Abstract
Background
Cryptic Epitopes (CE) are peptides derived from the translation of one or more of the five alternative reading frames (ARFs; 2 sense and 3 antisense) of genes. Here, we compared response rates to HIV-1 specific CE predicted to be restricted by HLA-I alleles associated with protection against disease progression to those without any such association.
Methods
Peptides (9–11mer) were designed based on HLA-I binding algorithms for B*27, B*57 or B*5801 (protective alleles) and HLA-B*5301 or B*5501 (non-protective allele) in all five ARFs of the nine HIV-1 encoded proteins. Peptides with >50% probability of being an epitope (n=231) were tested for T cell responses in an IFN-γ ELISpot assay. PBMC samples from HIV-1 seronegative donors (n=42) and HIV-1 seropositive patients with chronic clade B infections (n=129) were used.
Results
Overall, 16%, 2%, and 2% of CHI patients had CE responses by IFN-γ ELISpot in the protective, non-protective, and seronegative groups, respectively (p=0.009, Fischer’s exact test). Twenty novel CE specific responses were mapped (median magnitude of 95 SFC/106 PBMC) and the majority were both anti-sense derived (90%) as well as represented ARFs of accessory proteins (55%). CE-specific CD8 T cells were multifunctional and proliferated when assessed by intracellular cytokine staining.
Conclusions
CE responses were preferentially restricted by the protective HLA-I alleles in HIV-1 infection suggesting that they may contribute to viral control in this group of patients.
Keywords: Cryptic epitopes, alternate reading frames, chronic HIV-1 infection, protective alleles, HLA-B*53, HLA-B*57
INTRODUCTION
Development of a vaccine for HIV-1 that confers protection against HIV-1 infection or delays disease progression remains a formidable challenge1–3. Perhaps the biggest hurdle to an efficacious vaccine development is the enormous sequence diversity of HIV-1 and the propensity of the virus to escape cytotoxic CD8 T lymphocytes (CTL) and/or antibody recognition4–7,8. For a CTL based vaccine, novel approaches to vaccine design are currently being investigated in an attempt to circumvent this obstacle9–14. A dominant theme common to these studies is the use of vaccines designed to enhance the breadth (targeting more epitopes) of T-cell responses. The rationale for this line of research largely stems from the immunogenicity results obtained in non-human primates that have suggested that for a CTL based HIV-1 vaccine to be effective, it will need to induce an enhanced breadth of CD8 T-cell responses 15,16.
Several studies in human and non-human primates have shown that sense and antisense transcription and translation are fairly common likely owing to aberrations in gene expression that are driven by one or more of the multiple molecular mechanisms 17–32. Involvement of multiple reading frames is biologically plausible as among the 6 potential reading frames encoded in a double stranded DNA, one is designated as the ORF (open reading frame; frame usually associated with a functional protein) and five others represent ARFs (alternate reading frames) 33–38. Most HIV-1 epitopes, comprehensively characterized and studied to date in context of infection and vaccination, are those derived from known HIV-1 proteins encoded by the primary ORFs of the HIV-1 viral genome (traditional epitopes or TE). Cryptic epitopes (CE) represent a class of non-conventional epitopes, which have been shown in prior studies to be immunologically relevant in context of viral infections and cancers 30,32,39–63. Furthermore, studies in HIV-1 and SIV have shown that CE specific CTLs are under immune pressure to escape immunosurveillance 39,40,52,64,65. Despite the known immunogenicity of CE and its potential to increase the T cell breadth, most HIV-1 vaccines in clinical development do not induce pertinent responses directed against these epitopes 41.
A plethora of data in HIV-1 infection has now established a definite association of certain HLA class I alleles and disease progression66–74. Broadly, two groups of alleles can be delineated based on their disparate disease outcomes: protective allele (HLAs-B*27, B*57 and B*58) and non-protective allelic group (HLAs-B*53 and B*55) 66–74. Although studies on protective alleles and recognition of traditional epitopes are abundant, very few studies have addressed this association for cryptic epitopes. The first study that evaluated HLA-I restricted CE was done by Cardinaud et al 42 where CTL responses to CE were observed in HLA-B*07 expressing HIV-1 infected individuals. Using a selected set of CE (9–11 mers) based on HLA-I associated HIV-1 polymorphisms; we and others have previously shown that CE are frequently targeted during HIV-1 infection39,40. A recent paper illustrated enhanced ARF encoded immune responses in HIV infected patients receiving antiretroviral therapy (ART) 44. Similar to our prior work 39 most of the peptides used in this study were derived from reverse frames. The T-cell responses in this study were examined in a small subset of HIV-1 infected individuals expressing HLA A*02, B*07 and B*58 alleles 44. However, no study to date has assessed the frequency, functionality and biological significance of CE in context of specific HLA class I alleles associated with differential disease outcome.
In the current study, we compared predicted CE specific responses presented by “protective” (HLAs-B*58, B*57 and B*27) and the “non-protective” alleles (B*53 and B*55) in disease progression. We demonstrate that CD8 T-cell targeting by protective- CE is not uncommon and may therefore contribute to not only an increased breadth of responses but also durable viral control in individuals who are endowed with protective HLA-I alleles.
MATERIALS AND METHODS
Cryptic epitope prediction
Using consensus clade B sequences and the Epipred program (http://boson.research.microsoft.com/bio/epipred.aspx), we predicted HLA-I restricted (9–11mer peptides) that bind HLA-B*27, B*57, B*5801 (protective) or B*5301, B*5501 (non-protective) alleles for all the five alternative reading frames of the nine HIV-1 encoded proteins (ARF-CE, Table 1). A total of 231 predicted epitopes with posterior probability (pp) >0.5 were synthesized and tested for immunogenicity. Posterior probability of a predicted peptide is a statistical measure of an amino-acid sequence being a true epitope, as estimated from the interaction of peptide and HLA sequence characteristics 75. The epipred model was trained on all optimally defined (“A-list) HLA-epitope pairs listed in the Los Alamos database, in addition to all HLA-epitope pairs listed in the IEDB database (www.iedb.org) as eliciting a functional response. Negative training data were derived from randomly sampled peptides from the human proteome and from HLA-peptide pairs shown to exhibit low binding affinity and deposited in IEDB.
Table 1.
