Abstract
Background
As a traditional Chinese medicine herb, Chonglou (Paris polyphylla var. chinensis) has been used as anticancer medicine in China in recent decades, as it can induce cell cycle arrest and apoptosis in numerous cancer cells. The saponins extract from the rhizoma of Chonglou [Rhizoma Paridis saponins (RPS)] is known as the main active component for anticancer treatment. However, the molecular mechanism of the anticancer effect of RPS is unknown.
Material/Methods
The present study evaluated the effect of RPS in non-small-cell lung cancer (NSCLC) A549 cells using the 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT) assay and flow cytometry. Subsequently, the expression of several genes associated with cell cycle and apoptosis were detected by reverse transcription-quantitative polymerase chain reaction (qRT-PCR) and Western blotting.
Results
RPS was revealed to inhibit cell growth, causing a number of cells to accumulate in the G 1 phase of the cell cycle, leading to apoptosis. In addition, the effect was dose-dependent. Moreover, the results of qRT-PCR and Western blotting showed that p53 and cyclin-dependent kinase 2 (CDK2) were significantly downregulated, and that BCL2, BAX, and p21 were upregulated, by RPS treatment.
Conclusions
We speculated that the RPS could act on a pathway, including p53, p21, BCL2, BAX, and CDK2, and results in G1 cell cycle arrest and apoptosis in NSCLC cells.
MeSH Keywords: Apoptosis; Carcinoma, Non-Small-Cell Lung; Cell Cycle Checkpoints; Saponins
Background
Lung cancer occurs frequently in males and females, with ~520 000 new cases generated and 450 000 fatalities from lung cancer/year in China [1]. Over 80% of lung cancer patients are diagnosed as having non-small-cell lung cancer (NSCLC) at advanced stages of the disease [2]. Despite significant progress toward understanding lung cancer in the past 2 decades, NSCLC has an extremely poor 5-year survival rate [3,4], as evidenced by the finding that the epidermal growth factor receptor (EGFR) tyrosine kinase and anaplastic lymphoma kinase (ALK) inhibitors, including gefitinib, erlotinib, and crizotinib, have shown superior improvement of survival time and life quality in the subset of patients harboring EGFR or ALK mutations [5–8]. Regardless, gefitinib and crizotinib has improved the progression-free and overall survival in patients; however, drug resistance has remained a significant problem affecting patient survival [9]. Thus, new, longer-lasting, targeted therapeutic strategies are required.
Natural products have been the mainstay of cancer chemotherapy for the past 30 years, and their mechanisms of action require in-depth analysis [10,11]. Chonglou (Paris polyphylla var. chinensis), a traditional Chinese medicine herb, has been applied in the treatment of various types of cancer, including lung cancer, breast cancer, brain tumors, and digestive system carcinomas in recent decades [12,13]. The main constituents of the saponins extract from the rhizoma of Chonglou, known as Rhizoma Paridis saponins (RPS), were identified as polyphyllin D, formosanin C, dioscin, Paris H, and Paris VII. Numerous studies have proved that RPS was the main active ingredient for anticancer treatments [14–16]. These extracts could induce apoptosis, affect cell cycle distribution, inhibit angiogenesis, and improve the immune function in cancer cells [12–16]. However, due to the complexity and various actions of herbal components, the explicit antitumor mechanisms of RPS remain unknown.
In the present study, the antitumor effect and mechanism of RPS were examined on NSCLC A549 cells, and cell proliferation, cell cycle, and apoptosis were measured by 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT) assay and flow cytometry, and the expression level of the genes and proteins associated with the cell cycle and apoptosis were detected by reverse transcription-quantitative polymerase chain reaction (qPCR) and Western blotting. Finally, a pathway was identified that could be affected by RPS and results in G1 cell cycle arrest and apoptosis in NSCLC cells.
Material and Methods
RPS extraction
Chonglou rhizomes were ground to powder and 20 g of the powder was extracted twice with 30 ml of 80.0% alcohol under reflux in a water bath for 1 h. The combined extracts were filtered and concentrated by a rotary evaporator (De Hua Materials Testing Co., Ltd., Chengdu, China). Distilled water (250 ml) was added to the crude extract and the sample was extracted by water-saturated butanol (500 ml) for 12 h. Finally, the water-saturated butanol was concentrated to sediment, which was the RPS.
