Abstract
Background
The PENPACT-1 trial compared virologic thresholds to determine when to switch to second-line antiretroviral therapy (ART). Using PENPACT-1 data, we aimed to describe HIV-1 drug resistance accumulation on first-line ART by virologic threshold.
Methods
PENPACT-1 had a 2x2 factorial design, randomizing HIV-infected children to start protease inhibitor (PI) versus non-nucleoside reverse transcriptase inhibitor (NNRTI) based ART, and switch at a 1000c/ml versus 30000c/ml threshold. Switch-criteria were: not achieving the threshold by week 24, confirmed rebound above the threshold thereafter, or CDC-C event. Resistance tests were performed on samples ≥1000c/ml before switch, re-suppression and at 4-year/trial-end.
Results
Sixty-seven children started PI-based ART and were randomized to switch at 1000c/ml (PI-1000), 64 PIs and 30000c/ml (PI-30000), 67 NNRTIs and 1000c/ml (NNRTI-1000), and 65 NNRTI and 30000c/ml (NNRTI-30000). Ninety-four (36%) children reached the 1000c/ml switch-criteria during 5 years follow-up. In 30000c/ml threshold arms, median time from 1000c/ml to 30000c/ml switch-criteria was 58 (PI) versus 80 (NNRTI) weeks (P=0.81). In NNRTI-30000 more NRTI resistance mutations accumulated than other groups. NNRTI mutations were selected before switching at 1000c/ml (23% NNRTI-1000, 27% NNRTI-30000). Sixty-two children started abacavir+lamivudine, 166 lamivudine+zidovudine or stavudine, and 35 other NRTIs. The abacavir+lamivudine group acquired fewest NRTI mutations. Of 60 switched to second-line, 79% PI-1000, 63% PI-30000, 64% NNRTI-1000 and 100% NNRTI-30000 were <400c/ml 24 weeks later.
Conclusion
Children on first-line NNRTI-based ART who were randomized to switch at a higher virologic threshold developed the most resistance, yet re-suppressed on second-line. An abacavir+lamivudine NRTI combination seemed protective against development of NRTI resistance.
Keywords: drug resistance, children, virologic switch-criteria, second-line antiretroviral therapy
Introduction
Pediatric guidelines [1–3] recommend HIV-1 infected children initiate antiretroviral therapy (ART) early in life. Since ART dramatically reduces mortality, duration of this treatment is likely to be long; potentially for many decades. Historically, children have tended to be maintained on failing therapies longer than adults, due to challenges with adherence and limited treatment options. This is particularly true in resource-limited settings where HIV-1 RNA monitoring is generally not available. HIV-1 drug resistance is known to increase with continuation of the same ART regimen in the presence of detectable viremia. Therefore, long-term treatment success requires effective first-line therapies, minimization of resistant virus on these therapies, and preservation of second line options. Careful consideration is required when sequencing ART regimens, taking into account first-line ART exposure, acquisition of resistance mutations on first-line, and exposure to ART as part of programs to reduce mother to child transmission (MTCT).
The PENPACT-1 trial [4] is the only long-term strategy trial in children or adults to assess effectiveness of first-line ART regimens as well as randomized RNA thresholds (1000 or 30000c/ml) to determine when to switch to second-line. Over time, a 1000c/ml threshold has been adopted to define virologic failure, followed by prompt switch to second-line in adults, but direct evidence for this threshold remains weak. When PENPACT-1 was designed in the early 2000s, drug choice for children was limited, and switch to second-line could be delayed due to concerns that drug options would be quickly exhausted, therefore a threshold ~1.5log10c/ml higher than 1000c/ml, above assay variation, was chosen to reflect practice at the time. In children, current recommendations for when to switch still vary, with the consolidated WHO guidelines [1] recommending a switch time consistent with adults and the US and European guidelines [2,3] instead recommending assessment of reasons for virologic failure and consideration of drug availability, resistance profiles, adherence issues and readiness of the family/child to switch.
Using data on all children from PENPACT-1, we explored resistance profiles after first-line ART by randomized switch-criteria based on RNA threshold, as well as second-line treatment response and drug options in children. The hypothesis was that more resistance mutations would accumulate on first-line ART in children randomized to the higher threshold, and this would influence second-line response as well as available second-line drug options.
