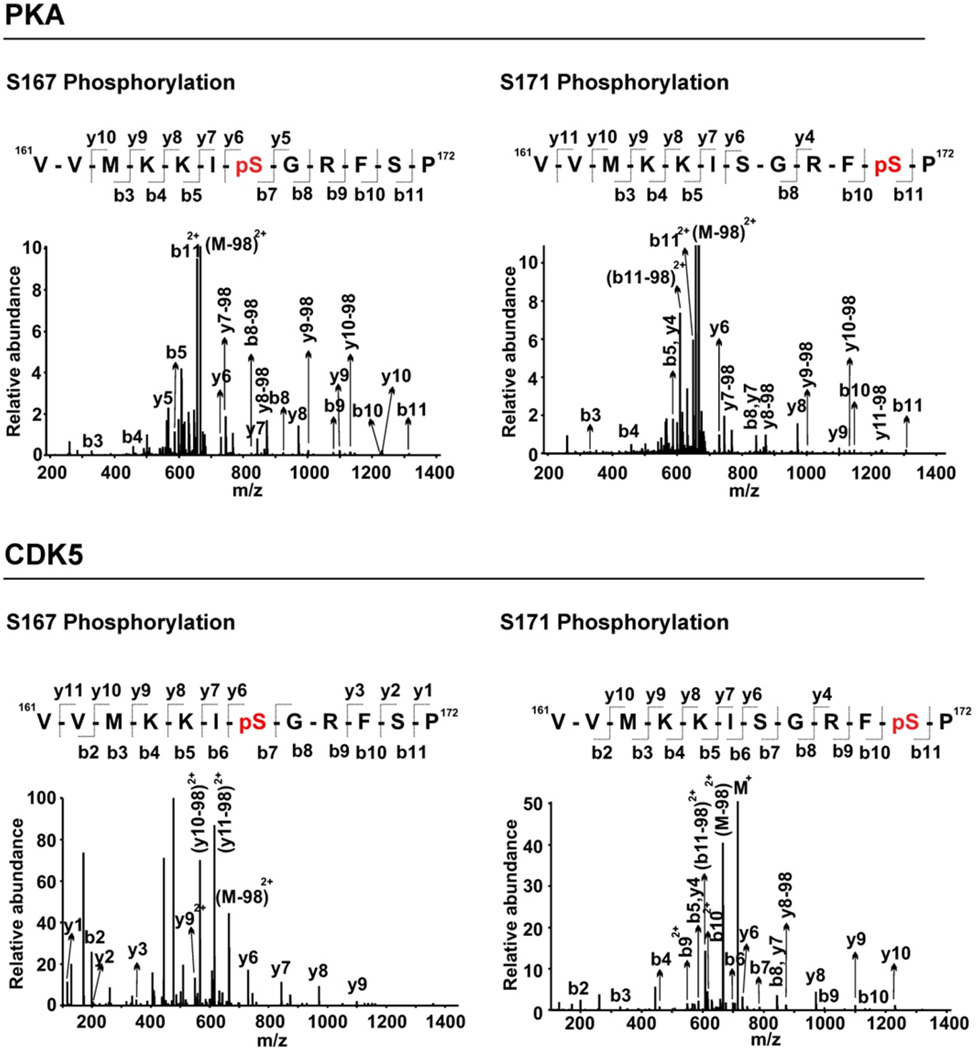
Peptide containing the entire intracellular region (VVMKKISGRFSP) of PDPN was incubated with PKA, CDK5, or both PKA and CDK5 along with [γ-32]ATP for 10 minutes. Representative MS/MS spectrum of PKA and CDK5 treated samples show doubly-charged ion (m/z 714.87) corresponding to the phosphorylated PDPN peptide sequence with phosphorylation at S167 or S171 as indicated. Observed y-ion and b-ion series confirming the peptide sequence and phosphorylation sites are also shown.
Figure 3. PKA phosphorylates S167 or S171, while CDK5 preferentially phosphorylates S171 in the PDPN intracellular domain.
Peptide containing the entire PDPN intracellular region was treated with PKA or CDK5 as indicated, and analyzed by LC-MS/MS to detect specific phosphorylation sites. Data are shown as the percent of PDPN peptide phosphorylated at serine residues position 167 or 171 based on at least 100 MS spectra counts.
