Abstract
The endosomal innate receptor CD158d (KIR2DL4) induces cellular senescence in human NK cells in response to soluble ligand (HLA-G or agonist antibody). These senescent NK cells display a senescence-associated secretory phenotype (SASP) and their secretome promotes vascular remodeling and angiogenesis. To understand how CD158d initiates signaling for a senescence response, we mapped the region in its cytoplasmic tail that controls signaling. We identified a conserved TRAF6 binding motif, which was required for CD158d-induced NF-κB activation and IL-8 secretion, for TRAF6 association with CD158d, and for TRAF6 recruitment to CD158d+ endosomes in transfected cells. The adaptor TRAF6 is known to couple proximal signals from receptors such as endosomal TLRs and CD40 through the kinase TAK1 for NF-κB-dependent pro-inflammatory responses. siRNA-mediated silencing of TRAF6 and TAK1, and inhibition of TAK1 blocked CD158d-dependent IL-8 secretion. Stimulation of primary, resting NK cells with soluble Ab to CD158d induced TRAF6 association with CD158d, induced TAK1 phosphorylation, and inhibition of TAK1 blocked the CD158d-dependent reprogramming of NK cells that produces the SASP signature. Our results reveal that a prototypic TLR and TNF-receptor signaling pathway is used by a killer cell immunoglobulin-like receptor that promotes secretion of pro-inflammatory and pro-angiogenic mediators as part of a unique senescence phenotype in NK cells.
Introduction
Natural killer (NK) cells carry out their effector functions through their cytolytic activity and cytokine secretion, and have important roles in immune defense and in reproduction (1, 2). CD158d (KIR2DL4) is a unique member of the killer cell Ig-like receptor (KIR) family and is expressed by all NK cells and a subset of T cells (3). Unlike most other KIR family members that are expressed at the cell surface, CD158d resides primarily in endosomes, from where it generates pro-inflammatory/pro-angiogenic signals in response to its ligand, soluble HLA-G (4). A signaling cascade involving the DNA damage kinase DNA-PKcs, Akt, and NF-κB is initiated upon CD158d engagement (5). This endosomal signaling results in the induction of cellular senescence in primary, resting NK cells and the production of a characteristic senescence associated secretory phenotype (SASP) (6). The secretome of these metabolically active senescent NK cells contain factors, including cytokines and chemokines that promote vascular remodeling and angiogenesis. NK cell reprogramming towards a SASP has implications at sites of HLA-G expression, such as in in early pregnancy and in certain tumors (7). NK cells, which are abundant at the implantation site, may sense the invasion of fetal trophoblast cells by responding to HLAG, a non-classical MHC molecule produced by these fetal cells in the early weeks of pregnancy (8). Endosomal signaling for a senescence response and sustained SASP may promote the vascular remodeling required for successful placentation. In support of this, a senescence signature was seen in a retrospective analysis of microarrays of decidual NK cells isolated from first trimester abortions that were compared to peripheral blood NK cells (6, 9).
How CD158d initiates NF-κB signaling for a senescence response is not known. Here we show that a short stretch of amino acids in the cytoplasmic tail of CD158d recruits the adaptor TRAF6 (tumor necrosis factor receptor-associated factor 6), which is an essential node in TLR signaling. We demonstrate that this recruitment of TRAF6 regulates NF-κB signaling in response to KIR2DL4, and that its interacting partner kinase TAK1 (transforming growth factor β-activated kinase 1) is required for the senescence response induced by KIR2DL4. Thus, we show the unexpected usage of signaling effectors of the TLR family by an endosomal innate immune receptor on NK cells.
Materials and Methods
Cell culture
HEK293T cells were obtained from ATCC (American Type Culture Collection) and cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% FBS. Cells were transiently transfected using LipofectAMINE 2000 (Invitrogen), according to the manufacturer's instructions. 293T-2DL4-GFP cells are HEK293T cells that were stably transfected with a plasmid encoding a fusion protein of 2DL4 and GFP, (4). Human polyclonal NK cells were isolated from peripheral blood lymphocytes from anonymous donors at the NIH Department of Transfusion Medicine, under an NIH Institutional Review Board-approved protocol, with informed consent. NK cells were isolated using the Negative Selection Human NK Cell Enrichment Kit (Stem Cell Technologies). NK cells were greater than 97% CD3− and CD56+. Freshly isolated NK cells (“resting NK”) were cultured in Iscove's modified DMEM containing 10% human serum without added IL-2 or feeders. Resting NK cells were incubated with control IgG1 (MOPC21), or agonist anti-KIR2DL4 (mAb #33) at 10 μg/ml for 16 h. NKL cells (a gift from M. Robertson, Indiana School of Medicine, Indianapolis, IN) were cultured in RPMI 1640 medium containing 10% fetal calf serum, 1% glutamine, 1% sodium pyruvate and 200 U/ml of recombinant IL-2. NKL cells were rested for 48 h in the absence of IL-2 and in reduced fetal calf serum (2%) prior to stimulation with anti-2DL4 antibodies.
Antibodies and reagents
The following Abs were used: anti-TRAF6: H-274 (used for immunoblotting and immunocytochemistry), D-10 (used for immunoprecipitation); HRP-conjugated anti-rabbit and anti-mouse Abs from Santa Cruz Biotechnology; anti-actin Ab from BD Biosciences; Alexa Fluor 488-conjugated anti-HA (6E2), anti-TAK1, anti-phospho-TAK1 (Thr187), anti-phospho-p38 MAPK (Thr180/Tyr182), anti-phospho-IκBα (Ser32/36), and anti-MOPC21 Abs from Cell Signaling Technology; anti-KIR2DL4 mAb (MAB2238) from R&D Systems; anti-KIR2DL4 mAbs #33 (IgG1), #36 (IgM) and #64 (IgM) were produced in our laboratory (10); anti-HA and Alexa Fluor 594-conjugated anti-HA (16B12) Abs from Covance; anti-GFP and anti-rabbit Alexa Fluor 564 Abs from Molecular Probes; and anti-Triad3A Ab from Abcam. HRP-conjugated Protein-A was from BD Biosciences and was used for Western blot detection of immunoprecipitated cell extracts. For immunocytochemistry experiments, ProLong Anti-fade kit and Image-iT™ FX Signal Enhancer (used to improve signal-to-noise ratio of immunolabeled cells) were purchased from Molecular Probes. TAK1 inhibitor (5Z)-7-Oxozeaenol was obtained from Tocris Bioscience.