Methods
PENPACT-1 was a multicenter phase 2/3, randomized, open-label, 2 by 2 factorial trial enrolling HIV-1 infected children from clinical centers in Europe and North and South America, with a minimum follow-up of 4 years [4]. Children were naïve to ART, although infants who had received <56 days of ART to reduce MTCT were eligible. Children were simultaneously randomized to start ART with two NRTIs plus either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI) and to switch from first-line to second-line ART at an RNA threshold of 1000c/ml (low-threshold) or 30000c/ml (higher-threshold). First-line ART was defined as the initial regimen, allowing drug substitutions (ideally within the same class) for non-virologic reasons (e.g. toxicity). The initial regimen was chosen by the treating clinician according to the randomized group. Children were switched to second-line if the RNA threshold (<1000c/ml or <30000c/ml) was not achieved by week 24, or if after an initial decline in RNA by week 24 there was a confirmed RNA rebound at/above the randomized threshold. Switch to second-line was also required if the child experienced a new CDC stage C event at/after week 24. For analysis purposes, children were defined in both arms as reaching the ‘1000 criteria’ and ‘30000 criteria’ using the above definition. Children who received first-line containing a PI were strongly encouraged to switch to a second-line containing an NNRTI, and vice-versa. Children had RNA measured at enrollment, weeks 8, 16, 24, and then 12 weekly until the last enrolled child reached 4 years.
Baseline resistance tests were performed on samples collected within 84 days before randomization. During follow-up, the overall aim was to evaluate new HIV-1 drug resistance mutations accumulated on first-line. As children were randomized to early (low-threshold) versus later (higher-threshold) switch-points, requirements for resistance testing aimed to identify additional mutations accumulated if a policy to switch at the ‘30000 criteria’ compared to the ‘1000 criteria’ was applied. To capture this, resistance tests were required in both RNA threshold arms, while children were on ART, at 1) the last sample with RNA ≥1000c/ml before switch, 2) the last sample after confirmed RNA ≥1000c/ml (e.g. if not switched because ‘30000 criteria' not met and RNA re-suppressed to <1000c/ml), and 3) samples with RNA ≥1000c/ml at 4 years or trial end. To further visualize the requirements for switch and resistance testing, we have provided a Supplemental Figure displaying a set of example RNA profiles. It can be seen that children in the higher-threshold arm would be tested later when we hypothesize more resistance mutations will have accumulated. It can also be noted, that some RNA profiles required multiple tests per child. Major resistance mutations were defined according to International AIDS Society-USA (IAS) guidelines [5]. New mutations on first-line were accumulated over post-baseline tests [6]. Susceptibility to specific ART drugs was defined as fully susceptible, potential low-level, low-level, intermediate and high-level resistance by the genotypic resistance interpretation algorithm available on the Stanford University HIV drug resistance database webpage [7]. Genotypic sensitivity from this algorithm was formulated for current WHO recommended second-lines.
In this analysis, unlike the primary publication [4], 4 children who started a drug class (PI or NNRTI) different to their randomization were grouped based on the drug class actually started, rather than the strict intent-to-treat definition based on randomized class. Proportions of children reaching criteria were tested using chi-squared tests, time to reach switch-criteria and to actual switch used Kaplan-Meier methods and log-rank tests, and comparison of time to resistance tests and RNA levels used Wilcoxon rank-sum tests. Poisson regression, without a time offset, tested differences in number of mutations by group. The model assumed children not requiring resistance tests did not develop mutations and excluded those with missing test results. For second-line efficacy, a child was considered successful if they achieved <400c/ml 24 weeks after switch. Proportions of children <400c/ml were calculated from Kaplan-Meier curves and comparisons used Cox regression. Analysis used Stata statistical software.
Results
Baseline Characteristics and First-line ART
Table 1 shows baseline age, HIV-1 RNA, resistance, HIV-1 subtype, prior ART use for reduction of MTCT and first-line ART for the 263 children enrolled in PENPACT-1 initiating therapy and included in this analysis. Other baseline characteristics have previously been published [4]. Median (range) age at start of ART was 6.5 (0.1-17.8) years. Thirty-nine children (15%) received ART for reduction of MTCT; most zidovudine prophylaxis alone and only 5 (2%) received single-dose nevirapine. Four percent (10/239) of baseline samples tested retrospectively had ≥1 major mutation. Of children starting PI based ART, 65 (50%) started lopinavir/ritonavir and 64 (49%) nelfinavir, whereas for children starting NNRTIs, 82 (62%) started efavirenz and 50 (38%) nevirapine. For NRTIs, 166 (63%) initiated lamivudine+zidovudine or stavudine, 62 (24%) abacavir+lamivudine and 35 (13%) other NRTI combinations (mainly lamivudine+didanosine).