Peptide pools of predicted CE, previously described TE or OLPs tested in immunoassays.
| Sourcea | Pool nameb | Typec | HLA-Id | Pool Size | Locatione | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Restriction | Associated Prognosis | Sense | Antisense | ||||
|
| |||||||
| ARFs | B*27CE | CE | B*27 | Protective | 30 | 11 (37%) | 19 (63%) |
| B*57CE | CE | B*57 | Protective | 39 | 4 (10%) | 35 (90%) | |
| B*58CE | CE | B*58 | Protective | 90 | 10 (11%) | 80 (89%) | |
| B*5301/B*5501 | CE | B*5301 or B*5501 | Non-protective | 72 | 12 (17%) | 60 (83%) | |
|
| |||||||
| Gene | B*27 TE | TE | B*27 | Protective | 17 | 17 (100%) | |
| B*57/58 TE | TE | B*57 or B*58 | Protective | 32 | 32 (100%) | ||
| Gag | OLP | Multiple | Both | 123 | 123 (100%) | ||
| Pol-N | OLP | Multiple | Both | 125 | 125 (100%) | ||
| Pol-C | OLP | Multiple | Both | 124 | 124 (100%) | ||
ARF=alternative reading frame
CE= cryptic epitope, TE= traditional epitope, and Pol-N and Pol-C contain peptides representing the N or C terminal regions of the protein, respectively
OLP= overlapping peptides
alleles associated with disease progression in multiple prior studies
sense or forward and antisense or reverse alternate reading frames; number (%) of sense and antisense is based on pool size
Study cohort
PBMC samples from clade B HIV-1 chronically (n=129, Table 2) infected individuals were used for immune assays. These individuals were enrolled from the 1917 HIV-1 clinic at the University of Birmingham at Alabama. In addition, PBMC from healthy seronegative donors (n=42) from the Alabama Vaccine Research Clinic (AVRC) were used as controls. IRB approval was obtained and all participants consented for this study. Details on the demographics and clinical features of these individuals are shown in detail in Table 2.
Table 2.
Demographic and clinical charateristics of the chronically HIV-1 infected cohort.
| Protective alleles (PA) | Number | % | Non-protective alleles (NPA) | Number | % |
|---|---|---|---|---|---|
|
| |||||
| Total | 79 | 100 | Total | 50 | 100 |
| B*57 | 47 | 59 | B*53 | 46 | 92 |
| B*27 | 17 | 22 | B*55 | 4 | 8 |
| B*58 | 13 | 16 | |||
| B*27/B*57 | 2 | 3 | |||
|
| |||||
| Gender | Gender | ||||
| Female | 32 | 41 | Female | 31 | 62 |
| Male | 47 | 59 | Male | 19 | 38 |
|
| |||||
| Race | Race | ||||
| Caucasian | 38 | 45 | Caucasian | 4 | 8 |
| African American | 41 | 55 | African American | 45 | 90 |
| Other | 0 | 0 | Other | 1 | 2 |
|
| |||||
| On ART | On ART | ||||
| Yes | 34 | 43 | Yes | 35 | 70 |
| No | 45 | 57 | No | 15 | 30 |
|
| |||||
| Median* | Median* | ||||
| CD4 | 543 | CD4 | 490 | ||
| Plasma viral load | 1186 | Plasma viral load | 1309 | ||
Absolute CD4 T-cell count (X106/l); viral load (RNA copies/ml)
HIV-1 and non-HIV-1 antigens
The predicted protective and non-protective allele specific CE were synthesized in a 96 well peptide array format from New England peptides (Gardner, MA). Individual stock peptides were reconstituted at 10–40mM in DMSO. The immunogenicity was evaluated for each specific HLA specific pool (Table 1). For B*58, both pools and subpools were tested initially. As positive controls, pools representing immunodominant epitopes, previously shown to be restricted by the protective alleles in the Los Alamos database, were used (ORF-TE, Table 1). In addition, overlapping peptides (OLP, 15 mers overlapping by 11) for Gag (# 8117) and Pol (#6208) from NIH AIDS reagent program were also used (ORF-OLP, Table 1). Due to protein length, the latter was split in 2 pools (Pol-N OLP and Pol-C OLP). For the non-HIV-1 antigens, we used CEF (9-mers, #9808, 2ug/ml) and CMV pp65 peptide pool (15 mers overlapping by 11, #11549, 5 μg/ml), both from NIH AIDS Reagent program. These peptides were tested in both HIV-1 seronegative and seropositive individuals.
IFN-γ ELISpot assay
An ex-vivo IFN-γ ELISpot assay was performed as described previously 39,66,76. In brief, cryopreserved PBMC were thawed and allowed to rest overnight at 37° C. Nitrocellulose 96-well plates were also coated overnight at 4° C with anti-IFN-γ monoclonal antibody. The following day, PBMCs (100,000 cells/well) in duplicate were incubated with appropriate peptide pool or single peptide at 2uM or 10uM respectively for 20–22 hours at 37° C in 5% CO2. The media or unstimulated controls were plated in quadruplicate. After washing, biotinylated anti-IFN-γ was added to the plates for 2 hours at room temperature. Following another round of washing, SA-conjugated alkaline phosphatase was added for 1 hr. and then NBT/BCIP added for color development. Individual cytokine-producing cells were counted by the ImmunoSpot CTL ELISpot reader. The criteria for a positive response was >50 SFC/106 PBMC and 2 times the unstimulated control. In addition, PHA was used as a positive control (duplicate) and samples with a PHA response of less than 500 SFC/106 were excluded from analysis.
Polychromatic flow cytometry (PFC)
Cryopreserved PBMC were stained in a PFC assay as described previously 39. In brief, PBMC were washed in RPMI containing 10% human AB sera (R-10 media) and co-stimulatory monoclonal antibodies (anti-CD28 and anti-CD49d; Becton Dickinson, San Jose, CA) at 1 ug/ml each and 50U/ml of Benzonase (Novagen, Madison, WI) were added to each tube containing 1×106 PBMC in 500μl R-10 media. For co-culture, CD107a-FITC was added. Cells were pulsed with the appropriate peptide, monensin and brefeldin for 12 hours at 37°C. Staphylococcus enterotoxin B (SEB) [1 ug/ml] was used as a positive control. The cells were stained for the dead cell dye marker and surface labeled for 20 min before Cytofix/cytoperm reagent was added. The fluorescent labeled antibodies used were: anti-CD3 (Alexa Fluor 780) and anti-CD8 (V500). After 20 min, the cells were labeled with intracellular antibodies i.e. anti-IL2 (APC), anti-TNFα (PECy7), anti-IFN-γ (Alexa Fluor 700) and anti-perforin (PE) for 20 min at room temperature. CD14 and CD19 labeled with PercpCy5.5 were used as a dump channel. The cells were fixed in 2% paraformaldehyde and analyzed on an LSR II flow cytometer. At least 100,000 CD3+ events were acquired and the data was analyzed using FlowJo Version 8.6.2 software. Lymphocytes were analyzed based on forward and side scatter profiles, and the gates were set based on the media control (irrelevant peptides) and applied to all samples from the same individual. Cytokines produced were measured from CD3+CD8+ cells. A response was considered positive if the value was greater than >2X the media control for that individual with a magnitude of ≥0.05%. All fluorochrome-conjugated antibodies were obtained from Becton Dickinson, San Jose, CA, USA.
In Vitro expansion of antigen specific CD8 T-cells
Peptide specific CD8 T-cells lines were expanded for 14 days in-vitro using monocytes as APC as described before 77. In brief, after 2 rounds of weekly antigenic stimulation, the CD8 T-cells were re-stimulated with the cognate antigen in the presence of co-stimulatory antibodies and intracellular transport inhibitors for 6 hours and analyzed as mentioned above. For HLA-I restriction experiments, HLA-class I matched and mismatched BLCL pulsed for 1 hr with the cognate peptide (10uM). The BLCL were washed three times before adding to the in-vitro expanded CD8 T-cells. The effector (CD8 T-cells) and targets (BLCL) were co-cultured at 5:1 (E:T) ratio for 6 hours in the presence of co-stimulatory antibodies and intracellular transport inhibitors as described above under ICS and the production of IFN-γ from CD8 T-cells was quantified.