Molecular constructs
The HA-tagged wild-type 2DL4 in the pDisplay vector (Stratagene) was previously described (4). Mutations in the cytoplasmic tail of 2DL4 were generated from the pDisplay-2DL4 expression vector using a Stratagene Quick Change site-directed mutagenesis kit according to the manufacturer's instructions. The following primers were used: forward 5'-GAGGACTCTGATGAAGCAGACCCTCAGGAGGTG-3' and reverse 5'-CACCTCCTGAGGGTCTGCTTCATCAGAGTCCTC-3' to generate a Q270A mutation; forward 5'-TCTGATGAACAAGACGCTCAGGAGGTGACATAC-3' and reverse 5'-GTATGTCACCTCCTGAGCGTCTTGTTCATCAGA-3' to generate a P272A mutation; forward 5'-GAACAAGACCCTCAGGCGGTGACATACGCACAG-3' and reverse 5'-CTGTGCGTATGTCACCGCCTGAGGGTCTTGTTC-3' to generate a E274A mutation; forward 5'-CCTCAGGAGGTGACAGCCGCACAGTTGGATCAC-3' and reverse 5'-GTGATCCAACTGTGCGGCTGTCACCTCCTGAGG-3' to generate a Y277A mutation; forward 5'-CAGGAGGTGACATACGCAGCGTTGGATCACTGCATTTTC-3' and reverse 5'-GAAAATGCAGTGATCCAACGCTGCGTATGTCACCTCCTG-3' to generate a Q279A mutation. The 2DL4 7A construct was generated using another cytoplasmic tail mutant (denoted 2DL4 5A) as a backbone. The 2DL4 5A mutant was generated by changing a. a. 271-275 (DPQEV to AAAAA). The primers used to generate the 2DL4 5A construct were: forward 5'-TCTGATGAACAAGCCGCTGCGGCGGCGACATACGCACAG-3' and reverse 5'-CTGTGCGTATGTCGCCGCCGCAGCGGCTTGTTCATCAGA-3'. Using 2DL4 5A mutation as a backbone, the 2DL4 7A construct was generated using the following primers: forward 5'-GCCGCTGCGGCGGCGGCAGCCGCACAGTTGGATCAC-3' and reverse 5'-GTGATCCAACTGTGCGGCTGCCGCCGCCGCAGCGGC-3'.
Immunostaining and confocal microscopy
Forty-eight hours after transfection with a plasmid encoding wildtype or mutant HA-2DL4, HEK293T cells or 293T-2DL4-GFP cells were incubated with an Alexa Fluor 488-conjugated mouse anti-HA (6E2) Ab or an Alexa Fluor 594-conjugated mouse anti-HA (16B12) Ab respectively, for two hours to allow for receptor internalization. Cells were allowed to settle onto poly-D-lysine-coated culture slides (BD Biosciences) for 30 min at room temperature. For co-localization experiments involving 2DL4 and endogenous TRAF6, cells were then fixed in PBS and 4% paraformaldehyde for 30 min followed by permeabilization with 0.2% Triton X-100 in PBS for 15 min. Image-it™ FX Signal Enhancer (Molecular Probes) was added to cells for 30 min, and cells were blocked for one hour at room temperature or overnight at 4°C. Cells were incubated with rabbit anti-TRAF6 H-274 Ab (Santa Cruz Biotechnology) for three hours at room temperature, incubated with anti-rabbit Alexa Fluor 564 secondary Ab (Molecular Probes) for one hour at room temperature, washed and mounted in slides with ProLong Anti-Fade reagent. Cells were imaged using a confocal laser-scanning microscope (Axiovert 200M LSM 510 META; Zeiss, Jena, Germany). Quantitative assessment of fluorophore co-localization was performed using Image J software (http://rsbweb.nih.gov/ij/). A region of interest from a non-cell area was traced using the tools in Image J, allowing for the exclusion of background/non-specific fluorescence from the image. Pearson's correlation coefficients were calculated using the Intensity Correlation Analysis plugin to determine the degree of correlation between channels one and two. A Pearson's value of zero indicates no co-localization while a value of one indicates perfect co-localization of all pixels.
RNA interference
HEK293T cells were transfected with siRNAs (Dharmacon) at a final concentration of 100 nM using Lipofectamine 2000 (Invitrogen). The siRNAs used were: ONTARGETplus SMARTpool siRNA (consists of four individual siRNA oligos) for TRAF6 and TAK1, ON-TARGETplus individual siRNA duplexes for TRAF6 (J-004712-09-0005) and TAK1 (J-003790-19-0005) and two custom synthesized individual siRNAs for Triad3A (Triad3A siRNA #1: sense AAGUGCUCAGUAGUCAGGACAUU and antisense UGUCCUGACUACUGAGCACUUUU; Triad3A siRNA #2: sense UCUGGACCGAUCCCACUGAAUU and antisense UUCAGUGGGAUCGGUCCAGAUU). ON-TARGETplus non-targeting siRNA pool (consists of four individual non-targeting siRNAs) was used a negative control in all siRNA experiments. Forty-eight hours after transfection with siRNA, HEK293T cells were transfected with 0.5 μg of 2DL4 plasmid DNA. 24 h after 2DL4 transfection and 72 h from the start of siRNA transfection, the culture medium was tested for the production of IL-8 by ELISA. Cell lysates from the siRNA-transfected cells were analyzed by immunoblotting to assess knockdown efficiency of target proteins.