Table 1.
Baseline Age, HIV-1 RNA, IAS Resistance Mutations, HIV-1 Subtype, ART for reduction of MTCT and First-line ART by Randomized Switch-threshold
| Randomized switch-threshold | |||||||
|---|---|---|---|---|---|---|---|
| Low (n=134) | Higher (n=129) | Total (n=263) | |||||
| Age (years), median (IQR) [range] | 6.1 | 6.9 | 6.5 | ||||
| (2.5–13.1) | (3.1–12.5) | (2.8–12.9) | |||||
| [0.3–17.8] | [0.1–17.5] | [0.1–17.8] | |||||
| HIV-1 RNA (c/ml), median (IQR) | 99646 | 119356 | 107813 | ||||
| (35488–446704) | (28732–434369) | (31251–446704) | |||||
| Baseline resistance test availablea | 121 | 100% | 118 | 100% | 239 | 100% | |
| Any major mutations | 7 | 6% | 3 | 3% | 10 | 4% | |
| 1 or 2 mutations | 7 | 2 | 9 | ||||
| ≥3 mutations | 0 | 1 | 1 | ||||
| NNRTI K103N | 1 | 1 | 2 | ||||
| NNRTI V108I | 1 | 0 | 1 | ||||
| NNRTI Y181C | 2 | 1 | 3 | ||||
| NNRTI Y188L | 1 | 0 | 1 | ||||
| PI Q58E | 1 | 0 | 1 | ||||
| NNRTI K101P Y181C | 1 | 0 | 1 | ||||
| NRTI D67N K219Q, NNRTI K103N | 0 | 1 | 1 | ||||
| HIV-1 subtype availableb | 128 | 100% | 121 | 100% | 249 | 100% | |
| HIV-1 subtype | B | 52 | 41% | 49 | 41% | 101 | 41% |
| C | 13 | 10% | 12 | 10% | 25 | 10% | |
| F | 25 | 20% | 23 | 19% | 48 | 19% | |
| A/CRF_AG/D/G | 25 | 20% | 27 | 22% | 52 | 21% | |
| unclassified | 13 | 10% | 10 | 8% | 23 | 9% | |
| ART use prior to randomization for reduction of MTCTc | 22 | 100% | 17 | 100% | 39 | 100% | |
| In utero (to the mother) | 4 | 18% | 6 | 35% | 10 | 26% | |
| 1 day | ZDV+NVP | 0 | 1 | 1 | |||
| 1–24 weeks | ZDV+3TC+NVP | 2 | 2 | 4 | |||
| 1–2 weeks | ZDV+3TC+NFV | 1 | 1 | 2 | |||
| 7–28 weeks | ZDV | 1 | 2 | 3 | |||
| Delivery/Post partum (to the infant) | 22 | 100% | 16 | 94% | 38 | 97% | |
| single-dose | NVP, 1–4 weeks ZDV+3TC | 0 | 2 | 2 | |||
| single-dose | NVP, 3–6 weeks ZDV | 2 | 1 | 3 | |||
| 1–12 weeks | ZDV | 19 | 12 | 31 | |||
| 5–6 weeks | ZDV, 3–6 weeks 3TC | 1 | 1 | 2 | |||
| First-line NNRTI ART | 67 | 100% | 65 | 100% | 132 | 100% | |
| EFV with | 43 | 64% | 39 | 60% | 82 | 62% | |
| ABC+3TC | 15 | 10 | 25 | ||||
| ZDV+3TC | 17 | 18 | 35 | ||||
| d4T+3TC | 6 | 3 | 9 | ||||
| other NRTIsd | 5 | 8 | 13 | ||||
| NVP with | 24 | 36% | 26 | 40% | 50 | 38% | |
| ABC+3TC | 3 | 6 | 9 | ||||
| ZDV+3TC | 15 | 11 | 26 | ||||
| d4T+3TC | 3 | 8 | 11 | ||||
| other NRTIse | 3 | 1 | 4 | ||||
| First-line PI ART | 67 | 100% | 64 | 100% | 131 | 100% | |
| LPV/r with | 29 | 43% | 36 | 56% | 65 | 50% | |
| ABC+3TC | 10 | 12 | 22 | ||||
| ZDV+3TC | 10 | 20 | 30 | ||||
| d4T+3TC | 6 | 4 | 10 | ||||
| other NRTIsf | 3 | 0 | 3 | ||||
| NFV with | 37 | 55% | 27 | 42% | 64 | 49% | |
| ABC+3TC | 3 | 3 | 6 | ||||
| ZDV+3TC | 12 | 9 | 21 | ||||
| d4T+3TC | 13 | 10 | 23 | ||||
| other NRTIsg | 9 | 5 | 14 | ||||