Statistics
Comparisons of continuous variables within each group were done using the non-parametric Wilcoxon rank sum test. Analyses of variables between each group were performed using the non-parametric Mann-Whitney U test. Differences in the responder frequencies were compared using Fishers exact test.
RESULTS
Predicted peptides for HLA-I alleles with opposing disease outcomes
The total number of the CE tested along with a select set of HLA-I restricted traditional epitopes (TE) and overlapping peptides (OLPs) representing the entirety of Gag and Pol are detailed in Table 1. The evaluated peptides included representatives from all nine known HIV proteins and were enriched in the antisense direction (63–90%, Table 1) for all HLA alleles but B*27 (when correcting for the number of alternative reading frames in the sense vs. antisense directions).
Frequency of CE responses in individuals with favorable HLA subtypes
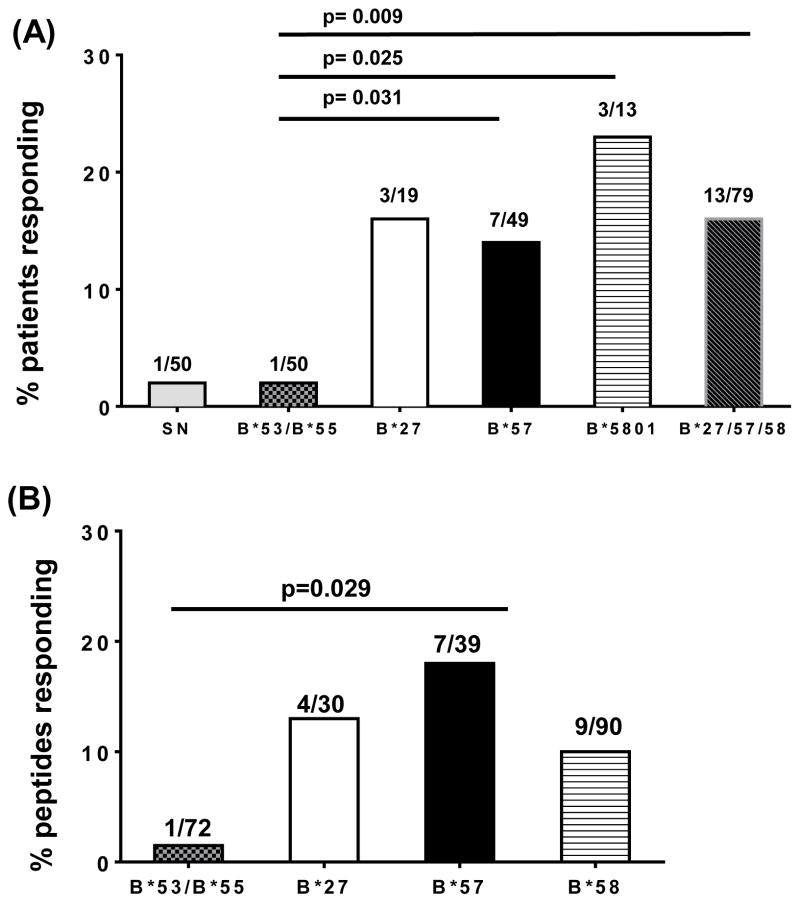
We evaluated CE specific immunogenicity in PBMC obtained from 42 HIV seronegative donors and 129 chronically HIV-1 infected individuals with B*27, B*57 or B*5801 (protective, n=79) or HLA-B*5301 or B*55 (non-protective, n=50) alleles. We first evaluated T-cell responses in 42 HIV-1 seronegative donors who were predominately Caucasian (30/42, 71%), females (25/42, 60%) with a median age of 42 years. Among the seronegative donors, HLA-I information was available for 39 of which thirteen had alleles of interest i.e. B*27, B*57, B*58, B*53 and/or B*55. HIV-1 seronegative donors elicited a high frequency (25/42, 60%) responses to the non-HIV-1 antigens represented by CMV-pp65 and/or CEF peptide pools; data not shown. However, a CE specific T-cell response was seen in only one seronegative donor (2%). The single seronegative donor that responded had a positive CE specific response to an HLA-B*58 pol subpool, and the net magnitude of the response was 85 SFC/106 PBMC. This sub-pool response could not be mapped to a single peptide.
The demographics and clinical features of the chronically HIV-1 infected individuals whose samples were used in this study are shown in Table 2. Our cohort was enriched with individuals with HLA-B*57 and B*53 among the protective and non-protective groups, respectively. Although both allelic groups were composed of African Americans, the non-protective group was largely African American, female and receiving ART; this composition is reflective of the high HIV-1 disease burden observed for this racial group in US. The median CD4 and plasma viral load were similar between the 2 allelic groups (Table 2). The relatively preserved CD4 T cells and low viral load in both groups is likely due to the fact that most patients in our HIV clinic are being treated with antiretroviral therapy (ART) unless their infection is fairly well controlled. Initially peptides in pools were tested (Table 1) and positive responses were enumerated using a criteria of >50 SFC/106 PBMC and 2 times the unstimulated control. These responses were further mapped to the sub-pool or to the single peptide level.
Overall 16% of the patients with a protective allele responded to at least one CE (Figure 1A). For each protective allele, the overall response rate was 16%, 14% and 23% for B*27, B*57 and B*5801, respectively (Figure 1A). At the peptide level, 13%, 18% and 10% responses were detected for all B*27, B*57 and B*58 restricted peptides tested respectively (Figure 1B). Furthermore, nearly all of these mapped peptides were antisense derived (90%,) and were predominately located in the ARFs of HIV-1 accessory genes (>55%, Nef, Vpr, Vif and Vpu); data not shown. Regarding the magnitude of response in the protective allele group, the median magnitude of the response was higher at the single peptide level compared to the sub-pool and pools of peptide (data not shown, p<0.05).
Figure 1. Patient and peptide responder frequency data for CE specific T-cell responses as assessed in an IFN-γ ELISpot assay in a cohort of chronically HIV infected individuals.
(A) Percentage patient responder frequency for each group of individuals carrying non-protective (B*53/55) and protective (B*27/57/58) HLA-class I alleles is shown. SN= HIV seronegative donors; (B) Response frequency (%) elicited to each peptide pool. The fraction on top of each bar indicates the number of positive responses over total tested for that group.
For the non-protective allele group, we evaluated responses in a total of 50 CHI individuals with HLA-B*53 (N=46) and HLA-B*55 (N=4). The total number of peptide tested was 72, with 57 and 9 peptides being unique to B*53 and B*55, respectively, and 6 common to both alleles. In this group, we observed only one response to a B*53 peptide (2%) and the pool which contained it. Taken together, the overall response rate for the protective allele and non-protective allele group was 16% and 2%, respectively (p= 0.009, Figure 1A) despite similar median posterior probability (0.60) of predicted epitopes. On the other hand, no differences were observed comparing either the plasma viral loads or the absolute CD4 T cell counts when comparing the individuals with CE responses to those without such responses (1,243 and 1,430 RNA copies/ml and 458 and 571 cells/mm3, respectively). Furthermore CE responses were similar among patients on and off ART (data not shown).