Cell lines and cell culture
The NSCLC A549 cell line was provided by West China-Frontier Pharma Tech Co., Ltd. (Chengdu, China). The cells were maintained as monolayers at 37°C in an atmosphere containing 5% CO2/O2 in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Life Technologies, Rockville, MD, USA) containing 10% heat-inactivated fetal bovine serum (FBS, Gibco Life Technologies) and 1% penicillin/streptomycin (Gibco Life Technologies). For RPS treatment, the cells were plated for 48 h in DMEM containing 10% FBS. The medium was subsequently changed to DMEM containing 5% charcoal-dextran-treated FBS with various concentrations of RPS.
MTT assay
The cytotoxicity of RPS in the A549 cell line was assessed using the MTT assay. Cells (5×103 cells/well) were plated in 96-well plates in 100 μl DMEM with various concentrations of RPS (0.5, 1.0, and 2.0 mg/ml) for 24, 48, 72, 96, and 120 h, and subsequently, an equivalent volume of MTT (0.5 mg/ml) was added to each well. After 4-h incubation at 37°C, the cells were centrifuged at 2000 rpm for 5 min, followed by the addition of 100 ml dimethylsulfoxide to each well to dissolve the formed formazan crystals by agitation for 10 min. The absorbance at 490 nm was measured using an ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA).
Flow cytometry
A549 cells were treated with various concentrations of RPS (0.5, 1.0, and 2.0 mg/ml) for 24 h. A total of 3×105–5×105 cells were washed with chilled phosphate-buffered saline and resuspended in 1X binding buffer (100 ml). A total of 5 μl of annexin V (AV)-fluorescein isothiocyanate solution and 1 μl of dissolved propidium iodide (PI) were added to the cell suspensions to investigate whether the growth inhibition of RPS was caused by apoptosis. Subsequently, the cells were gently vortexed and incubated at room temperature in the dark for 15 min. Following this, 400 μl of chilled binding buffer was added and mixed gently prior to the examination of the cell preparations by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA, USA).
RT-qPCR
Genes were validated by RT-qPCR assays. The RT-qPCR reactions were performed on a Roche LightCycler Instrument 1.5, using a LightCycler® FastStart DNA Master PLUS SYBR-Green I kit (Roche cat. no. 03515885001; Roche Diagnostics Australia Pty Limited, Castle Hill, Australia). Briefly, the reactions had a 15 μl volume: 7.5 μl Master mix, 0.1 μl forward primer and reverse primer, 1 μl cDNA sample and 6.3 μl ddH2O were prepared. Each sample was run in triplicate. The RT-qPCR program was set to 95°C for 5 min and subsequently 45 cycles of 95°C for 10 s, 55–60°C for 35 s and 72°C for 40 s. At the end of each program, a melting curve analysis was performed. In addition, the data were automatically analyzed by the system and an amplification plot was generated for each cDNA sample at the end of each qPCR run. The reference gene was β-actin. The primers of each gene used are shown in Table 1.
Table 1.
The PCR primers of genes.