| Other protease inhibitor | 1 | 1% | 1 | 2% | 2 | 2% | |
| ZDV+3TC+high-dose ritonavir | 0 | 1 | 1 | ||||
| ddI+3TC+fosamprenavir/ritonavir | 1 | 0 | 1 | ||||
Includes 1 test where only the protease gene was sequenced.
Available from 239 baseline resistance tests and 10 resistance tests during follow-up.
For 3/5 children who received single-dose NVP, the mother also received NVP in utero.
10 ZDV+ddI, 2 3TC+ddI, 1 ddI+FTC
1 ZDV+ddI, 1 ZDV+ABC, 2 ddI+d4T
1 ZDV+ddI, 2 ZDV+ABC
13 ZDV+ddI, 1 ddI+d4T
Children are displayed in 2 groups defined by their randomized switch threshold (low=1000c/ml, higher=30000c/ml). IAS = International AIDS Society-USA, MTCT = mother-to-child transmission, ART= antiretroviral therapy, PI = protease inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, ZDV = zidovudine, NVP = nevirapine, 3TC = lamivudine, EFV = efavirenz, ABC = abacavir, d4T = stavudine, LPV/r = lopinavir/ritonavir, NFV = nelfinavir, ddI = didanosine, FTC = emtricitabine
Children Reaching the 1000c/ml and 30000c/ml switching criteria
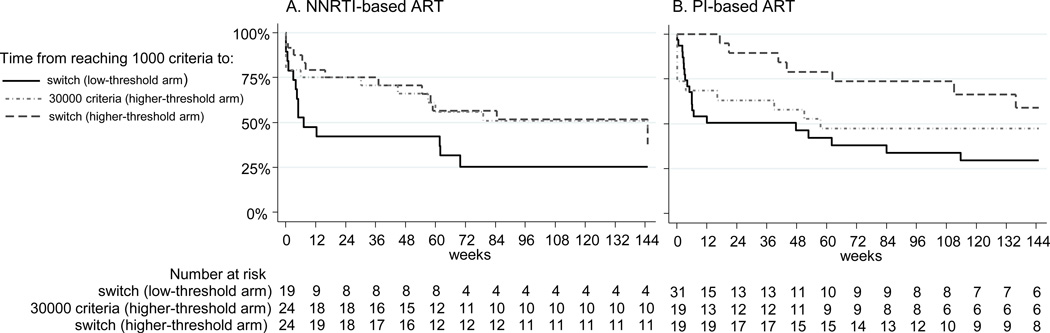
By trial end, at a median follow-up of 5.0 years (IQR 4.2–6.0), 94 children (36%) had reached the ‘1000 criteria’ (51 low-threshold, 43 higher-threshold, chi-squared P=0.42). These 94 children were evenly distributed by drug class; 51 (54%) started PIs and 43 (46%) NNRTIs (chi-squared P=0.42). Median RNA when the ‘1000 criteria’ were met was 13505c/ml for those on PIs and 9800c/ml for NNRTIs (Wilcoxon rank-sum P=0.49). As expected, most children in the low-threshold arm switched soon after reaching the ‘1000 criteria’ (median time to switch after reaching ‘1000 criteria’: 12 weeks). This time was similar in children starting PIs (12 weeks) and NNRTIs (8 weeks, log-rank P=0.60) [Figure 1, solid-line]. Of 43 children in the higher-threshold arm who reached the ‘1000 criteria’, 3 (7%) switched before subsequently reaching the ‘30000 criteria’, 22 (55%) reached the ‘30000 criteria’ before trial end (18 switched), and the remaining 18 (42%) neither reached the ‘30000 criteria’ nor switched. The median time from reaching the ‘1000 criteria’ to the ‘30000 criteria’ was 80 weeks [Figure 1, dotted-dashed-line]. This observed time was longer for those starting NNRTIs (median 80 weeks) compared to PIs (median 58 weeks), although not significantly different (log-rank P=0.81). However, there was an observed shorter time from reaching the ‘1000 criteria’ to switch for those on NNRTIs (25th percentile: 17 weeks) compared to PIs (25th percentile: 63 weeks, log-rank P=0.16) [Figure 1, solid-dashed-line]. The median RNA when the ‘30000 criteria’ were met was 54991c/ml (NNRTIs 44550c/ml versus PIs 69649c/ml, Wilcoxon rank-sum P=0.09).