Novel CD8 cryptic epitope specific responses targeted during chronic HIV-1 infection
In our study, we identified a total of twenty novel 9–10 mer peptides restricted by one or more of the protective alleles (Table 3) in seven HIV-1 infected individuals. These responses were restricted nearly equally by each of the 3 protective alleles studied (range 20–45 %).
Table 3.
List of novel immunogenic cryptic epitopes (CE)
| Cryptic epitope | ||||
|---|---|---|---|---|
|
| ||||
| Patient ID | Sequence | Name | HLA-Ia | ARF-frameb |
|
| ||||
| CMI-1 | HLLAQLSFF | HF9 | B57 | Nef-RF6 |
| YMNCYQDNF | YNF9 | B57 | Pol-RF4 | |
| YCMDFQAQF | YQF9 | B57 | Pol-RF6 | |
| NYCYYCCYY | NY9 | B57 | Vpu-RF4 | |
|
| ||||
| CMI-2 | FYFSSPSIY | FIY9 | B5801 | Env-RF4 |
|
| ||||
| CMI-3 | WPLVFWGLF | WF9 | B57 | Vif-RF5 |
| YYGPHNYCYY | YY10 | B57 | Vpu-RF4 | |
| FYEYYGPHNY | FNY10 | B5801 | Vpu-RF4 | |
|
| ||||
| CMI-4 | RQWQQFHQYY | RY10 | B27 | Pol-RF3 |
| TRLYTFRRK | TK9 | B27 | Pol-RF3 | |
| HRFYYSLTL | HL9 | B27 | Pol-RF4 | |
|
| ||||
| CMI-5 | IYIWCFTKL | IL9 | B27 | Vif-RF5 |
| FPKPEALFW | FW9 | B57 | Gag-RF4 | |
|
| ||||
| CMI-6 | FPHFQQPFF | FF9 | B5801 | Gag-RF5 |
| LPLWEGQIF | LF9 | B5801 | Gag-RF5 | |
| GLFYLLWLNW | GW10 | B5801 | Nef-RF4 | |
|
| ||||
| CMI-7 | FPCSNPHPVY | FVY10 | B5801 | Vif-RF4 |
| HPLAFLEIY | HY9 | B5801 | Vif-RF5 | |
| DPNASLFLLY | DY10 | B5801 | Vif-RF6 | |
| YPRKMSNSF | YF9 | B5801 | Vpr-RF4 | |
EpiPred predicted HLA-I restriction
alternate reading frame of an HIV-1 gene and frame number (RF3=sense and RF4–6=antisense)
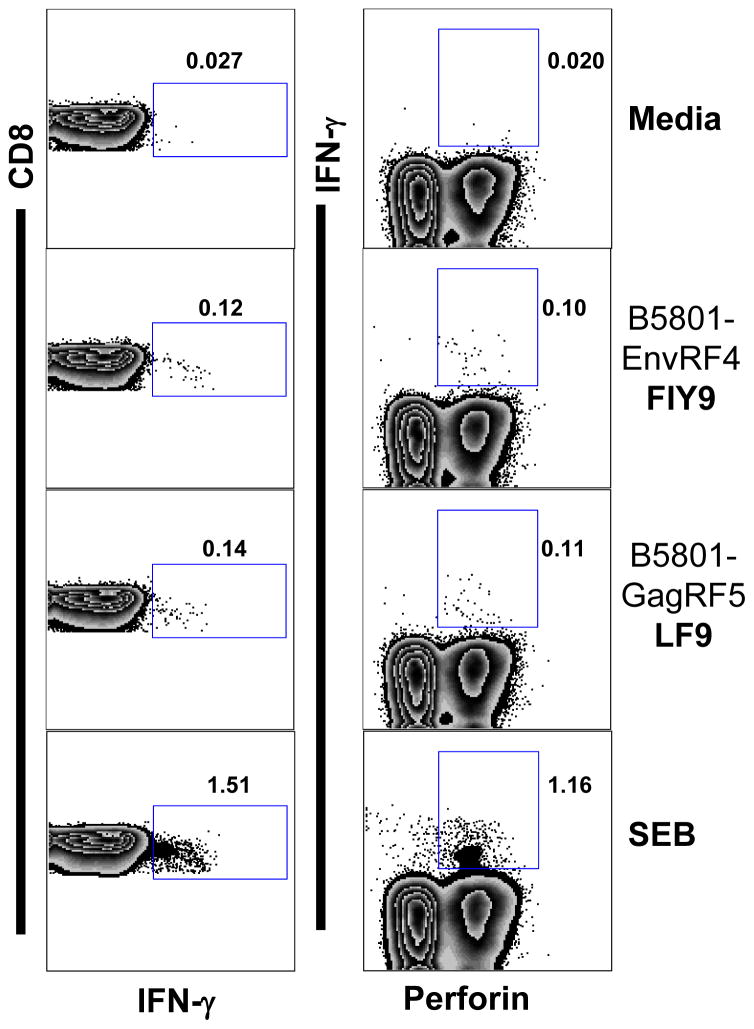
Polychromatic flow cytometry was used to further characterize the CE specific CD8 T-cell responses. Using an ex-vivo ICS assay, our data showed that these CD8 T-cells elicit multifunctional cytokine and effector responses (Figure 2). In addition to IFN-γ, CE specific cells produce cytokines and effectors molecules including IL-2, TNF-α, CD107 and granzyme-B and perforin. Following our ex-vivo analyses (Figure 2), we expanded the CE specific CD8 T-cells in culture using autologous peptide pulsed monocytes as antigen presenting cells (APC). These cells readily proliferate and retain their multifunctionality (Supplementary figure 1). Using a similar approach for CE-CD8 T-cell expansion, we were also able to confirm the HLA-I restriction of CE to be similar to the computational prediction (Supplementary figure 2).
Figure 2. Functionality of CE specific CD8 T-cell responses restricted by protective alleles as measured in an ex-vivo ICS assay.
PBMC from a chronically HIV infected donor (HLA-A: 0201/0205, HLA-B:0702/5703, HLA-C:0701/0702) were stimulated with 10uM peptide in presence of co-stimulatory antibodies and intracellular transport inhibitors for 12 hours at 37°C. Representative data showing IFN-γ and perforin production from CE specific CD8 T-cells in response to B*5801 restricted Env RF4 (FIY9= FYFSSPSIY) and B*5801 restricted Gag RF5 (LF9= LPLWEGQIF) peptides. Response to SEB is shown as a positive control. Using Fischer’s exact test, p-value for FIY9 response relative to media was 1.1 × 10−6 for IFN-γ, and 4.0 × 10−6 for perforin. For LF9 response, the p-values for media compared to IFN-γ and perforin were 2.7 × 10−5 and 4.4 × 10−5 respectively. This patient was on ART and the plasma viral load and absolute CD4 counts were 49 and 611 respectively.