The percent of PDPN peptide phosphorylated at S167 or S171 was calculated from spectra counts to determine if PKA and CDK5 preferentially phosphorylate specific serine residues. As shown in Figure 3, PKA phosphorylated PDPN on S167 or S171 equally well. However, CDK5 phosphorylated S171 four fold more than S167. These data indicate that PKA can phosphorylate PDPN equally well on either S167 or S171, whereas CDK5 preferentially phosphorylates PDPN on S171.
Both S167 and S171 residues on PDPN are phosphorylated to inhibit cell migration
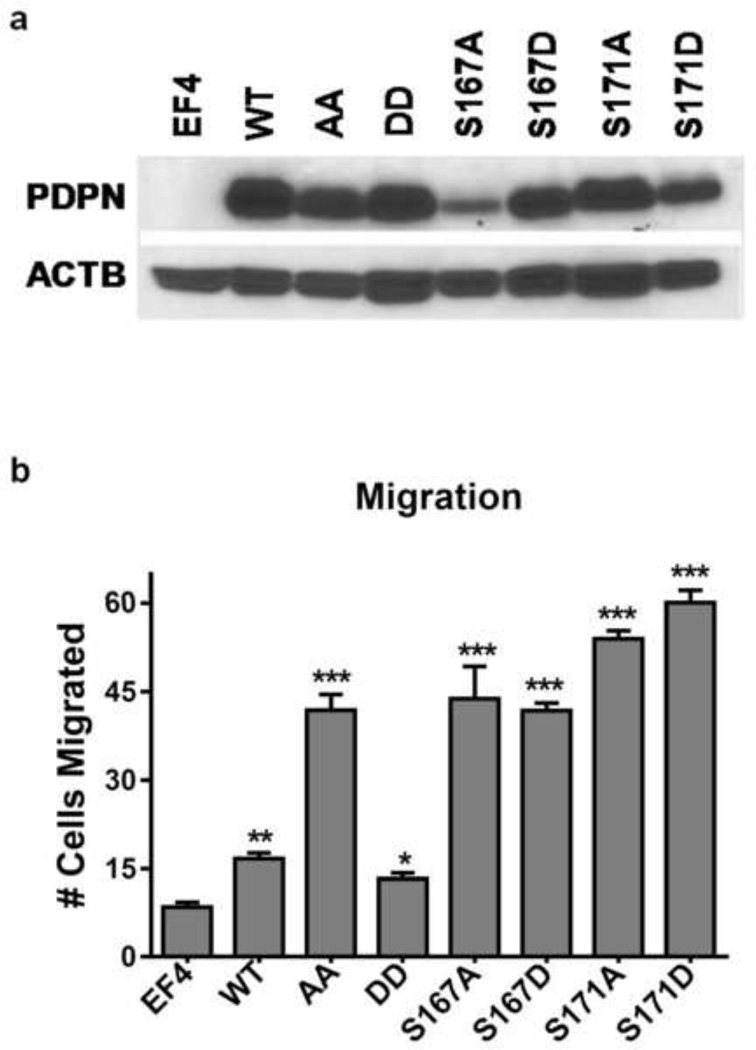
We used homozygous null PDPN knockout mouse embryonic fibroblasts (PdpnKo cells) to evaluate how modification of each PDPN intracellular serine residue affects cell migration. As shown in Figure 4b, cells expressing wild-type PDPN (PdpnWT) migrated about 2 fold more than control PdpnKo cells transfected with empty parental vectors (PdpnEF4). Substitution of both intracellular serines to nonphosphorylatable alanines (PdpnAA cells) increased cell migration about 3 fold compared to PdpnWT cells. In contrast to cells expressing nonphosphorylatable PDPN, migration of cells expressing PDPN with both serines mutated to phosphomimetic aspartate (PdpnDD) was comparable to control transfectants. These data are consistent with previous studies indicating that phosphorylation of PDPN can decrease cell migration [18].
Figure 4. Both serine residues in the intracellular domain of PDPN are phosphorylated to decrease cell migration.