Figure 1.
Kaplan-Meier curves displaying the time from reaching the 1000 criteria to switch in the low-threshold arm (solid-line), time from the 1000 criteria to the 30000 criteria in the higher-threshold arm (dotted-dashed-line), and time from the 1000 criteria to switch in the higher-threshold arm (solid-dashed-line) by first-line NNRTI-based or PI-based antiretroviral therapy (ART). The 1000 criteria were defined as not achieving HIV-1 RNA <1000c/ml by week 24, confirmed rebound ≥1000c/ml thereafter or CDC stage C event. The 30000 criteria were defined as not achieving HIV-1 RNA <30000c/ml by week 24, confirmed rebound ≥30000c/ml thereafter or CDC stage C event. Children in the low-threshold arm were randomized to switch at the 1000 criteria, and those in the higher-threshold arm to switch at the 30000 criteria. Ninety-four children reached the 1000 criteria during the trial, but 93 children are displayed in the figure as 1 child on PI-based first-line ART randomized to switch at the low-threshold ended follow-up on the same day as reaching the 1000 criteria. PI=protease inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor
Resistance Tests Required and Performed
In total, 107 children required resistance tests (Table 2). This included 90/94 children reaching the ‘1000 criteria’ and 17 additional children. The 4 children reaching the ‘1000 criteria’ not requiring tests were due to 1 child switching at a CDC stage C event with suppressed RNA and 3 being off ART for all RNAs ≥1000c/ml. The 17 additional tests were due to single RNA ≥1000c/ml at 4 years, trial end or before switch. These 107 children required 127 tests on first-line; 90 (84%) required one test, 14 (13%) two tests and 3 (3%) three tests. The reasons for requiring resistance tests were 1) last sample with RNA ≥1000c/ml before switch (n=58), 2) last sample after confirmed RNA ≥1000c/ml (e.g. if not switched because ‘30000 criteria' not met and RNA re-suppressed to <1000c/ml) (n=24), and 3) samples with RNA ≥1000c/ml at 4 years or trial end (n=45) (See the Supplemental Figure for example RNA profiles and resistance testing requirements). Overall, 101/127 (80%) tests were available for 87/107 (81%) children. The 20 children with missing test results were similarly distributed across first-line regimen and randomized switch-thresholds (chi-squared on 3 degrees of freedom P=0.76). For 87 children with available resistance tests on first-line, median time from randomization to last resistance test was 72 weeks in the low-threshold and 124 weeks in the higher-threshold arm (Wilcoxon rank-sum P=0.009). For PIs this was 50 versus 121 weeks (P=0.01) and for NNRTIs 95 versus 148 weeks (P=0.35).
Table 2.