Immunoprecipitation and immunoblotting
Cells were washed with ice-cold PBS and lysed in 1% NP40 lysis buffer containing 50 mM Tris, 140 mM NaCl, and protease-inhibitor cocktail (Roche). Cell lysates were pre-cleared with control IgG and protein G-agarose beads for one hour, followed by incubation with various antibodies at 4°C. Protein G-agarose beads were then added and allowed to bind for one hour at 4°C. Immunoprecipitates were collected by brief centrifugation, washed six times, and eluted by boiling in 2X LDS sample buffer (Invitrogen). For blotting, equivalent amounts of proteins (40 μg per sample) were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane. The membrane was blocked overnight with 5% non-fat dry milk or 5% BSA in PBST (PBS plus 0.1% Tween-20) at 4°C, probed overnight with primary antibody, washed five times (5 min each) with PBST, and incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for one hour. For immunoblots of immunoprecipitated samples, blots were incubated with Protein A-HRP (BD Biosciences). After washing five times (5 min each) with PBST, blots were developed with ECL (Thermo Scientific Pierce). For biochemical detection of the association between TRAF6 and 2DL4, freshly isolated resting NK cells or NKL cells that were rested for 48 h were stimulated with either control IgM TEPC 183 (cIgM) or anti-KIR2DL4 mAbs (#36 + #64) for 16 h. Total lysates were prepared as above and immunoprecipitated with anti-KIR2DL4 (MAB2238, IgG2a) or cIgG (29E4.G7, IgG2a) covalently coupled to Protein G agarose beads. Western blotting was carried out as described above. For biochemical detection of TAK1 phosphorylation, NK cells were stimulated with either cIg or anti-2DL4 (mAb #33) at 10 μg/ml for 16 h in the presence of 1 μM of TAK1 inhibitor. Total cell lysates were blotted as described above.
Reporter Assays
To assess reporter gene expression, HEK293T cells were transfected with the relevant siRNAs and 2DL4 plasmids as described above. NF-κB-luciferase reporter construct in pLuc MCS vector (Stratagene) and the constitutively expressing Renilla plasmid were transfected 48 h after the first set of transfections. After 48 h, cell lysates were analyzed with the Dual Luciferase Reporter Assay System (Promega) to measure firefly and Renilla luciferase activities. To normalize the responses of each sample to the efficiency of transfection, Renilla luciferase was used as an internal reference.
IL-6 and IL-8 secretion assays
24 or 48 h after transfection of HEK293T cells, culture supernatants were removed and tested for IL-8 by ELISA (Biosource) according to the manufacturer's instructions. After 24 hours of stimulation of primary, resting NK cells with either cIg or anti-2DL4 (#33) mAbs at 10 μg/ml, supernatants were tested for IL-6 by ELISA using the LEGEND MAX IL-6 ELISA kit (Biolegend).
Real time PCR
Total RNA from resting NK cells stimulated with mAbs in the presence or absence of TAK1 inhibitor for 16 h was isolated using the RNeasy Mini-Kit (Qiagen) and real-time PCR was performed using the iQ-SYBR Green SuperMix Kit (Bio-Rad) with the iCycler sequence detection system (Bio-Rad) using GAPDH as the internal control. The primers used have been described previously (6).
Statistical analysis
The statistical examination of results was performed using unpaired two-tailed Student's t-tests with GraphPad Prism Software. Data are represented as mean ± SEM. P values below 0.05 were considered as statistically significant.
Results
Mutational analysis of the CD158d cytoplasmic tail identifies a TRAF6 binding motif required for signaling
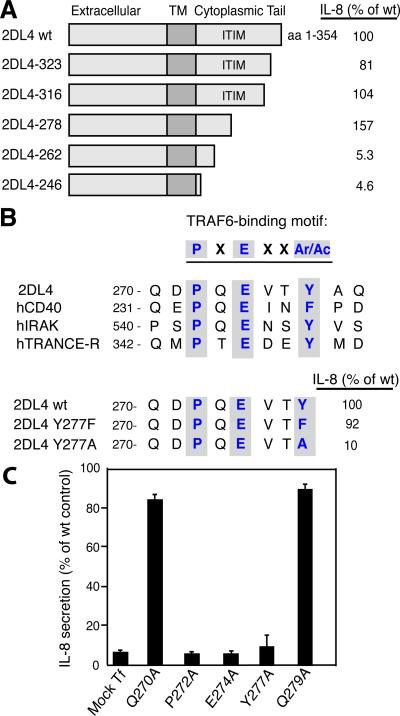
Earlier work showed that transfection of CD158d (hereafter referred to as 2DL4) into human HEK293T cells results in 2DL4 transport to endosomes, where it signals for the constitutive production of IL-8 (4). Our previous work has shown that DNA-PKcs is required for signaling by 2DL4 in both 293T-2DL4 cells and in primary NK cells (5). The sharing of this unusual requirement of a DNA damage response (DDR) kinase for an endosomal signaling pathway between primary NK cells and 293T cells expressing 2DL4, as well as several other shared features, supports the use of 293T-2DL4 cells as a tool to examine specific aspects of the signaling pathway. This 2DL4-dependent secretion of IL-8 by HEK293T cells, which requires the 2DL4 cytoplasmic tail (4), provided a convenient assay to map the amino acid residues that were required for signaling. Sequential truncations of the 2DL4 cytoplasmic tail were engineered and tested in HEK293T cells for induction of IL-8 secretion (Fig. 1A). Each one of the five cytoplasmic truncation mutants localized in endosomal vesicles, as determined by confocal microscopy (unpublished observations), consistent with our earlier finding that endosomal localization of 2DL4 is controlled by the Ig domains (4). Notably, mutant 2DL4-278, in which the cytoplasmic tail is truncated within the single immunoreceptor tyrosine-based inhibition motif (ITIM), retained the ability to induce IL-8 secretion, indicating that the ITIM was not required for 2DL4-mediated signaling. Further truncations of the cytoplasmic tail, as in mutants 2DL4-262 and 2DL4-246, did not induce IL-8 secretion by HEK293T cells. Therefore, a region essential for receptor function was narrowed down to a short stretch of a. a. 263-278.
FIGURE 1. Mutagenesis of the 2DL4 cytoplasmic domain identifies critical residues for signaling.
A, The 2DL4 cytoplasmic tail truncations, as indicated, were transfected into HEK293T cells. After 48 h, IL-8 secretion was measured by ELISA. IL-8 secreted after transfection of wt 2DL4 was 430 pg/ml. The data shown is representative of 2 to 4 experiments for each truncation. B, The TRAF6-binding motif P-X-E-X-X-Ar/Ac, (Ar, aromatic; Ac, acidic) in the 2DL4 cytoplasmic tail is aligned with similar motifs in human CD40, IRAK, and TRANCE-R. The aromatic Y277 in the 2DL4 cytoplasmic tail was mutated to either aromatic F or aliphatic A. IL-8 secretion was measured 48 h after transfection of HEK293T cells with 2DL4 and the Y277F or Y277A mutants. IL-8 secreted after transfection of wt 2DL4 was 1750 pg/ml. The data shown is representative of 2 experiments. C, Plasmids encoding point mutations of the indicated 2DL4 cytoplasmic tail residues were transfected into HEK293T cells. After 48 h, IL-8 secretion was measured by ELISA. Mock Tf: Mock-transfected cells. Results represent the mean ± SEM of triplicate samples from one representative experiment out of 3 experiments. IL-8 secreted after transfection of wt 2DL4 ranged from 888 to 1110 pg/ml.