| Gene | Sequence (5′–>3′) | Length |
|---|---|---|
| β-actin | Forward primer | CATGTACGTTGCTATCCAGGC |
| Reverse primer | CTCCTTAATGTCACGCACGAT | |
| P53 | Forward primer | GAGGTTGGCTCTGACTGTACC |
| Reverse primer | TCCGTCCCAGTAGATTACCAC | |
| BCL2 | Forward primer | GGTGGGGTCATGTGTGTGG |
| Reverse primer | CGGTTCAGGTACTCAGTCATCC | |
| p21 | Forward primer | CGATGGAACTTCGACTTTGTCA |
| Reverse primer | GCACAAGGGTACAAGACAGTG | |
| BAX | Forward primer | CCCGAGAGGTCTTTTTCCGAG |
| Reverse primer | CCAGCCCATGATGGTTCTGAT | |
| Cylin A | Forward primer | TGGAAAGCAAACAGTAAACAGCC |
| Reverse primer | GGGCATCTTCACGCTCTATTT | |
| Cylin B1 | Forward primer | TTGGGGACATTGGTAACAAAGTC |
| Reverse primer | ATAGGCTCAGGCGAAAGTTTTT | |
| Cylin D1 | Forward primer | TGGAGCCCGTGAAAAAGAGC |
| Reverse primer | TCTCCTTCATCTTAGAGGCCAC | |
| Cylin E | Forward primer | GCCAGCCTTGGGACAATAATG |
| Reverse primer | CTTGCACGTTGAGTTTGGGT | |
| CDK2 | Forward primer | GTACCTCCCCTGGATGAAGAT |
| Reverse primer | CGAAATCCGCTTGTTAGGGTC | |
| CDK4 | Forward primer | CTGGTGTTTGAGCATGTAGACC |
| Reverse primer | GATCCTTGATCGTTTCGGCTG | |
| CDK6 | Forward primer | CCAGATGGCTCTAACCTCAGT |
| Reverse primer | AACTTCCACGAAAAAGAGGCTT |
Western blotting
Cell lysates were separated by SDS-PAGE in 8% Tris-glycine gels (Invitrogen Life Technologies, Carlsbad, CA, USA) and transferred to a nitrocellulose membrane. To determine the expression levels of p53, cyclin-dependent kinase 2 (CDK2), BCL2, BAX and p21, blots were probed with their specific antibodies [diluted with 5% bovine serum albumin (BSA) to 1:1,000]. Membranes were probed with horseradish peroxidase-labeled anti-rabbit secondary antibody (diluted with 5% BSA to 1:1000; Cell Signaling Technology, Inc., Danvers, MA, USA). Antibody binding was detected by enhanced chemiluminescence detection kit (Amersham International PLC, Buckinghamshire, UK)
Results
RPS inhibits the proliferation of A549 cells
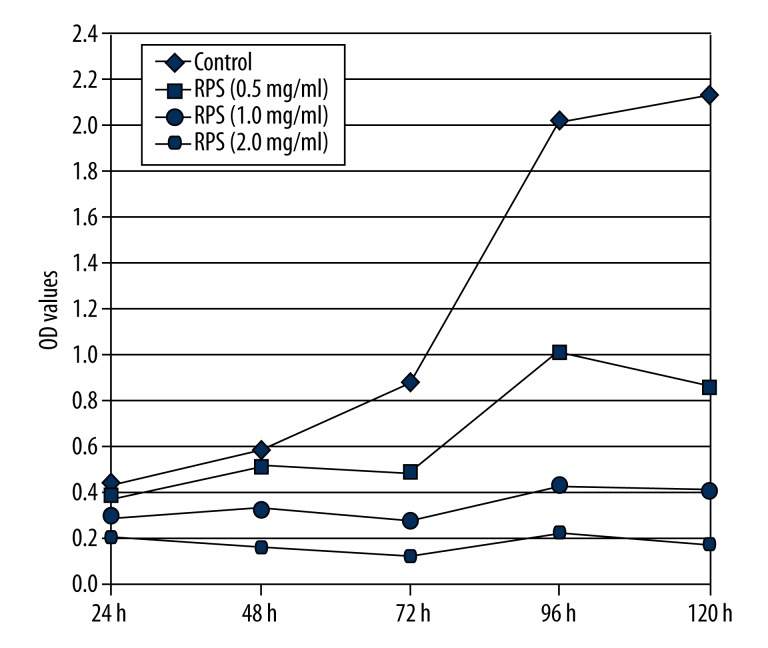
As shown in Figure 1, accompanied with the increasing incubation times, the optical density (OD) values of the RPS-treated cells were significantly lower compared with the control cells and showed an inverse correlation between OD values and concentrations of RPS, which indicate that RPS could inhibit the proliferation of A549 cells in a dose-dependent manner. Additionally, the A549 cells almost stop proliferation when concentrations of RPS were >1.0 mg/ml.
Figure 1.
Effect of Rhizoma Paridis saponins (RPS) on the proliferation of A549 cells. OD, optical density.