(a) PDPN and β-actin were detected by Western Blotting of protein (10 µg/lane) from homozygous null Pdpn knock-out mouse embryonic fibroblasts transfected with empty parental vectors (EF4), wild type Pdpn (WT), Pdpn with both intracellular serines mutated to alanine (AA), Pdpn with both intracellular serines mutated to aspartate (DD), or Pdpn with each of the 2 serines mutated to alanine or aspartate (S167A, S167D, S171A, S171D) as indicated. (b) Cell migration was measured by wound healing assays, and quantitated as the number of cells that entered 400 × 400 µm field in the center of a wound within 18 hours (mean+SEM, n=3). Single, double, and triple asterisks indicate p<0.05, 0.01, or 0.001 by t-Test compared to EF4 cells, respectively.
After examining double mutants, we generated cells expressing PDPN with single site mutations to examine the effects of phosphorylation of individual intracellular serine residues on cell migration. PdpnKo cells were transfected with PDPN constructs with S167 or S171 mutated to nonphosphorylatable alanine (PdpnS167A or PdpnS171A) and phosphomimetic constructs with these serines mutated to aspartate (PdpnS167D or PdpnS171D). After confirming PDPN expression by Western Blotting (Figure 4a), cell migration was evaluated by wound healing assays.
As shown in Figure 4b, cells expressing PDPN with either serine S167 or S171 mutated to alanine (PdpnS167A or Pdpn171A) migrated over twice as well as cells expressing wild type PDPN (PdpnWT). These data suggest that both of these serines need to be phosphorylated to effectively inhibit cell motility. As shown in Figure 4b, cells expressing any construct with a single serine of PDPN mutated to alanine or aspartate migrated comparably to cells expressing nonphosphorylatable PDPN - with both sites mutated to alanine (PdpnAA). As discussed below, these data indicate that both intracellular serines on PDPN are phosphorylated to decrease cell migration, and phosphomimetic residues can not fully compensate for these phosphorylation events.
Discussion
PDPN has emerged as a functionally relevant tumor biomarker and chemotherapeutic target [25, 35, 36]. Several ligands can bind to the extracellular region of PDPN to promote transformed cell migration and tumorigenesis [37–40]. Accordingly, this extracellular region can be targeted by reagents to inhibit tumor cell growth and motility [15, 25, 27, 28].
Regardless of extracellular signals, the intracellular region of PDPN must also direct events that affect cell migration. The two conserved serine residues in the short PDPN intracellular tail present a clear potential for biological relevance. Results from studies presented here indicate that phosphorylation of either serine in the PDPN tail is not sufficient to inhibit cell migration; they both need to be phosphorylated to inhibit cell motility.
Interestingly, PdpnWT cells treated with a PKA inhibitor migrated about 50% more than control cells, while CDK5 inhibition decreased cell migration by about 50% (Supplemental Figure 1). However, CDK5 regulates cell proliferation and morphology in addition to migration, and these pleiotropic effects may cause toxicities that prohibit cell motility in manners unrelated to PDPN signaling [41–43]. In addition, cells expressing phosphomimetic PDPN constructs with either intracellular serine mutated to aspartate (PdpnS167D or Pdpn171D) migrated twice as well as cells with wild type PDPN. Clearly, these phosphomimetic mutations do not reproduce PDPN modifications that result from phosphorylation events. These data are consistent with studies indicating that phosphate residues affect protein function in many ways, including alterations to potential adaptor binding sites and changes to the overall chemical environment that are not accomplished by changes of serines to aspartate or glutamate residues [44–46].
Taken together, these results suggest a scenario, outlined in Figure 6, in which PKA and CDK5 phosphorylate PDPN S167 or S171, respectively, in order to decrease cell motility. Previous studies found that PKA can phosphorylate PDPN on one or both intracellular serines to decrease cell migration [18]. Here, we show that both serines need to be phosphorylated in order to decrease cell migration. Moreover, these data indicate that PKA can phosphorylate PDPN on either serine, while CDK5 appears to phosphorylate S171. Therefore, a coordinated effort by unrelated kinases is required to phosphorylate PDPN in order to inhibit cell motility.
Identification of PKA and CDK5 as kinases that phosphorylate specific residues on PDPN to affect cell migration should help elucidate mechanisms that control processes including embryonic development, tumorigenesis, and chemotherapeutic treatments. For example, chemotherapeutic agents such as disulfiram and CARP-1 functional mimetics have recently been found to induce PDPN phosphorylation. Moreover, PDPN phosphorylation induced by these anticancer reagents leads to PDPN degradation and mesothelioma cell growth inhibition [31, 47]. Further elucidation of how PDPN phosphorylation controls cell growth and motility should yield fundamental insights into many important biological and biomedical processes.