Examination of this region revealed a canonical TRAF6-binding motif of the general form PxExx-(Ar/Ac), with either an aromatic (Ar) or an acidic (Ac) residue at the last position (11) (Fig. 1B). Further mutagenesis of this region was performed to test its role in signaling by 2DL4. Mutation of Y277 to A, converting the last residue of the TRAF6 binding motif from an Ar/Ac to an aliphatic amino acid resulted in a loss of signaling ability by the receptor (Fig. 1B). In contrast, mutation of Y277 to F did not affect signaling. Our results are so far consistent with a TRAF6 binding site in which replacement of an aromatic Y277 with another aromatic residue, F, is not expected to disrupt the TRAF6 binding site (11).
Next, we performed alanine-scanning mutagenesis of residues that form the putative TRAF6 binding site. HA-tagged 2DL4 mutants were transfected into HEK293T cells and IL-8 secretion was examined after 48 h (Fig. 1C). Immunoblotting for the HA tag was performed in every experiment to verify equivalent levels of transfected 2DL4-HA in HEK293T cells (unpublished data). Expression of mutants of each of the consensus residues in the TRAF6 binding motif, P272A, E274A, and Y277A abrogated 2DL4-mediated production of IL-8 (Fig. 1C). Thus, we identified a TRAF6 binding motif in the cytoplasmic tail of 2DL4 that is required for signaling.
TRAF6 associates with 2DL4 and is recruited to 2DL4+ endosomes
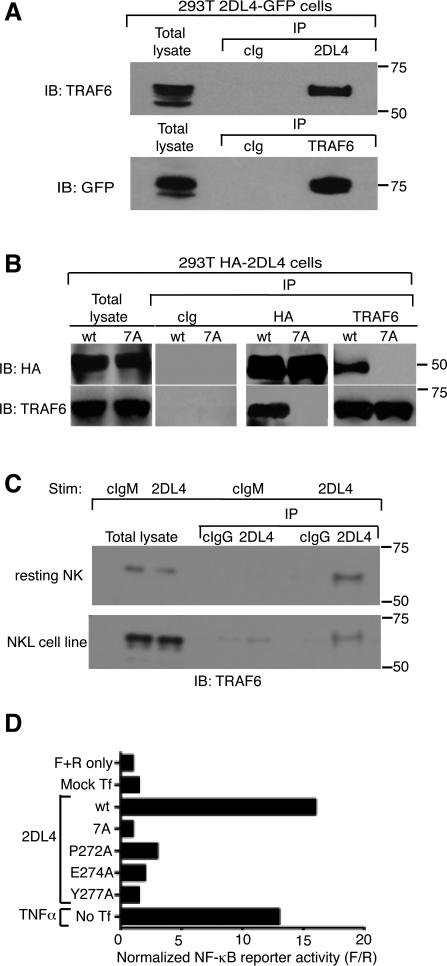
To test association of TRAF6 with 2DL4, HEK293T cells stably expressing 2DL4 tagged with GFP (293T–2DL4-GFP) were used for co-immunoprecipitation studies. Immunoprecipitation of lysates with antibodies against either 2DL4 or TRAF6, followed by immunoblotting for the presence of TRAF6 or 2DL4-GFP, respectively, revealed an association between TRAF6 and 2DL4-GFP (Fig. 2A). The specificity of this association was evaluated with a 2DL4 mutant lacking the TRAF6 binding motif, in which residues 271-277 were mutated to alanine, denoted 7A. To exclude the possibility that GFP contributed to the TRAF6 association, a 2DL4 tagged with an HA epitope (HA-2DL4) was used. Cell lysates of HEK293T cells transfected with plasmids encoding wild-type HA-2DL4 (wt) or mutated HA-2DL4 (7A) were immunoprecipitated using anti-TRAF6 or anti-HA antibodies, and immunoblotted for HA (to detect 2DL4) and TRAF6 (Fig. 2B). Co-immunoprecipitation of TRAF6 with HA-2DL4 was observed only in cells transfected with HA-2DL4 wt, and not with the HA-2DL4 7A mutant (Fig. 2B). These results demonstrate that TRAF6 associates with 2DL4, and that this interaction depends on amino acids 271-77.
FIGURE 2. A canonical TRAF6-binding motif in the 2DL4 cytoplasmic tail is required for NF-κB activation.
A, TRAF6 associates with 2DL4. Cell lysates from HEK293T–2DL4-GFP cells were immunoprecipitated (IP) with control antibody (cIg), anti-2DL4 antibody, or anti-TRAF6 antibody, as indicated, and analyzed by immunoblotting (IB) with antibodies against TRAF6 and GFP. B, Mutagenesis of the TRAF6-binding region in the cytoplasmic tail of 2DL4 abrogates binding. Wild-type (wt), HA-tagged 2DL4 (HA-2DL4) and the 7A mutant (amino acids 271-277 replaced by alanine) were transfected in HEK293T cells. After 48 h, cell lysates were immunoprecipitated with control antibody (cIg), or antibodies against HA or TRAF6 followed by immunoblotting using anti-HA and anti-TRAF6 antibodies. C, TRAF6 association with 2DL4 in resting NK cells. Resting NK cells (top panel) or rested NKL cells (bottom panel) were stimulated with IgM Abs to 2DL4 and control IgM (cIgM) as indicated for 16 h and lysed. Lysates were immunoprecipitated as indicated with control Ab (cIgG) or anti-2DL4 IgG Ab and analyzed by immunoblotting with antibodies against TRAF6. D, NF-κB reporter activity in HEK293T cells transfected with either wt 2DL4 or the indicated 2DL4 mutants. Mock Tf: mock transfection. F+R: Firefly and Renilla luciferase constructs only. Stimulation of untransfected cells with TNF-α (10 ng/ml) during the final 5 hours of culture served as a positive control. These data are representative of 3 independent experiments.