RPS induces cell apoptosis in A549 cells
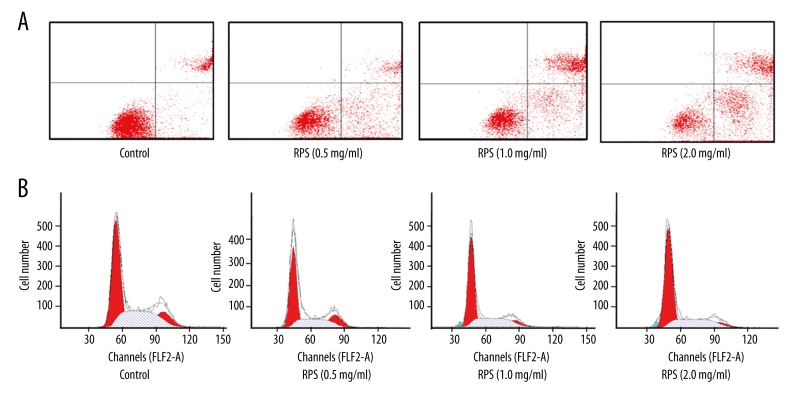
As shown in Table 2 and Figure 2A, the percentages of apoptotic cells were significantly increased in RPS treatment groups and showed a positive correlation with concentrations of RPS, indicating that RPS could induce the apoptosis of A549 cells in a dose-dependent manner.
Table 2.
Effect of RPS on the apoptosis of A549 cells.
| Groups | UL (%) (monocyte) | UR (%) (necrotic cells, late stage apoptotic cells) | LL (%) (live cells) | LR (%) (early apoptotic cells) |
|---|---|---|---|---|
| Control | 0.01 | 8.79 | 87.03 | 4.17 |
| RPS (0.5 mg/ml) | 0.08 | 28.9 | 48.89 | 22.13 |
| RPS (1.0 mg/ml) | 0.21 | 25.8 | 35.17 | 38.82 |
| RPS (2.0 mg/ml) | 1.16 | 28.56 | 23.79 | 46.49 |
UL – upper left; LL – lower left; UR – upper right; LR – lower right.
Figure 2.
Effect of Rhizoma Paridis saponins (RPS) on the apoptosis and cell cycle of A549 cells. Effect of RPS on the (A) apoptosis and (B) cell cycle of A549 cells.
RPS induces the G1 cell cycle arrest in A549 cells
As shown in the results in Figure 2B and Table 3, the treatment of A549cells with RPS caused the accumulation of cells at the G1 phase of the cell cycle, in addition to a significant decrease in the number of cells in the S phase. These trends were enhanced with increased concentrations of RPS. However, there was no significant change for the number of cells in the G2 phase, which indicated that RPS could induce cell cycle arrest of A549 cells in G1 phase.
Table 3.
Effect of RPS on the cell cycle of the A549 cells.
| Groups | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|
| Control | 50.84±0.03 | 35.35±2.89 | 13.81±3.34 |
| RPS (0.5 mg/ml) | 63.57±4.93* | 24.11±5.17* | 12.32±2.19 |
| RPS (1.0 mg/ml) | 70.79±6.07* | 17.13±4.87* | 12.08±5.12 |
| RPS (2.0 mg/ml) | 74.05±3.61* | 14.19±2.85* | 11.76±1.17* |
P<0.05 as determined by a student’s t-test compared with the control group.