Major IAS Resistance Mutations Accumulated on first-line ART
| PI- low |
PI- higher |
NNRTI- low |
NNRTI- higher |
Poisson P-value* |
|
|---|---|---|---|---|---|
| Total children | 67 | 64 | 67 | 65 | |
| Number requiring tests** | 34 | 22 | 26 | 25 | |
| Number with test results | 28 | 17 | 20 | 22 | |
| Included in analysis* | 61 | 59 | 61 | 62 | |
| NRTI resistance | |||||
| 1 or 2 mutations | 11 (18%) | 7 (12%) | 12 (20%) | 12 (19%) | any difference <0.001 |
| ≥3 mutations | 0 (0%) | 1 (2%) | 0 (0%) | 7 (11%) | |
| PI or NNRTI resistance | |||||
| 1 or 2 mutations | 10 (16 %) | 4 (7%) | 13 (21%) | 12 (19%) | PI versus |
| ≥3 mutations | 0 (0%) | 0 (0%) | 1 (2%) | 5 (8%) | NNRTI <0.001 |
| ABC+3TC |
3TC+ ZDV/d4T |
Other (mainly ZDV+ddI) |
Poisson P-value* |
||
| Total children | 62 | 166 | 35 | ||
| Number requiring tests | 15 | 67 | 25 | ||
| Number with test results | 9 | 59 | 19 | ||
| Included in analysis* | 56 | 158 | 29 | ||
| NRTI resistance | |||||
| 1 or 2 mutations | 4 (7%) | 32 (20%) | 6 (21%) | any difference <0.01 |
|
| ≥3 mutations | 1 (2%) | 7 (4%) | 0 (0%) | ||
| Thymidine analogue mutations (TAMs) | |||||
| 1 or 2 TAMs | 0 (0%) | 6 (4%) | 6 (21%) | ||
| ≥3 TAMs | 0 (0%) | 4 (3%) | 0 (0%) | ||
| K65R | 1 (2%) | 0 (0%) | 0 (0%) | ||
| L74R | 1 (2%) | 1 (1%) | 0 (0%) | ||
| Y115F | 1 (2%) | 0 (0%) | 0 (0%) | ||
| M184V/I | 5 (9%) | 39 (25%) | 0 (0%) | ||
Analysis assumes those not requiring tests were not resistant, and excludes those with unavailable resistance results.
Resistance tests were required on first-line, while children were on ART, at 1) the last sample with RNA ≥1000c/ml before switch, 2) the last sample after confirmed RNA ≥1000c/ml (e.g. if not switched because ‘30000 criteria' not met and RNA re-suppressed to <1000c/ml), and 3) samples with RNA ≥1000c/ml at 4 years or trial end. IAS major resistance mutations were accumulated across multiple tests per child on first-line, where appropriate. The table displays number of children requiring tests and the number of children with test results, rather than the absolute number of tests performed.
Children are displayed in 4 groups defined by the class of ART initiated as first-line (PI-based versus NNRTI-based) and their randomized switch threshold (low=1000c/ml, higher=30000c/ml). IAS = International AIDS Society-USA, ART = antiretroviral therapy, PI = protease inhibitor, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitor, ABC = abacavir, 3TC = lamivudine, ZDV = zidovudine, d4T = stavudine, ddI = didanosine