To examine the association of TRAF6 and 2DL4 in NK cells and to test whether this association required 2DL4 stimulation, freshly isolated resting NK cells were incubated with cIgM or agonist IgM antibodies to 2DL4 for 16 h followed by immunoprecipitation of lysates with IgG2a cIgG or anti-2DL4 Abs that were directly coupled to beads. Immunoblotting for TRAF6 revealed association in cells stimulated with anti-2DL4 Abs but not with control Ab (Fig. 2C). Similar analysis was also carried out in the IL-2 dependent NKL cell line that was rested for 48 h in the absence of IL-2 in medium containing reduced serum (2% FCS). A low basal association between 2DL4 and TRAF6 was detected in NKL, which was increased upon stimulation with anti-2DL4 Abs (Fig. 2C). These results show that TRAF6 associates with 2DL4 in NK cells, and that in primary resting NK cells, this association is induced by engagement of 2DL4.
To test if the TRAF6 binding site in 2DL4 is required for NF-κB activation, we used a reporter assay for NF-κB activation. Plasmids encoding 2DL4 wt or 2DL4 mutated at the three consensus amino acids in the TRAF6 binding motif were transfected in HEK293T cells together with a reporter plasmid for NF-κB-dependent promoter activation. 2DL4 expression induced an NF-κB dependent reporter activity comparable to that induced by TNF-α, a potent inducer of NF-κB activation (Fig. 2D). In contrast, the three single amino acid mutants (P272A, E274A, and Y277A) as well as the 7A mutant failed to activate NF-κB. Thus, the TRAF6 binding motif was required for NF-κB dependent reporter activity induced by 2DL4.
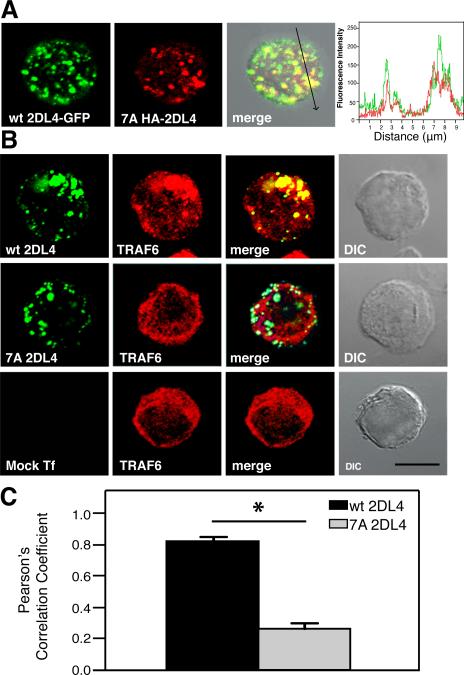
2DL4 resides in and signals from Rab5-positive early endosomes (4). Even though trafficking of 2DL4 to endosomes is controlled by its lumenal Ig domains (4), we tested whether the lack of signaling by the TRAF6 binding motif mutants could be due to mislocalization. HEK293T cells that stably express gfp-tagged 2DL4 (2DL4-gfp) were transiently transfected with a plasmid encoding the HA-2DL4 7A mutant. The 7A mutant was labeled using endocytosed HA Ab. Imaging by confocal microscopy showed that the endocytosed HA-2DL4 7A mutant was in vesicular structures that co-localized with endosomes containing stably expressed wild-type 2DL4-GFP (Fig. 3A). In addition, each of the single alanine substitutions that affected 2DL4 signaling (Fig. 1C) by disrupting the TRAF6-binding site also co-localized with endosomal wild-type 2DL4 (unpublished observations). Thus, disruption of the TRAF6-binding site does not result in mislocalization of 2DL4.
FIGURE 3. TRAF6 colocalizes with endosomal 2DL4.
A, The endocytosed 7A 2DL4 mutant colocalizes with wt 2DL4-gfp in HEK293T cells. Intact cells were incubated with anti-HA Alexa Fluor 594 at 37°C for 2 h, fixed and analyzed by confocal microscopy. The arrow in the third panel indicates the axis along which the fluorescence intensities shown in the fourth panel were traced. B, An intact TRAF6-binding domain is required for co-localization with endosomal 2DL4. HEK293T cells were mock-transfected (bottom panel) or transiently transfected with plasmids encoding either wt 2DL4 (top panel) or 7A 2DL4 (middle panel). Intact cells were incubated with anti-HA Alexa Fluor 488, fixed, permeabilized, stained with anti-TRAF6 antibody plus secondary antibody conjugated with Alexa Fluor 564, and analyzed by confocal microscopy. wt and 7A 2DL4 are shown in green, and endogenous TRAF6 is shown in red. Scale bar: 20 μM C, Pearson's Correlation Coefficient for co-localization of endogenous TRAF6 with wt or 7A 2DL4 in the transfected HEK293T cells. Results are presented as mean ± SEM of 12 cells transfected with WT 2DL4 and 10 cells transfected with 7A 2DL4 (*P<0.0001; Student's t-test).
The cytosolic adaptor TRAF6 can be recruited to endosomes and mediate signaling by various receptors. For example, TRAF6 is recruited to endosomal IL-1 receptor complexes in the presence of NADPH oxidase-generated reactive oxygen species, leading to NF-κB activation (12, 13) TRAF6 also interacts with CD40 that has been internalized into endosomal vesicles and transmits CD40-dependent proinflammatory signals (14). We therefore investigated the colocalization of endogenous TRAF6 with endosomal HA-2DL4 wt or HA-2DL4 7A in transfected HEK293T cells (Fig. 3B). While TRAF6 displayed diffuse cytosolic staining in mock-transfected cells, a fraction of TRAF6 was localized to endosomes containing 2DL4 upon expression of wild-type 2DL4. In contrast, in cells transfected with HA-2DL4 7A, the TRAF6 distribution pattern remained cytosolic. Quantitative co-localization analysis gave a Pearson's Correlation Coefficient (PCC) of 0.820 ± 0.020 (n = 12 cells) for wild-type 2DL4-transfected cells and 0.265 ± 0.026 (n = 10 cells) for 7A-transfected cells (Fig. 3C). Thus, an intact TRAF6-binding domain in 2DL4 is required for the recruitment of TRAF6 to endosomes containing 2DL4.