Supplementary Material
Wound healing experiments were performed on confluent monolayers of PdpnWT cells treated with the PKA inhibitor H-89 or the CDK5 inhibitor Roscovitine Data are shown as the migration compared to nontreated controls plus SD (n=2).
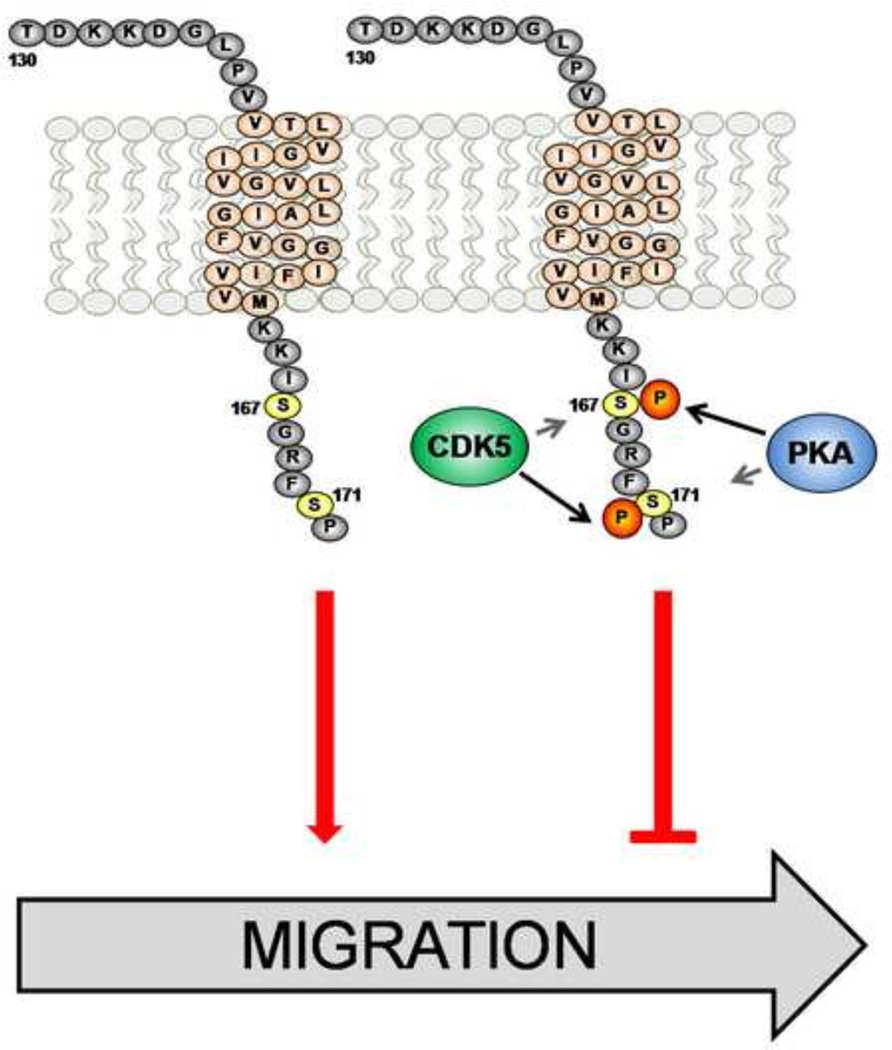
Figure 5. Phosphorylation of specific serines on the PDPN intracellular domain affect cell migration.
PKA can phosphorylate serines S167 or S171 on the intracellular domain of PDPN, whereas CDK5 preferentially phosphorylates S171. However, phosphorylation of both intracellular serines on PDPN is required to inhibit PDPN mediated cell migration. Thus, PKA and CDK5 may phosphorylate PDPN S167 and S171, respectively, in order to decrease cell motility.
Highlights.
We examined the dynamics of PDPN phosphorylation and its role in cell migration.
PKA and CDK5 phosphorylate the intracellular domain of PDPN.
PKA can phosphorylate PDPN on S167 or S171.
CDK5 preferentially phosphorylates PDPN on S171.
Both S167 and S171 residues on PDPN are phosphorylated to inhibit cell motility.