Effect of RPS on the expression of genes associated with cell cycle and apoptosis
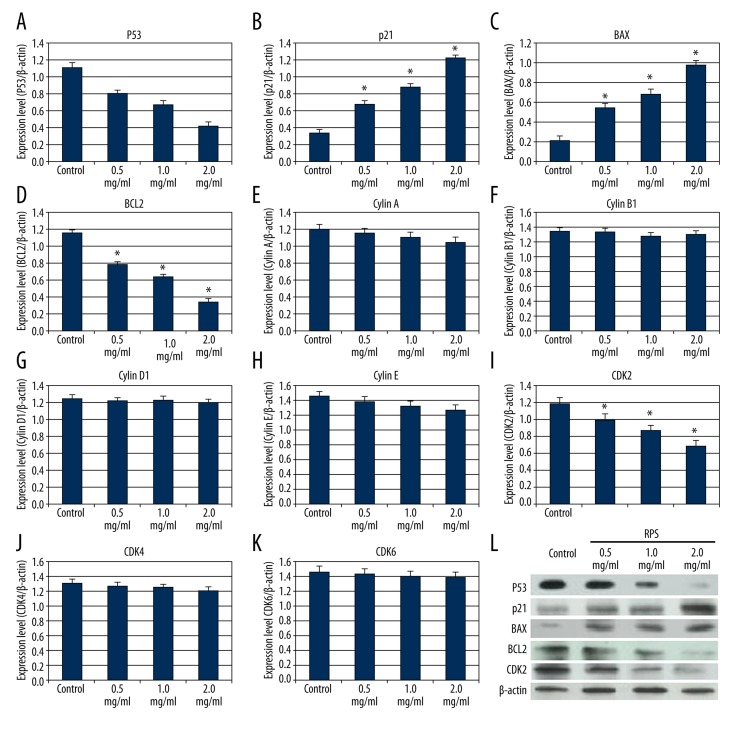
As shown in Figure 3, the expression of p53, BCL2 and CDK2 were significantly downregulated by RPS treatment (Figure 3A, 3D, 3I). In comparison, the expression of p21 and BAX were significantly upregulated by RPS treatment (Figure 3B, 3C). However, the expression of cyclins A/B1/D1/E and CDK4/6 did not show any clear changes (Figure 3E–3H, 3J, 3K). Subsequently, Western blotting was used to detect the protein expression of p53, p21, BAX, BCL2, and CDK2, and the results verified that the expression of the 5 proteins changed in accordance with the qPCR results (Figure 3L).
Figure 3.
Effect of Rhizoma Paridis saponins (RPS) on expression of cell cycle and apoptosis-related genes. Results of (A–K) quantitative polymerase chain reaction for genes and (L) Western blotting for the proteins. * P<0.05, as determined by a Student’s t-test compared with the control group.
Discussion
RPS has extensive medicinal value, including antimicrobial, anthelmintic, hemostasis, immunoregulation, analgesia, and anticancer effects [17–20]. However, the complexity and varying action of components of RPS has limited its extensive application and hindered the study of their underlying molecular mechanism(s). A number of studies have focused on identifying and analyzing the compounds in RPS to research the anticancer bioactivators of Chonglou [21–23].
Certain studies have shown that RPS has an anticancer effect by regulation of the cell cycle and apoptosis [14,24,25]. However, the underlying mechanism of RPS remains unknown. The present study investigated the effect of RPS in NSCLC A549 cells by the MTT assay and flow cytometry, and identified that RPS could inhibit cell growth and induce G1 cell cycle arrest and apoptosis in A549 cells in a dose-dependent manner, indicating that RPS has an evident effect in NSCLC treatment. Subsequently, to investigate the molecular mechanism of the effect for RPS treatment, expression of genes (p53, p21, BAX, BCL2, CDK2/4/6, and cyclins A/B1/D1/E) associated with proliferation, cell cycle arrest, and apoptosis were detected using qPCR and Western blotting. Finally, p53, p21, BAX, BCL2 and CDK2 were identified to have significant expression changes. In carcinogenesis, p53 is known as a ‘superstar’ gene, which can regulate cell growth, DNA repair, angiogenesis, and apoptosis by participating in various cell signaling pathways [26,27], and p21 is a downstream protein of p53, which is a key inhibitor of the CDKs and can regulate the cell cycle by adjusting the activity of cyclin/CDK complexes [28]. CDK2, a member of the CDK family, is an important kinase in the G0/G1 cell cycle [29]. BAX and BCL2 are also downstream proteins of p53, and cell apoptosis depends on the intracellular balance between BCL2 and BAX activity [30]. Evidently, expression changes of those 5 genes can perturb cell growth and apoptosis. NSCLC is a heterogeneity disease that is associated with numerous gene variations and expression imbalance, and disturbance of multiple biological functions, including cell cycle, apoptosis, angiogenesis, and immune escape [31,32].