DISCUSSION
Our understanding of cryptic epitopes (CE) has significantly improved since the first antisense protein was described in 1988 “as a novel protein on the genomic DNA plus strand” 25. Antisense transcription for HIV-1 was later shown 35,78,79 and He et al elegantly demonstrated its common occurrence in the human genome 23. Although traditionally derived epitopes (TE) have long been considered to be the sole source of HLA-I presented immunogens, the functional relevance of the ARF encoded cryptic epitopes (CE) has only recently begun to emerge. Therefore, this would imply that the total breadth of epitope presentation has heretofore been largely under estimated.
Consistent with our previous findings that HLA-I CE are commonly targeted in HIV-1 infection 39, we observed a significant rate of CE targeting, especially from anti-sense derived peptides. Our data shows a bias towards the preferential targeting of CE restricted by protective compared to non-protective alleles. This observation could have been skewed by two factors. Firstly, it is possible that the binding prediction algorithms for protective allele are better than non-protective allele since the former group is much better studied. However, this is unlikely to be the main reason since the peptides in both group were selected based on a posterior probability of >0.5 and the number of peptides tested in each group were comparable. Furthermore, the prediction models used in the current manuscript have worked well previously for both allelic groups (i.e. PA and NPA) 39,40,80. Secondly, based on the observation that at a population level protective alleles are associated with more traditional epitopes that have evidence of escape 81, it can be speculated that the protective allele group has the ability to target more CE as well. Conversely, patients with HLA types associated with rapid disease progression recognize a small fraction of the TE restricted by the non-protective alleles 72. It is therefore not surprising that the CE restricted by these alleles are also not frequently targeted.
Although previous work 39,40 has shown that the breadth of CE specific CTL do correlate with markers of disease progression, we were unable to see a significant correlation between CE targeting and plasma viral load. One possible explanation is that the median viral load in our entire cohort was very low making correlations difficult. Nevertheless, multiple prior studies have shown that overall, individuals with protective alleles have lower viral load compared to individuals who lack these HLA-I types 82,83,84,85,86. Moreover, this effect can be seen early following viral infection suggesting that CE responses could perhaps be partly responsible for the enhanced viral control afforded to these patients.
There are fairly convincing data that the protective alleles’ favorable effect on disease progression is in a large part attributed to HIV-1 Gag targeting 87–89,90,82, although targeting 4,91,92 of other proteins has been shown to be important during acute infection. HIV-1 and SIV specific CE CD8 T cell responses have also been directed towards ARFs of all of the viral proteins that have been analyzed 39,40,44,46,54,93,94. Consistent with prior work, the current study demonstrated CE responses to ARFs of all of the genes analyzed, including the accessory genes 44,46. Interestingly, targeting CE in the ARFs of accessory genes such as Tat, Nef, and Vif was also frequently seen in macaques including those exhibiting elite control of SIV 95–98. Therefore, CE targeting ARFs can be an important source of immmunogens for enhancing the overall repertoire of virus specific T cell responses.
The preferential targeting of antisense peptides is consistent with our prior work that showed that there is potential to encode more antisense peptides 39 relative to sense direction. A recent study also found a higher number of predicted epitopes were derived from antisense ARFs 44. In the current study, we found that nearly half of CE specific responses were targeted towards peptides translated from the ARFs of accessory genes (vif, vpr, vpu and nef) followed by pol antisense ARFs. The latter is in line with our previous data 39 showing that the potential number of antisense pol-CE far outweighs the number of pol TE. In parallel, the multi-functionality of CE specific T cell responses is comparable with those seen for TE making CE attractive as viable immunogens that can likely act in concert with TE to broaden the overall CD8 T-cell response.
Taken together, data from this study suggests that there are multifaceted players involved in exacting viral control in individuals endowed with protective alleles. Some of the attributes working in tandem or in concert to control a viral infection include the ability to mount fitness cost imposing CD8 T-cell responses, elicit multifunctional responses 70, superior ability of responses to cross-recognize variants 99,100, and a broader TCR usage. Perhaps a greater breadth of responses via CE may yet be another dimension to this equation. In summary, understanding the full extent of immune targeting of protein products derived from ARFs not only provides insight into the full extent of virus specific CTL responses to HIV-1 but could yield information which can be effectively utilized to increase the breadth of future CTL based HIV-1 vaccines.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT:
This work was supported by the NIAID: RO1 A1 084772 (Goepfert) and P30 A1 027767 (Bansal)
We appreciate the help provided by Marion Spell for flow cytometric acquisition.
Footnotes
CONFLICT OF INTEREST:
All authors declare no financial conflict of interest for this study.
PRESENTATION OF WORK:
This work was presented at the AIDS Vaccine meeting, October 7 – 10, 2013, Barcelona, Spain, poster # P12.22 and at the AIDS Vaccine 2011 meeting, Sep 12–15, Bangkok, Thailand, poster # P17.06
AUTHOR CONTRIBUTIONS:
Conceived and designed the experiments: Anju Bansal and Paul Goepfert
Peptide predictions: Jonathan Carlson and Anne Bet
Performed the experiments: Anju Bansal, Tiffanie Mann, Sarah Sterrett, Binghao Peng, Anne Bet
Analyzed the data: Anju Bansal, Tiffanie Mann, Sarah Sterrett, Binghao Peng and Paul Goepfert
Wrote the paper: Anju Bansal and Paul Goepfert
References
- 1.Cohen J. AIDS research. More woes for struggling HIV vaccine field. Science. 2013 May 10;340(6133):667. doi: 10.1126/science.340.6133.667. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012 Apr 5;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997 Feb;3(2):205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 5.Goulder PJ, Phillips RE, Colbert RA, et al. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997 Feb;3(2):212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 6.Goulder PJ, Walker BD. The great escape - AIDS viruses and immune control. Nat Med. 1999 Nov;5(11):1233–1235. doi: 10.1038/15184. [DOI] [PubMed] [Google Scholar]