TRAF6 silencing impairs 2DL4-mediated signaling in HEK293T cells
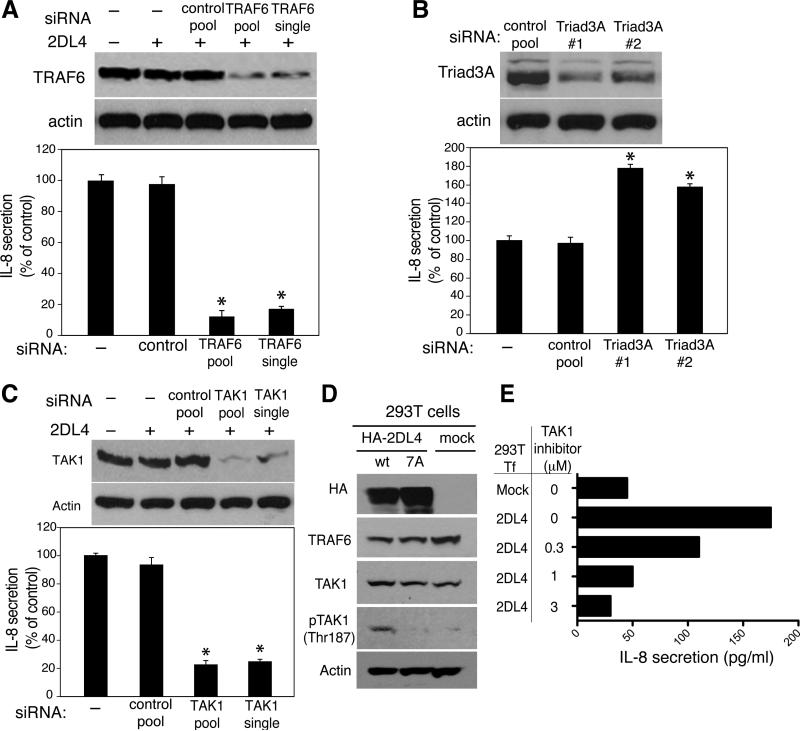
To study the role of TRAF6 in 2DL4-mediated signaling, we tested the effects of TRAF6 silencing on 2DL4-induced IL-8 secretion in HEK293T cells. Transfection of siRNA for TRAF6 led to a reduction in TRAF6 levels in both pool and individual duplex TRAF6 siRNA–transfected cells (Fig. 4A, top panel). This knockdown of TRAF6 expression using pooled TRAF6 siRNA or individual duplex TRAF6 siRNA markedly reduced 2DL4-mediated IL-8 secretion, relative to mock-transfected cells (Fig. 4A). These results indicate that TRAF6 is required for endosomal signaling by 2DL4.
FIGURE 4. TRAF6 and TAK1 silencing impairs 2DL4-mediated signaling.
A, siRNA-mediated silencing of endogenous TRAF6 in HEK293T cells impairs 2DL4-mediated IL-8 secretion. Total cell lysates of HEK293T cells transfected with the indicated siRNAs and HA-2DL4 were analyzed for TRAF6 expression by immunoblotting. Actin was used as a loading control. IL-8 secretion by these cells was measured by ELISA. Values are mean ± SEM of two replicates per experimental group. The data shown are representative of at least eight independent experiments (*P<0.0006, compared with 2DL4-induced IL-8 secretion by cells transfected with negative control siRNA; Student's t-test). B, Triad3A silencing enhances IL-8 production by 2DL4 in HEK293T cells. Silencing of endogenous Triad3A in HEK293T cells was as described for TRAF6 and monitored by immunoblotting. IL-8 secretion by HEK293T cells was measured by ELISA 72 h post-transfection of Triad3A siRNA. Values are mean ± SEM of triplicate samples from two independent experiments. (*P<0.0001, compared with control siRNA; Student's t-test). C, Silencing of TAK1 in HEK293T cells by siRNA was monitored after 72 h by immunoblotting. IL-8 secretion was measured by ELISA after 72 h. Values are mean ± SEM of duplicate samples from two independent experiments. (*P=0.003, compared with control siRNA; Student's t-test). D, Lysates of HEK293T cells transfected with plasmids encoding wt HA-2DL4 or 7A HA-2DL4 for 48 h, were analyzed by immunoblotting, using the indicated antibodies. Actin was used as a loading control. E, TAK1 inhibitor (5Z-7-Oxozeanol) blocks 2DL4-mediated IL-8 secretion in HEK293T cells. 24 h after transfection with HA-2DL4, medium in the cultures was replaced with fresh medium containing 1 μM TAK1 inhibitor. 48 h later, supernatants were tested for IL-8 secretion by ELISA.
A positive role for the E3 ubiquitin ligase TRAF6 in promoting 2DL4-mediated signaling is in contrast to the role of Triad3A, another E3 ubiquitin ligase, which is involved in the negative regulation of 2DL4-mediated cytokine production. Triad3A promotes polyubiquitylation and degradation of 2DL4 (15). To compare the effects of TRAF6 and Triad3A on 2DL4-mediated signaling, both were silenced in parallel (Fig. 4B). In contrast to the marked reduction in 2DL4-induced IL-8 secretion after TRAF6 silencing (Fig. 4A), Triad3A silencing increased the relative 2DL4-induced IL-8 secretion as compared to mock-transfection (Fig. 4B). This is consistent with its role in promoting degradation of 2DL4 (15). Our data show that TRAF6 acts as a positive regulator of 2DL4 signaling, while Triad3A is a negative regulator.
Downstream of innate receptors, TRAF6 activates TAK1, which results in the activation of the classical NF-κB pathway (16). The requirement for TRAF6 in the 2DL4 signaling pathway is consistent with earlier evidence that 2DL4 signaling activates NF-κB and promotes the nuclear translocation of p65 (5).