Acknowledgements
This work was funded in part by support from the Foundation of UMDNJ, the Osteopathic Heritage Foundation, and the Northarvest Bean Growers Association to G.S.G., National Institutes of Health Grant R01 CA 58530 to W.T.M, and HL 083034 to M.I.R. Mass spectrometry data were obtained from a Q-Exactive MS instrument funded in part by NIH Grant NS 046593 for the support of the Rutgers Neuroproteomics Core Facility.
Abbreviations
- PDPN
Podoplanin
- PKA
Protein Kinase A
- CDK5
Cyclin dependent Kinase 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nose K, Saito H, Kuroki T. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1990;1:511–518. [PubMed] [Google Scholar]
- 2.Gandarillas A, Scholl FG, Benito N, Gamallo C, Quintanilla M. Induction of PA2.26, a cell-surface antigen expressed by active fibroblasts, in mouse epidermal keratinocytes during carcinogenesis. Mol. Carcinog. 1997;20:10–18. doi: 10.1002/(sici)1098-2744(199709)20:1<10::aid-mc3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Honma M, Minami-Hori M, Takahashi H, Iizuka H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Regulation by TGF-beta and STAT-3 activating cytokines, IFN-gamma, IL-6, and IL-22. J. Dermatol. Sci. 2012;65:134–140. doi: 10.1016/j.jdermsci.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Fujii M, Honma M, Takahashi H, Ishida-Yamamoto A, Iizuka H. Intercellular contact augments epidermal growth factor receptor (EGFR) and signal transducer and activator of transcription 3 (STAT3)-activation which increases podoplanin-expression in order to promote squamous cell carcinoma motility. Cellular signalling. 2013;25:760–765. doi: 10.1016/j.cellsig.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Shen Y, Chen CS, Ichikawa H, Goldberg GS. SRC induces podoplanin expression to promote cell migration. J. Biol. Chem. 2010;285:9649–9656. doi: 10.1074/jbc.M109.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br. J. Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat. Med. 2004;10:175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 8.Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raica M, Cimpean AM, Ribatti D. The role of podoplanin in tumor progression and metastasis. Anticancer Res. 2008;28:2997–3006. [PubMed] [Google Scholar]
- 10.Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev. Biol. 2003;256:61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- 11.Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MA, Nicoloso MS, Shimizu M, Rossi S, Gopisetty G, Molina JR, Carlotti C, Jr, Tirapelli D, Neder L, Brassesco MS, Scrideli CA, Tone LG, Georgescu MM, Zhang W, Puduvalli V, Calin GA. miR-29b and miR-125a regulate podoplanin and suppress invasion in glioblastoma. Genes Chromosomes. Cancer. 2010;49:981–990. doi: 10.1002/gcc.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer. 2005;113:899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 14.Tsuneki M, Yamazaki M, Maruyama S, Cheng J, Saku T. Podoplanin-mediated cell adhesion through extracellular matrix in oral squamous cell carcinoma. Lab Invest. 2013;93:921–932. doi: 10.1038/labinvest.2013.86. [DOI] [PubMed] [Google Scholar]
- 15.Ochoa-Alvarez JA, Krishnan H, Pastorino JG, Kephart D, Lee JJ, Retzbach EP, Shen Y, Fatahzadeh M, Baredes S, Kalyoussef E, Honma M, Adelson ME, Kaneko MK, Kato Y, Young MA, Deluca-Rapone L, Shienbaum AJ, Yin K, Jensen LD, Goldberg GS. Antibody and lectin target podoplanin to inhibit oral squamous carcinoma cell migration and viability by distinct mechanisms. Oncotarget. 2015 doi: 10.18632/oncotarget.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. J. Cell Mol. Med. 2009;13:1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammed RA, Martin SG, Gill MS, Green AR, Paish EC, Ellis IO. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am. J. Surg. Pathol. 2007;31:1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan H, Ochoa-Alvarez JA, Shen Y, Nevel E, Lakshminarayanan M, Williams MC, Ramirez MI, Miller WT, Goldberg GS. Serines in the intracellular tail of podoplanin (PDPN) regulate cell motility. The Journal of biological chemistry. 