- 7.Leslie AJ, Pfafferott KJ, Chetty P, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004 Mar;10(3):282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 8.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003 Mar 20;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 9.Barouch DH, Korber B. HIV-1 vaccine development after STEP. Annu Rev Med. 2010;61:153–167. doi: 10.1146/annurev.med.042508.093728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barouch DH, O’Brien KL, Simmons NL, et al. Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med. 2010 Mar;16(3):319–323. doi: 10.1038/nm.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong WP, Wu L, Wallstrom TC, et al. Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type 1 envelope DNA vaccination. J Virol. 2009 Mar;83(5):2201–2215. doi: 10.1128/JVI.02256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med. 2012;63:95–111. doi: 10.1146/annurev-med-042010-085643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santra S, Liao HX, Zhang R, et al. Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med. 2010 Mar;16(3):324–328. doi: 10.1038/nm.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santra S, Muldoon M, Watson S, et al. Breadth of cellular and humoral immune responses elicited in rhesus monkeys by multi-valent mosaic and consensus immunogens. Virology. 2012 Jul 5;428(2):121–127. doi: 10.1016/j.virol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, O’Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009 Jan 1;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson NA, Keele BF, Reed JS, et al. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol. 2009 Jul;83(13):6508–6521. doi: 10.1128/JVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruce AG, Atkins JF, Gesteland RF. tRNA anticodon replacement experiments show that ribosomal frameshifting can be caused by doublet decoding. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5062–5066. doi: 10.1073/pnas.83.14.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullock TN, Patterson AE, Franlin LL, Notidis E, Eisenlohr LC. Initiation codon scanthrough versus termination codon readthrough demonstrates strong potential for major histocompatibility complex class I-restricted cryptic epitope expression. J Exp Med. 1997 Oct 6;186(7):1051–1058. doi: 10.1084/jem.186.7.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Core LJ, Lis JT. Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science. 2008 Mar 28;319(5871):1791–1792. doi: 10.1126/science.1150843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008 Dec 19;322(5909):1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan BP, Bennink JR, Yewdell JW. Translating DRiPs: progress in understanding viral and cellular sources of MHC class I peptide ligands. Cellular and molecular life sciences: CMLS. 2011 May;68(9):1481–1489. doi: 10.1007/s00018-011-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan BP, Li L, Veltri CA, Ireland CM, Bennink JR, Yewdell JW. Distinct pathways generate peptides from defective ribosomal products for CD8+ T cell immunosurveillance. J Immunol. 2011 Feb 15;186(4):2065–2072. doi: 10.4049/jimmunol.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Vogelstein B, Velculescu VE, Papadopoulos N, Kinzler KW. The antisense transcriptomes of human cells. Science. 2008 Dec 19;322(5909):1855–1857. doi: 10.1126/science.1163853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malarkannan S, Horng T, Shih PP, Schwab S, Shastri N. Presentation of out-of-frame peptide/MHC class I complexes by a novel translation initiation mechanism. Immunity. 1999 Jun;10(6):681–690. doi: 10.1016/s1074-7613(00)80067-9. [DOI] [PubMed] [Google Scholar]
- 25.Miller RH. Human immunodeficiency virus may encode a novel protein on the genomic DNA plus strand. Science. 1988 Mar 18;239(4846):1420–1422. doi: 10.1126/science.3347840. [DOI] [PubMed] [Google Scholar]
- 26.Morris KV, Santoso S, Turner AM, Pastori C, Hawkins PG. Bidirectional transcription directs both transcriptional gene activation and suppression in human cells. PLoS genetics. 2008 Nov;4(11):e1000258. doi: 10.1371/journal.pgen.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saulquin X, Scotet E, Trautmann L, et al. +1 Frameshifting as a novel mechanism to generate a cryptic cytotoxic T lymphocyte epitope derived from human interleukin 10. J Exp Med. 2002 Feb 4;195(3):353–358. doi: 10.1084/jem.20011399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab SR, Shugart JA, Horng T, Malarkannan S, Shastri N. Unanticipated antigens: translation initiation at CUG with leucine. PLoS biology. 2004 Nov;2(11):e366. doi: 10.1371/journal.pbio.0020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Starck SR, Jiang V, Pavon-Eternod M, et al. Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science. 2012 Jun 29;336(6089):1719–1723. doi: 10.1126/science.1220270. [DOI] [PubMed] [Google Scholar]
- 30.Starck SR, Ow Y, Jiang V, et al. A distinct translation initiation mechanism generates cryptic peptides for immune surveillance. PLoS One. 2008;3(10):e3460. doi: 10.1371/journal.pone.0003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starck SR, Shastri N. Non-conventional sources of peptides presented by MHC class I. Cellular and molecular life sciences: CMLS. 2011 May;68(9):1471–1479. doi: 10.1007/s00018-011-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zook MB, Howard MT, Sinnathamby G, Atkins JF, Eisenlohr LC. Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J Immunol. 2006 Jun 1;176(11):6928–6934. doi: 10.4049/jimmunol.176.11.6928. [DOI] [PubMed] [Google Scholar]
- 33.Bukrinsky MI, Etkin AF. Plus strand of the HIV provirus DNA is expressed at early stages of infection. AIDS Res Hum Retroviruses. 1990 Apr;6(4):425–426. doi: 10.1089/aid.1990.6.425. [DOI] [PubMed] [Google Scholar]
- 34.Cavanagh MH, Landry S, Audet B, et al. HTLV-I antisense transcripts initiating in the 3′LTR are alternatively spliced and polyadenylated. Retrovirology. 2006;3:15. doi: 10.1186/1742-4690-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landry S, Halin M, Lefort S, et al. Detection, characterization and regulation of antisense transcripts in HIV-1. Retrovirology. 2007;4:71. doi: 10.1186/1742-4690-4-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larocque E, Halin M, Landry S, Marriott SJ, Switzer WM, Barbeau B. Human T-cell lymphotropic virus type 3 (HTLV-3)- and HTLV-4-derived antisense transcripts encode proteins with similar Tax-inhibiting functions but distinct subcellular localization. J Virol. 2011 Dec;85(23):12673–12685. doi: 10.1128/JVI.05296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laverdure S, Gross A, Arpin-Andre C, et al. HIV-1 antisense transcription is preferentially activated in primary monocyte-derived cells. J Virol. 2012 Dec;86(24):13785–13789. doi: 10.1128/JVI.01723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lefebvre G, Desfarges S, Uyttebroeck F, et al. Analysis of HIV-1 expression level and sense of transcription by high-throughput sequencing of the infected cell. J Virol. 2011 Jul;85(13):6205–6211. doi: 10.1128/JVI.00252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal A, Carlson J, Yan J, et al. CD8 T cell response and evolutionary pressure to HIV-1 cryptic epitopes derived from antisense transcription. J Exp Med. 2010 Jan 18;207(1):51–59. doi: 10.1084/jem.20092060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger CT, Carlson JM, Brumme CJ, et al. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J Exp Med. 2010 Jan 18;207(1):61–75. doi: 10.1084/jem.20091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bet A, Sterrett S, Sato A, Bansal A, Goepfert PA. Characterization of T-cell responses to cryptic epitopes in recipients of a noncodon-optimized HIV-1 vaccine. Journal of acquired immune deficiency syndromes. 