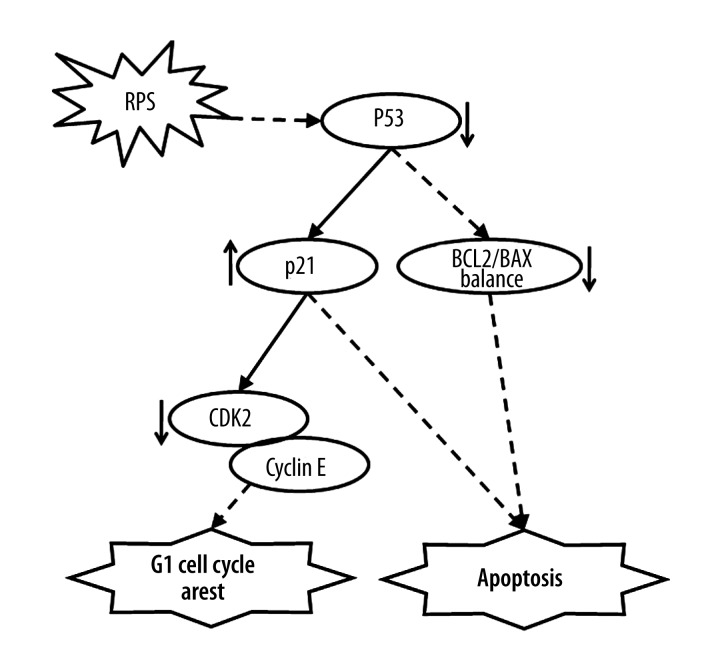
The present study showed that RPS could suppress proliferation by inducing cell cycle arrest and apoptosis of the NSCLC cells, and regulate the expression of p53, p21, BAX, BCL2, and CDK2. Therefore, in accordance with the cell cycle, apoptosis pathways, and the present experimental results, we speculate that there is a pathway including p53, p21, BAX, BCL2, and CDK2 that can be affected by RPS (Figure 4). In this pathway, RPS could directly or indirectly affect the target-regulated expression of p53, and subsequently change the expression of the downstream genes p21, BAX, BCL2, and CDK2, and lead to cell proliferation inhibition, cell cycle arrest, and apoptosis, finally inhibiting NSCLC progression.
Figure 4.
Mechanism of Rhizoma Paridis saponins (RPS) treatment for non-small-cell lung cancer. CDK2, cyclin-dependent kinase 2.
Conclusions
The present study demonstrated that RPS can inhibit NSCLC cell growth by inducing G1 cell cycle arrest and apoptosis in a dose-dependent manner; 5 associated differentially expressed genes (p53, p21, BAX, BCL2, and CDK2) were detected as being involved in these processes. Finally, the study established a pathway for RPS treatment to reveal the molecular mechanism of the anticancer effect of RPS at cellular and molecular levels (Figure 4), which is helpful for improving the medicinal application of the anticancer effect of Chonglou.