2013;288:12215–12221. doi: 10.1074/jbc.C112.446823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono S, Ishii G, Nagai K, Takuwa T, Yoshida J, Nishimura M, Hishida T, Aokage K, Fujii S, Ikeda N, Ochiai A. Podoplanin-positive cancer-associated fibroblasts could have prognostic value independent of cancer cell phenotype in stage I lung squamous cell carcinoma: usefulness of combining analysis of both cancer cell phenotype and cancer-associated fibroblast phenotype. Chest. 2013;143:963–970. doi: 10.1378/chest.12-0913. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Ishii G, Hoshino A, Hashimoto H, Neri S, Kuwata T, Higashi M, Nagai K, Ochiai A. Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. Biochem. Biophys. Res. Commun. 2012;422:194–199. doi: 10.1016/j.bbrc.2012.04.158. [DOI] [PubMed] [Google Scholar]
- 21.Neri S, Ishii G, Taira T, Hishida T, Yoshida J, Nishimura M, Nagai K, Ochiai A. Recruitment of podoplanin positive cancer-associated fibroblasts in metastatic lymph nodes predicts poor prognosis in pathological N2 stage III lung adenocarcinoma. Ann Surg Oncol. 2012;19:3953–3962. doi: 10.1245/s10434-012-2421-4. [DOI] [PubMed] [Google Scholar]
- 22.Kan S, Konishi E, Arita T, Ikemoto C, Takenaka H, Yanagisawa A, Katoh N, Asai J. Podoplanin expression in cancer-associated fibroblasts predicts aggressive behavior in melanoma. J Cutan Pathol. 2014;41:561–567. doi: 10.1111/cup.12322. [DOI] [PubMed] [Google Scholar]
- 23.Kato Y, Kaneko MK, Kuno A, Uchiyama N, Amano K, Chiba Y, Hasegawa Y, Hirabayashi J, Narimatsu H, Mishima K, Osawa M. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem. Biophys. Res. Commun. 2006;349:1301–1307. doi: 10.1016/j.bbrc.2006.08.171. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko MK, Kunita A, Abe S, Tsujimoto Y, Fukayama M, Goto K, Sawa Y, Nishioka Y, Kato Y. A chimeric anti-podoplanin antibody suppresses tumor metastasis via neutralization and antibody-dependent cellular cytotoxicity. Cancer Sci. 2012 doi: 10.1111/j.1349-7006.2012.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochoa-Alvarez JA, Krishnan H, Shen Y, Acharya NK, Han M, McNulty DE, Hasegawa H, Hyodo T, Senga T, Geng JG, Kosciuk M, Shin SS, Goydos JS, Temiakov D, Nagele RG, Goldberg GS. Plant lectin can target receptors containing sialic Acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PLoS. ONE. 2012;7:e41845. doi: 10.1371/journal.pone.0041845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, Hatake K, Fujita N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PloS one. 2013;8:e73609. doi: 10.1371/journal.pone.0073609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe S, Morita Y, Kaneko MK, Hanibuchi M, Tsujimoto Y, Goto H, Kakiuchi S, Aono Y, Huang J, Sato S, Kishuku M, Taniguchi Y, Azuma M, Kawazoe K, Sekido Y, Yano S, Akiyama S, Sone S, Minakuchi K, Kato Y, Nishioka Y. A novel targeting therapy of malignant mesothelioma using anti-podoplanin antibody. Journal of immunology. 2013;190:6239–6249. doi: 10.4049/jimmunol.1300448. [DOI] [PubMed] [Google Scholar]
- 28.Kato Y, Vaidyanathan G, Kaneko MK, Mishima K, Srivastava N, Chandramohan V, Pegram C, Keir ST, Kuan CT, Bigner DD, Zalutsky MR. Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl. Med. Biol. 2010;37:785–794. doi: 10.1016/j.nucmedbio.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. Journal of Cell Science. 2006;119:4541–4553. doi: 10.1242/jcs.03218. [DOI] [PubMed] [Google Scholar]
- 30.Cheriyan VT, Wang Y, Muthu M, Jamal S, Chen D, Yang H, Polin LA, Tarca AL, Pass HI, Dou QP, Sharma S, Wali A, Rishi AK. Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis. PLoS One. 2014;9:e93711. doi: 10.1371/journal.pone.0093711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal S, Cheriyan VT, Muthu M, Munie S, Levi E, Ashour AE, Pass HI, Wali A, Singh M, Rishi AK. CARP-1 functional mimetics are a novel class of small molecule inhibitors of malignant pleural mesothelioma cells. PLoS One. 2014;9:e89146. doi: 10.1371/journal.pone.0089146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen Y, Khusial PR, Li X, Ichikawa H, Moreno AP, Goldberg GS. Src utilizes Cas to block gap junctional communication mediated by connexin43. J. Biol. Chem. 2007;282:18914–18921. doi: 10.1074/jbc.M608980200. [DOI] [PubMed] [Google Scholar]