2014 Feb 1;65(2):142–150. doi: 10.1097/QAI.0b013e3182a9917e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardinaud S, Moris A, Fevrier M, et al. Identification of cryptic MHC I-restricted epitopes encoded by HIV-1 alternative reading frames. J Exp Med. 2004 Apr 19;199(8):1053–1063. doi: 10.1084/jem.20031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardinaud S, Starck SR, Chandra P, Shastri N. The synthesis of truncated polypeptides for immune surveillance and viral evasion. PLoS One. 2010;5(1):e8692. doi: 10.1371/journal.pone.0008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Champiat S, Raposo RA, Maness NJ, et al. Influence of HAART on Alternative Reading Frame Immune Responses over the Course of HIV-1 Infection. Plos One. 2012;7(6):e39311. doi: 10.1371/journal.pone.0039311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolan BP, Li L, Takeda K, Bennink JR, Yewdell JW. Defective ribosomal products are the major source of antigenic peptides endogenously generated from influenza A virus neuraminidase. J Immunol. 2010 Feb 1;184(3):1419–1424. doi: 10.4049/jimmunol.0901907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garrison KE, Champiat S, York VA, et al. Transcriptional errors in human immunodeficiency virus type 1 generate targets for T-cell responses. Clinical and vaccine immunology: CVI. 2009 Sep;16(9):1369–1371. doi: 10.1128/CVI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaur A, Green WR. Role of a cytotoxic-T-lymphocyte epitope-defined, alternative gag open reading frame in the pathogenesis of a murine retrovirus-induced immunodeficiency syndrome. J Virol. 2005 Apr;79(7):4308–4315. doi: 10.1128/JVI.79.7.4308-4315.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gnjatic S, Jager E, Chen WS, et al. CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients. P Natl Acad Sci USA. 2002 Sep 3;99(18):11813–11818. doi: 10.1073/pnas.142417699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho O, Green WR. Cytolytic CD8(+) T cells directed against a cryptic epitope derived from a retroviral alternative reading frame confer disease protection. J Immunol. 2006 Feb 15;176(4):2470–2475. doi: 10.4049/jimmunol.176.4.2470. [DOI] [PubMed] [Google Scholar]
- 50.Ho O, Green WR. Alternative translational products and cryptic T cell epitopes: expecting the unexpected. J Immunol. 2006 Dec 15;177(12):8283–8289. doi: 10.4049/jimmunol.177.12.8283. [DOI] [PubMed] [Google Scholar]
- 51.Li CW, Goudy K, Hirsch M, et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. P Natl Acad Sci USA. 2009 Jun 30;106(26):10770–10774. doi: 10.1073/pnas.0902269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maness NJ, Valentine LE, May GE, et al. AIDS virus-specific CD8(+) T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med. 2007 Oct 29;204(11):2505–2512. doi: 10.1084/jem.20071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maness NJ, Walsh AD, Piaskowski SM, et al. CD8+ T cell recognition of cryptic epitopes is a ubiquitous feature of AIDS virus infection. J Virol. 2010 Nov;84(21):11569–11574. doi: 10.1128/JVI.01419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maness NJ, Wilson NA, Reed JS, et al. Robust, vaccine-induced CD8(+) T lymphocyte response against an out-of-frame epitope. J Immunol. 2010 Jan 1;184(1):67–72. doi: 10.4049/jimmunol.0903118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayrand SM, Green WR. Non-traditionally derived CTL epitopes: exceptions that prove the rules? Immunology today. 1998 Dec;19(12):551–556. doi: 10.1016/s0167-5699(98)01342-5. [DOI] [PubMed] [Google Scholar]
- 56.Probst-Kepper M, Stroobant V, Kridel R, et al. An alternative open reading frame of the human macrophage colony-stimulating factor gene is independently translated and codes for an antigenic peptide of 14 amino acids recognized by tumor-infiltrating CD8 T lymphocytes. J Exp Med. 2001 May 21;193(10):1189–1198. doi: 10.1084/jem.193.10.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronsin C, Chung-Scott V, Poullion I, Aknouche N, Gaudin C, Triebel F. A non-AUG-defined alternative open reading frame of the intestinal carboxyl esterase mRNA generates an epitope recognized by renal cell carcinoma-reactive tumor-infiltrating lymphocytes in situ. J Immunol. 1999 Jul 1;163(1):483–490. [PubMed] [Google Scholar]
- 58.Shichijo S, Nakao M, Imai Y, et al. A gene encoding antigenic peptides of human squamous cell carcinoma recognized by cytotoxic T lymphocytes. J Exp Med. 1998 Feb 2;187(3):277–288. doi: 10.1084/jem.187.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang RF, Johnston SL, Zeng G, Topalian SL, Schwartzentruber DJ, Rosenberg SA. A breast and melanoma-shared tumor antigen: T cell responses to antigenic peptides translated from different open reading frames. J Immunol. 1998 Oct 1;161(7):3598–3606. [PubMed] [Google Scholar]
- 60.Wang RF, Parkhurst MR, Kawakami Y, Robbins PF, Rosenberg SA. Utilization of an alternative open reading frame of a normal gene in generating a novel human cancer antigen. J Exp Med. 1996 Mar 1;183(3):1131–1140. doi: 10.1084/jem.183.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinzierl AO, Maurer D, Altenberend F, et al. A cryptic vascular endothelial growth factor T-cell epitope: identification and characterization by mass spectrometry and T-cell assays. Cancer research. 2008 Apr 1;68(7):2447–2454. doi: 10.1158/0008-5472.CAN-07-2540. [DOI] [PubMed] [Google Scholar]
- 62.Schirmbeck R, Riedl P, Fissolo N, Lemonnier FA, Bertoletti A, Reimann J. Translation from cryptic reading frames of DNA vaccines generates an extended repertoire of immunogenic, MHC class I-restricted epitopes. J Immunol. 2005 Apr 15;174(8):4647–4656. doi: 10.4049/jimmunol.174.8.4647. [DOI] [PubMed] [Google Scholar]
- 63.Fissolo N, Riedl P, Reimann J, Schirmbeck R. DNA vaccines prime CD8+ T cell responses to epitopes of viral antigens produced from overlapping reading frames of a single coding sequence. Eur J Immunol. 2005 Jan;35(1):117–127. doi: 10.1002/eji.200425608. [DOI] [PubMed] [Google Scholar]
- 64.Cardinaud S, Consiglieri G, Bouziat R, et al. CTL Escape Mediated by Proteasomal Destruction of an HIV-1 Cryptic Epitope. Plos Pathog. 2011 May;7(5) doi: 10.1371/journal.ppat.1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walsh AD, Bimber BN, Das A, et al. Acute phase CD8+ T lymphocytes against alternate reading frame epitopes select for rapid viral escape during SIV infection. PLoS One. 2013;8(5):e61383. doi: 10.1371/journal.pone.0061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bansal A, Yue L, Conway J, et al. Immunological control of chronic HIV-1 infection: HLA-mediated immune function and viral evolution in adolescents. AIDS. 2007 Nov 30;21(18):2387–2397. doi: 10.1097/QAD.0b013e3282f13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, Ndhlovu ZM, Liu D, et al. TCR clonotypes modulate the protective effect of HLA class I molecules in HIV-1 infection. Nature immunology. 2012 Jul;13(7):691–700. doi: 10.1038/ni.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elahi S, Dinges WL, Lejarcegui N, et al. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat Med. 2011 Aug;17(8):989–995. doi: 10.1038/nm.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hendel H, Caillat-Zucman S, Lebuanec H, et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J Immunol. 1999 Jun 1;162(11):6942–6946. [PubMed] [Google Scholar]