Footnotes
Source of support: This research was supported by Support Project of National Science and Technology (No. 2011BAI13B02-6) Project of the Education Department in Sichuan (No. 13ZB0342), Project of Engineering Scientific Research of Chengdu University (No. 20819031)
References
- 1.Tan X, Qin W, Zhang L, et al. A 5-microRNA signature for lung squamous cell carcinoma diagnosis and hsa-miR-31 for prognosis. Clin Cancer Res. 2011;17(21):6802–11. doi: 10.1158/1078-0432.CCR-11-0419. [DOI] [PubMed] [Google Scholar]
- 2.Peng B, Wang YH, Liu YM, Ma LX. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med. 2015;8(3):3098–106. [PMC free article] [PubMed] [Google Scholar]
- 3.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3(4):242–49. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rittmeyer A. Quality of Life in patients with NSCLC receiving maintenance therapy. Cancers (Basel) 2015;7(2):950–62. doi: 10.3390/cancers7020817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 6.Ellis PM, Coakley N, Feld R, et al. Use of the epidermal growth factor receptor inhibitors gefitinib, erlotinib, afatinib, dacomitinib, and icotinib in the treatment of non-small-cell lung cancer: a systematic review. Curr Oncol. 2015;22(3):e183–215. doi: 10.3747/co.22.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juchum M, Günther M, Laufer SA. Fighting cancer drug resistance: Opportunities and challenges for mutation-specific EGFR inhibitors. Drug Resist Updat. 2015;20:12–28. doi: 10.1016/j.drup.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Kanaji N, Tadokoro A, Watanabe N, et al. Increases in serum CYFRA21-1 concentration during successful treatment with crizotinib. Am J Case Rep. 2014;15:480–84. doi: 10.12659/AJCR.891194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–95. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 10.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2001;2(2):143–48. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 11.Qiu JX, He YQ, Wang Y, et al. Plumbagin induces the apoptosis of human tongue carcinoma cells through the mitochondria-mediated pathway. Med Sci Monit Basic Res. 2013;19:228–36. doi: 10.12659/MSMBR.884004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21(11):1102–4. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Wu W, Zheng YL. Simultaneous determination of four steroidal saponins compounds in Paris ployphylla by high performance liquid chromatography reversed-phase. Shi Zhen Guo Yi Guo Yao. 2007;18(8):1896–97. [Google Scholar]
- 14.Zhang W, Zhang D, Ma X, et al. Paris saponin VII suppressed the growth of human cervical cancer Hela cells. Eur J Med Res. 2014;19:41. doi: 10.1186/2047-783X-19-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan LL, Zhang YJ, Gao WY, et al. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp Oncol. 2009;31(1):27–32. [PubMed] [Google Scholar]
- 16.Shuli M, Wenyuan G, Yanjun Z, et al. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm Res. 2011;34(1):43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 17.Wang GX, Han J, Zhao LW, et al. Anthelmintic activity of steroidal saponins from Paris polyphylla. Phytomedicine. 2010;17(14):1102–5. doi: 10.1016/j.phymed.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Hao J, Gao W, et al. Study on hemostatic activities of the rhizome of Paris bashanensis. Pharm Biol. 2013;51(10):1321–25. doi: 10.3109/13880209.2013.790065. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Mou Y, Shan T, et al. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules. 2010;15(11):7961–70. doi: 10.3390/molecules15117961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GuangLie C, WeiShi G, GaiLing H, JianPing C. Effect of Paris saponin on antitumor and immune function in U14 tumor-bearing mice. Afr J Tradit Complement Altern Med. 2013;10(3):503–7. doi: 10.4314/ajtcam.v10i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y, Kang LP, Liu YX, et al. Steroidal saponins from the rhizome of Paris polyphylla and their cytotoxic activities. Planta Med. 2009;75(4):356–63. doi: 10.1055/s-0028-1088380. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Liu H, Liu XT, et al. Qualitative and quantitative analysis of steroidal saponins in crude extracts from Paris polyphylla var. yunnanensis and P. polyphylla var. chinensis by high performance liquid chromatography coupled with mass spectrometry. J Pharm Biomed Anal. 2010;51(1):114–24. doi: 10.1016/j.jpba.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhang YJ, Gao WY, Yan LL. Anti-tumor constituents from Paris polyphylla var. yunnanensis. Zhongguo Zhong Yao Za Zhi. 2007;32(14):1425–28. [PubMed] [Google Scholar]
- 24.Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9(6):2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 25.Xiao X, Zou J, Bui-Nguyen TM, et al. Paris saponin II of Rhizoma Paridis – a novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6(4):201–11. doi: 10.5582/bst.2012.v6.4.201. [DOI] [PubMed] [Google Scholar]
- 26.Muller PA, Vousden KH. p53 mutations in cancer. Nat Cell Biol. 2013;15(1):2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 27.Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14(5):359–70. doi: 10.1038/nrc3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim TG, Lee SY, Huang Z, et al. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev Res. 2014;7(4):466–74. doi: 10.1158/1940-6207.CAPR-13-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson LF, Fortney J, Magro G, et al. Regulation of BAX and BCL-2 expression in breast cancer cells by chemotherapy. Breast Cancer Res Treat. 1999;55(2):107–17. doi: 10.1023/a:1006175811676. [DOI] [PubMed] [Google Scholar]
- 31.Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–46. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]