- 33.Shen Y, Jia Z, Nagele RG, Ichikawa H, Goldberg GS. SRC uses Cas to suppress Fhl1 in order to promote nonanchored growth and migration of tumor cells. Cancer Research. 2006;66:1543–1552. doi: 10.1158/0008-5472.CAN-05-3152. [DOI] [PubMed] [Google Scholar]
- 34.Patwardhan P, Shen Y, Goldberg GS, Miller WT. Individual Cas phosphorylation sites are dispensable for processive phosphorylation by Src and cellular transformation. J. Biol. Chem. 2006;281:20689–20697. doi: 10.1074/jbc.M602311200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita N, Takagi S. The impact of Aggrus/podoplanin on platelet aggregation and tumor metastasis. J. Biochem. 2012 doi: 10.1093/jb/mvs108. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan H, Miller WT, Goldberg GS. SRC points the way to biomarkers and chemotherapeutic targets. Genes Cancer. 2012;3:426–435. doi: 10.1177/1947601912458583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cueni LN, Detmar M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Experimental Cell Research. 2009;315:1715–1723. doi: 10.1016/j.yexcr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin-Villar E, Fernandez-Munoz B, Parsons M, Yurrita MM, Megias D, Perez-Gomez E, Jones GE, Quintanilla M. Podoplanin Associates with CD44 to Promote Directional Cell Migration. Mol. Biol. Cell. 2010;21:4387–4399. doi: 10.1091/mbc.E10-06-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-type Lectin Receptor CLEC-2. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuneki M, Maruyama S, Yamazaki M, Xu B, Essa A, Abe T, Babkair H, Cheng J, Yamamoto T, Saku T. Extracellular heat shock protein A9 is a novel interaction partner of podoplanin in oral squamous cell carcinoma cells. Biochemical and biophysical research communications. 2013;434:124–130. doi: 10.1016/j.bbrc.2013.03.057. [DOI] [PubMed] [Google Scholar]
- 41.Griffin SV, Hiromura K, Pippin J, Petermann AT, Blonski MJ, Krofft R, Takahashi S, Kulkarni AB, Shankland SJ. Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am J Pathol. 2004;165:1175–1185. doi: 10.1016/S0002-9440(10)63378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin H, Chen MC, Chiu CY, Song YM, Lin SY. Cdk5 regulates STAT3 activation and cell proliferation in medullary thyroid carcinoma cells. J Biol Chem. 2007;282:2776–2784. doi: 10.1074/jbc.M607234200. [DOI] [PubMed] [Google Scholar]
- 43.Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu DX, Feng J, Hou P, Yao R, Zhang Y, Huang B, Lu J. CDK5 is essential for TGF-beta1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013;3:2932. doi: 10.1038/srep02932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts-Galbraith RH, Ohi MD, Ballif BA, Chen JS, McLeod I, McDonald WH, Gygi SP, Yates JR, 3rd, Gould KL. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol Cell. 2010;39:86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter T. Why nature chose phosphate to modify proteins. Philos Trans R Soc Lond B Biol Sci. 2012;367:2513–2516. doi: 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dephoure N, Gould KL, Gygi SP, Kellogg DR. Mapping and analysis of phosphorylation sites: a quick guide for cell biologists. Mol Biol Cell. 2013;24:535–542. doi: 10.1091/mbc.E12-09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheriyan VT, Wang Y, Muthu M, Jamal S, Chen D, Yang H, Polin LA, Tarca AL, Pass HI, Dou QP, Sharma S, Wali A, Rishi AK. Disulfiram suppresses growth of the malignant pleural mesothelioma cells in part by inducing apoptosis. PloS one. 2014;9:e93711. doi: 10.1371/journal.pone.0093711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wound healing experiments were performed on confluent monolayers of PdpnWT cells treated with the PKA inhibitor H-89 or the CDK5 inhibitor Roscovitine Data are shown as the migration compared to nontreated controls plus SD (n=2).