TAK1 activity is required for 2DL4-mediated signaling
TRAF6-mediated activation of downstream kinases, including IκB kinase (IKK) and MAPKs, involves TAK1, a member of the MAP3K family (17, 18). TRAF6 activates TAK1, and in turn, phospho-TAK1 activates downstream signaling that leads to the activation of JNK, p38 MAPK, and NF-κB (19, 20). To determine whether TAK1 is required for 2DL4 signaling, the effect of TAK1 silencing on 2DL4-mediated IL-8 production was tested. Transfection with pooled or individual duplex TAK1 siRNAs, or a control non-targeting pool siRNA, was followed by transfection with a plasmid encoding 2DL4. TAK1 silencing (Fig. 4C, top panel) resulted in a significant reduction of 2DL4-mediated IL-8 secretion relative to mock-transfected cells (Fig. 4C). These results implicate TAK1 in 2DL4-mediated signaling, leading to IL-8 secretion.
To test if TAK1 activation is controlled by TRAF6 during 2DL4 signaling, HEK293T cells were transfected with a plasmid encoding either wild-type 2DL4 or the 7A mutant, which lacks the TRAF6-binding motif. After 24 h, phosphorylation of TAK1 at T187 was examined in total cell extracts (Fig. 4D). Expression of HA-2DL4 in HEK293T cells induced TAK1 phosphorylation at T187. In contrast, phosphorylation of TAK1 was almost undetectable in cells transfected with the 7A mutant, implicating the 2DL4-TRAF6 interaction in the activation of TAK1. These results show that an intact TRAF6-binding domain in the 2DL4 cytoplasmic tail is required for TAK1 activation in the 2DL4-TRAF6 signaling pathway.
We also examined the effects of a pharmacological inhibitor of TAK1, 5Z-7-oxozeaneol, on 2DL4-mediated IL-8 secretion in HEK293T cells. 5Z-7-oxozeaneol, a resorcylic lactone of fungal origin, inhibits the kinase activity of purified TAK1 while having no significant effect on the kinase activity of other members of the MAP3K family (21). In cells transfected with plasmid encoding 2DL4 and incubated in fresh media containing increasing concentrations (0.3 – 3 μM) of 5Z-7-oxozeaneol, there was a dose-dependent inhibition of IL-8 secretion with increasing concentrations of the TAK1 inhibitor (Fig. 4E). Collectively, these results indicate that TAK1 is a component of the TRAF6 signaling complex involved in 2DL4 signaling.
Inhibition of TAK1 blocks the 2DL4-dependent senescence response in primary NK cells
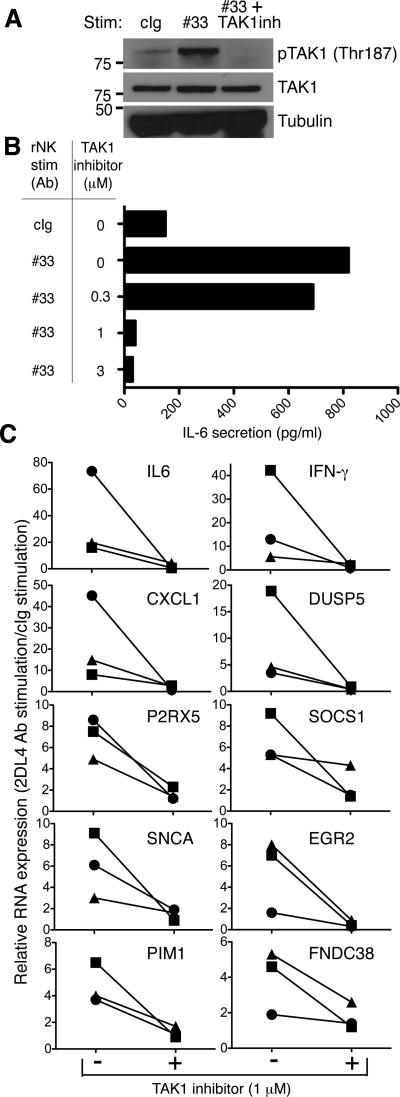
In the presence of soluble agonist Ab to 2DL4, primary NK cells undergo senescence and secrete cytokines such as IL-6, IL-8 and IFN-γ as part of their senescence secretome (6). It was not possible to silence TRAF6 or TAK1 in primary, resting NK cells, as these cells are not amenable to transfection with siRNA or plasmids. To examine the role of TAK1 in primary NK cells, we first tested if activation via 2DL4 resulted in TAK1 phosphorylation. Phosphorylation of TAK1 at T187 was detected upon 2DL4 activation by agonist Ab #33 (Fig. 5A). In the presence of the TAK1 inhibitor, resting NK cells showed no detectable phosphorylation of TAK1 at T187 (Fig. 5A). Furthermore, pharmacological inhibition of TAK1 in primary NK cells stimulated via 2DL4 prevented the secretion of IL-6 (Fig. 5B), a cytokine that is indispensible for establishing and maintaining the senescent state (22).
FIGURE 5. TAK1 inhibition in primary NK cells blocks the induction of SASP.
A, Freshly isolated primary NK cells were treated with either control IgG (cIg) or agonist Ab to 2DL4 (#33) for 16 h. TAK1 inhibitor 5Z-7-Oxozeanol (as indicated) was added 30 min before stimulation of cells by Ab #33. Total cell lysates were blotted for phospho-TAK1, TAK1 and tubulin. Data is representative of that obtained from 3 independent donors. B, IL-6 secretion of supernatants from cells treated as described in A was measured by ELISA. C, qRT-PCR analysis of genes induced during the senescence response mediated by 2DL4. Fold increase in gene expression (2DL4 stimulation/cIg stimulation) in the presence or absence of TAK1 inhibitor in resting NK cells of 3 donors, after stimulation for 16 h. Each symbol (circle, square, triangle) represents data from the same donor. Different scales are used according to strength of gene induction.
Sustained activation through 2DL4 induces a transcriptional signature of a senescence associated secretory profile (SASP) (6). SASP is a program that involves the secretion of an array of pro-inflammatory cytokines and immune modulators by senescent cells, including IL-6, IFN-γ, CXCL1 and P2RX5 (22, 23). To test if TAK1 was required for the 2DL4-mediated SASP of resting NK cells, we stimulated resting NK cells with agonist antibody to 2DL4 for 16 h in the presence or absence of 1 μM TAK1 inhibitor. Quantitative RT-PCR was used to test the relative expression of a set of 10 genes that contribute to the senescent signature of primary NK cells. Up-regulation of each of these 10 genes upon stimulation of NK cells with agonist Ab to 2DL4 was blocked in the presence of 1 μM TAK1 inhibitor (Fig. 5C). These results suggest that TAK1 is required for the SASP that results from the activation of primary NK cells by 2DL4.