- 70.Jansen CA, Kostense S, Vandenberghe K, et al. High responsiveness of HLA-B57-restricted Gag-specific CD8+ T cells in vitro may contribute to the protective effect of HLA-B57 in HIV-infection. Eur J Immunol. 2005 Jan;35(1):150–158. doi: 10.1002/eji.200425487. [DOI] [PubMed] [Google Scholar]
- 71.Jin X, Gao X, Ramanathan M, Jr, et al. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+-T-cell responses for groups of HIV-1-infected individuals with different HLA-B*35 genotypes. J Virol. 2002 Dec;76(24):12603–12610. doi: 10.1128/JVI.76.24.12603-12610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scherer A, Frater J, Oxenius A, et al. Quantifiable cytotoxic T lymphocyte responses and HLA-related risk of progression to AIDS. Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12266–12270. doi: 10.1073/pnas.0404091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Streeck H, Lichterfeld M, Alter G, et al. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J Virol. 2007 Jul;81(14):7725–7731. doi: 10.1128/JVI.00708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie J, Lu W, Samri A, et al. Distinct differentiation profiles of HIV-Gag and Nef-specific central memory CD8+ T cells associated with HLA-B57/5801 and virus control. AIDS. 2010 Sep 24;24(15):2323–2329. doi: 10.1097/QAD.0b013e32833e5009. [DOI] [PubMed] [Google Scholar]
- 75.Heckerman D, Kadie C, Listgarten J. Leveraging information across HLA alleles/supertypes improves epitope prediction. J Comput Biol. 2007 Jul-Aug;14(6):736–746. doi: 10.1089/cmb.2007.R013. [DOI] [PubMed] [Google Scholar]
- 76.Bansal A, Gough E, Sabbaj S, et al. CD8 T-cell responses in early HIV-1 infection are skewed towards high entropy peptides. AIDS. 2005 Feb 18;19(3):241–250. [PubMed] [Google Scholar]
- 77.Akinsiku OT, Bansal A, Sabbaj S, Heath SL, Goepfert PA. Interleukin-2 production by polyfunctional HIV-1-specific CD8 T cells is associated with enhanced viral suppression. Journal of acquired immune deficiency syndromes. 2011 Oct 1;58(2):132–140. doi: 10.1097/QAI.0b013e318224d2e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ludwig LB, Ambrus JL, Jr, Krawczyk KA, et al. Human Immunodeficiency Virus-Type 1 LTR DNA contains an intrinsic gene producing antisense RNA and protein products. Retrovirology. 2006;3:80. doi: 10.1186/1742-4690-3-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Michael NL, Vahey MT, d’Arcy L, et al. Negative-strand RNA transcripts are produced in human immunodeficiency virus type 1-infected cells and patients by a novel promoter downregulated by Tat. J Virol. 1994 Feb;68(2):979–987. doi: 10.1128/jvi.68.2.979-987.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Almeida CA, Bronke C, Roberts SG, et al. Translation of HLA-HIV associations to the cellular level: HIV adapts to inflate CD8 T cell responses against Nef and HLA-adapted variant epitopes. J Immunol. 2011 Sep 1;187(5):2502–2513. doi: 10.4049/jimmunol.1100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carlson JM, Brumme CJ, Martin E, et al. Correlates of protective cellular immunity revealed by analysis of population-level immune escape pathways in HIV-1. J Virol. 2012 Dec;86(24):13202–13216. doi: 10.1128/JVI.01998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serwanga J, Shafer LA, Pimego E, et al. Host HLA B*allele-associated multi-clade Gag T-cell recognition correlates with slow HIV-1 disease progression in antiretroviral therapy-naive Ugandans. PLoS One. 2009;4(1):e4188. doi: 10.1371/journal.pone.0004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaidya SA, Streeck H, Beckwith N, et al. Temporal effect of HLA-B*57 on viral control during primary HIV-1 infection. Retrovirology. 2013;10:139. doi: 10.1186/1742-4690-10-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Juarez-Molina CI, Payne R, Soto-Nava M, et al. Impact of HLA selection pressure on HIV fitness at a population level in Mexico and Barbados. J Virol. 2014 Sep;88(18):10392–10398. doi: 10.1128/JVI.01162-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salgado M, Simon A, Sanz-Minguela B, et al. An additive effect of protective host genetic factors correlates with HIV nonprogression status. Journal of acquired immune deficiency syndromes. 2011 Apr;56(4):300–305. doi: 10.1097/QAI.0b013e3182036f14. [DOI] [PubMed] [Google Scholar]
- 86.Frater AJ, Brown H, Oxenius A, et al. Effective T-cell responses select human immunodeficiency virus mutants and slow disease progression. J Virol. 2007 Jun;81(12):6742–6751. doi: 10.1128/JVI.00022-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goepfert PA, Lumm W, Farmer P, et al. Transmission of HIV-1 Gag immune escape mutations is associated with reduced viral load in linked recipients. J Exp Med. 2008 May 12;205(5):1009–1017. doi: 10.1084/jem.20072457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002 Mar;76(5):2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kiepiela P, Ngumbela K, Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007 Jan;13(1):46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 90.Martinez-Picado J, Prado JG, Fry EE, et al. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006 Apr;80(7):3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994 Sep;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009 Jun 8;206(6):1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maness NJ, Sacha JB, Piaskowski SM, et al. Novel translation products from simian immunodeficiency virus SIVmac239 Env-encoding mRNA contain both Rev and cryptic T-cell epitopes. J Virol. 2009 Oct;83(19):10280–10285. doi: 10.1128/JVI.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maness NJ, Valentine LE, May GE, et al. AIDS virus specific CD8+ T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med. 2007 Oct 29;204(11):2505–2512. doi: 10.1084/jem.20071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loffredo JT, Friedrich TC, Leon EJ, et al. CD8+ T cells from SIV elite controller macaques recognize Mamu-B*08-bound epitopes and select for widespread viral variation. PLoS One. 2007;2(11):e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mudd PA, Martins MA, Ericsen AJ, et al. Vaccine-induced CD8+ T cells control AIDS virus replication. Nature. 2012 Nov 1;491(7422):129–133. doi: 10.1038/nature11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Connor DH, Mothe BR, Weinfurter JT, et al. Major histocompatibility complex class I alleles associated with slow simian immunodeficiency virus disease progression bind epitopes recognized by dominant acute-phase cytotoxic-T-lymphocyte responses. J Virol. 2003 Aug;77(16):9029–9040. doi: 10.1128/JVI.77.16.9029-9040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sacha JB, Buechler MB, Newman LP, et al. Simian immunodeficiency virus-specific CD8+ T cells recognize Vpr- and Rev-derived epitopes early after infection. J Virol. 2010 Oct;84(20):10907–10912. doi: 10.1128/JVI.01357-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gillespie GM, Kaul R, Dong T, et al. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS. 2002 May 3;16(7):961–972. doi: 10.1097/00002030-200205030-00002. [DOI] [PubMed] [Google Scholar]
- 100.Yu XG, Lichterfeld M, Chetty S, et al. Mutually exclusive T-cell receptor induction and differential susceptibility to human immunodeficiency virus type 1 mutational escape associated with a two-amino-acid difference between HLA class I subtypes. J Virol. 2007 Feb;81(4):1619–1631. doi: 10.1128/JVI.01580-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.