Discussion
The NK receptor KIR2DL4 (CD158d) resides in endosomes and generates signals from this compartment using an unusual signaling pathway that does not involve the activation of PI3K or Src family kinases. It acts as a sensor of its ligand, soluble HLA-G, in the extracellular milieu and constitutive endocytosis of 2DL4 correlates with the uptake of sHLA-G into the same endosomes (24). Sustained signaling from these endosomes results in DNA damage response (DDR) signaling for a senescence response in NK cells and leads to an NF-κB dependent secretion of a distinctive profile of soluble mediators that both reinforce the senescence phenotype and make up the SASP (6). In this study, we have addressed how the NF-κB signaling for the SASP is regulated during activation via 2DL4.
This study has identified TRAF6 and TAK1 as proximal players in endosomal signaling by 2DL4 for a senescence response. Both molecules have known functions in innate TLR and TNF-receptor signaling pathways. While TRAF6 binds directly to TNF-R family member CD40, engagement of TLRs leads to recruitment of TIR domain containing adaptors (e.g. MyD88) that associate with IRAKs and TRAF6, leading to TAK1 phosphorylation for NF-κB activation (25). NF-κB in turn has been identified as a master regulator of senescence by controlling the SASP and promoting senescence (26, 27). We show here that 2DL4 bypasses the requirement for TIR domains by directly recruiting TRAF6 via a conserved binding motif in its cytoplasmic tail to activate TAK1 and induce NF-κB activation. Thus, there is inducible association of 2DL4 with TRAF6 in NK cells and TAK1 activation in response to engagement of 2DL4 can promote a senescence response in NK cells. To our knowledge, this is the first description of a receptor belonging to the KIR family coopting signaling effectors used by TLR and TNF-R family members.
What are the implications of this TRAF6-TAK1-mediated response at sites of HLA-G expression? As engagement of 2DL4 via this pathway is a molecular switch for a SASP, these signals are likely to promote vascular permeability and angiogenesis at sites of HLA-G expression, such as in the maternal decidua during early pregnancy. We have previously proposed that a positive role of senescence is to favor reproduction through sustained NK activation to remodel maternal vasculature early in pregnancy (28). HLA-G expression has also been detected de novo during viral infections, transplantation, autoimmunity and upon malignant transformation of healthy cells (7). How signaling by 2DL4 through this pathway influences NK cell functions at sites of de novo HLA-G expression such as tumors awaits further investigation.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH.
Abbreviations
- IKK
IκB kinase
- IRAK
IL-1 receptor associated kinase
- KIR
killer cell Ig-like receptor
- SASP
senescence associated secretory phenotype
- TAK1
transforming growth factor β activated protein kinase 1
- TRAF6
TNF receptor associated factor 6
REFERENCES
- 1.Parham P, Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat Rev Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long EO, Sik Kim H, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajagopalan S, Long EO. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front Immunol. 2012;3:258. doi: 10.3389/fimmu.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajagopalan S, Bryceson YT, Kuppusamy SP, Geraghty DE, van der Meer A, Joosten I, Long EO. Activation of NK cells by an endocytosed receptor for soluble HLA-G. PLoS Biol. 2006;4:e9. doi: 10.1371/journal.pbio.0040009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan S, Moyle MW, Joosten I, Long EO. DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Science Signaling. 2010;3:ra14. doi: 10.1126/scisignal.2000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Long EO. Cellular senescence induced by CD158d reprograms natural killer cells to promote vascular remodeling. Proc Natl Acad Sci U S A. 2012;109:20596–20601. doi: 10.1073/pnas.1208248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J. Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood. 2008;111:4862–4870. doi: 10.1182/blood-2007-12-127662. [DOI] [PubMed] [Google Scholar]
- 8.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2:656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 9.Koopman LA, Kopcow HD, Rybalov B, Boyson JE, Orange JS, Schatz F, Masch R, Lockwood CJ, Schachter AD, Park PJ, Strominger JL. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001;167:1877–1881. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 11.Ye H, Arron JR, Lamothe B, Cirilli M, Kobayashi T, Shevde NK, Segal D, Dzivenu OK, Vologodskaia M, Yim M, Du K, Singh S, Pike JW, Darnay BG, Choi Y, Wu H. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–447. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2- dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–154. doi: 10.1128/MCB.26.1.140-154.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284:33255–33264. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Chen J, Xiong Y, Da Q, Xu Y, Jiang X, Tang H. Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem Biophys Res Commun. 2006;345:106–117. doi: 10.1016/j.bbrc.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Miah SM, Purdy AK, Rodin NB, MacFarlane A. W. t., Oshinsky J, Alvarez-Arias DA, Campbell KS. Ubiquitylation of an internalized killer cell Ig-like receptor by Triad3A disrupts sustained NF-kappaB signaling. J Immunol. 2011;186:2959–2969. doi: 10.4049/jimmunol.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacker H, Tseng PH, Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev Immunol. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 19.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 21.Ninomiya-Tsuji J, Kajino T, Ono K, Ohtomo T, Matsumoto M, Shiina M, Mihara M, Tsuchiya M, Matsumoto K. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem. 2003;278:18485–18490. doi: 10.1074/jbc.M207453200. [DOI] [PubMed] [Google Scholar]
- 22.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9:81–94. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 23.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopalan S. Endosomal signaling and a novel pathway defined by the natural killer receptor KIR2DL4 (CD158d). Traffic. 2010;11:1381–1390. doi: 10.1111/j.1600-0854.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi T, Walsh MC, Choi Y. The role of TRAF6 in signal transduction and the immune response. Microbes and infection / Institut Pasteur. 2004;6:1333–1338. doi: 10.1016/j.micinf.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-kappaB promotes senescence and enhances chemosensitivity. Genes Dev. 2011;25:2125–2136. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovillain E, Mansfield L, Caetano C, Alvarez-Fernandez M, Caballero OL, Medema RH, Hummerich H, Jat PS. Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene. 2011;30:2356–2366. doi: 10.1038/onc.2010.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopalan S, Long EO. A positive role for senescence in reproduction? Aging. 2013;5:96–97. doi: 10.18632/aging.100538. [DOI] [PMC free article] [PubMed] [Google Scholar]