HIV-1 Resistance Mutations Accumulated on First-line ART
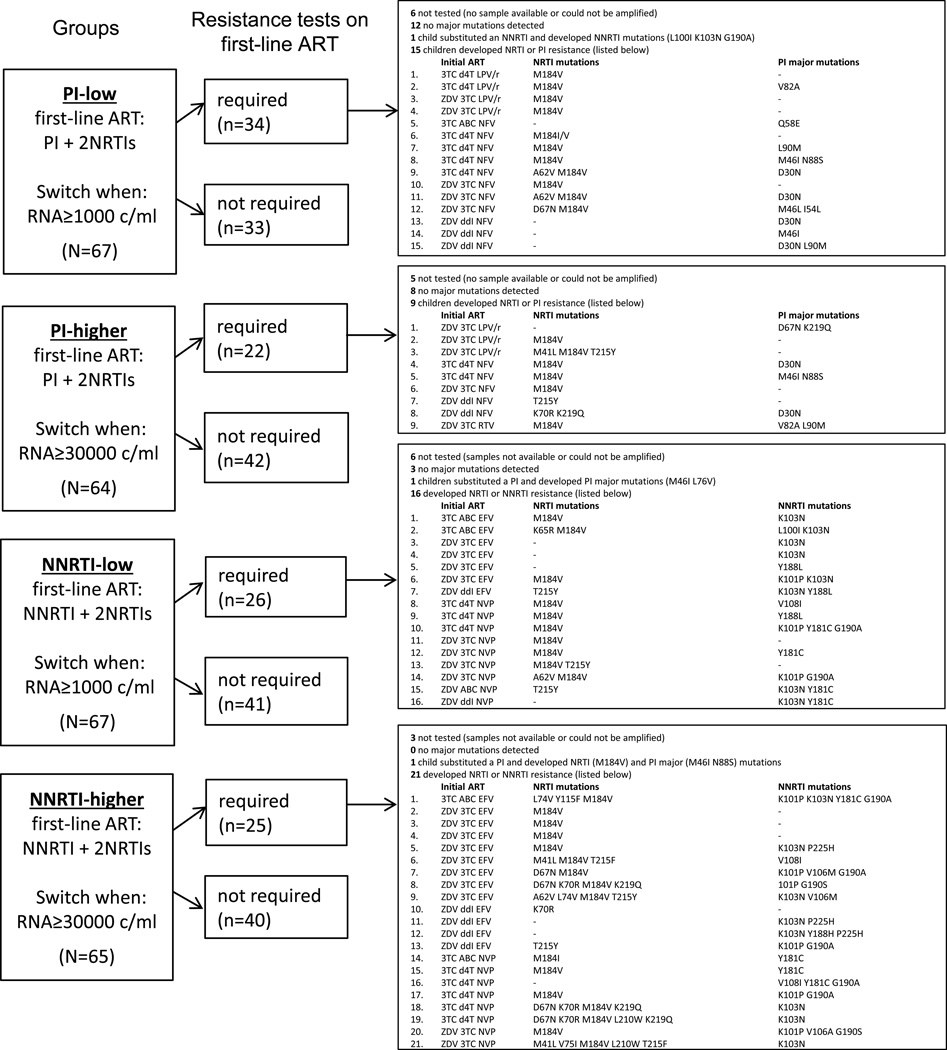
Table 2 displays new major IAS resistance mutations accumulated on first-line. More NRTI mutations accumulated in NNRTI-higher than the other 3 groups, with more children selecting ≥3 mutations in NNRTI-higher driving this difference (Poisson P<0.001). Overall, more NNRTI than PI mutations accumulated (Poisson P<0.001). It appeared that NNRTI mutations had already been selected before switching at the ‘1000 criteria’ as NNRTI-low had a similar number of mutations to NNRTI-higher. PI mutations were developed by 16% in PI-low and 7% in PI-higher; note more nelfinavir was administered in PI-low. For non-randomized NRTIs, 5 (9%) on abacavir+lamivudine, 39 (25%) on lamivudine+zidovudine or stavudine, and 6 (21%) on other NRTI combinations developed mutations (Poisson P<0.01).
Figure 2 provides a detailed description of first-line ART administered and mutations accumulated. It reveals, in PI-low and PI-higher, very few PI mutations were selected by children on lopinavir/ritonavir and relatively more on nelfinavir. On lopinavir/ritonavir the main NRTI mutation selected was M184V, but on nelfinavir additional NRTI mutations were accumulated. All 5 children who developed NRTI mutations on abacavir+lamivudine were on NNRTIs, whereas 22/39 (56%) children on lamivudine+zidovudine or stavudine with mutations were on NNRTIs and 17/39 (44%) on PIs.
Figure 2.
Major IAS resistance mutations accumulated on first-line antiretroviral therapy (ART). Children are displayed in 4 groups defined by the class of ART initiated as first-line (PI-based versus NNRTI-based) and their randomized switch threshold (low=1000c/ml versus higher=30000c/ml). Resistance tests were required on first-line in both randomized switch threshold arms, while children were on ART, at 1) the last sample with RNA ≥1000c/ml before switch, 2) the last sample after confirmed RNA ≥1000c/ml (e.g. if not switched because ‘30000 criteria' not met and RNA re-suppressed to <1000c/ml), and 3) samples with RNA ≥1000c/ml at 4 years or trial end. IAS=International AIDS Society-USA, PI=protease inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor, NRTI=nucleoside reverse transcriptase inhibitor, 3TC=lamivudine, d4T=stavudine, ZDV=zidovudine, ABC=abacavir, ddI=didanosine, LPV/r=lopinavir/ritonavir, NFV=nelfinavir, RTV=high-dose ritonavir, EFV=efavirenz, NVP=nevirapine,
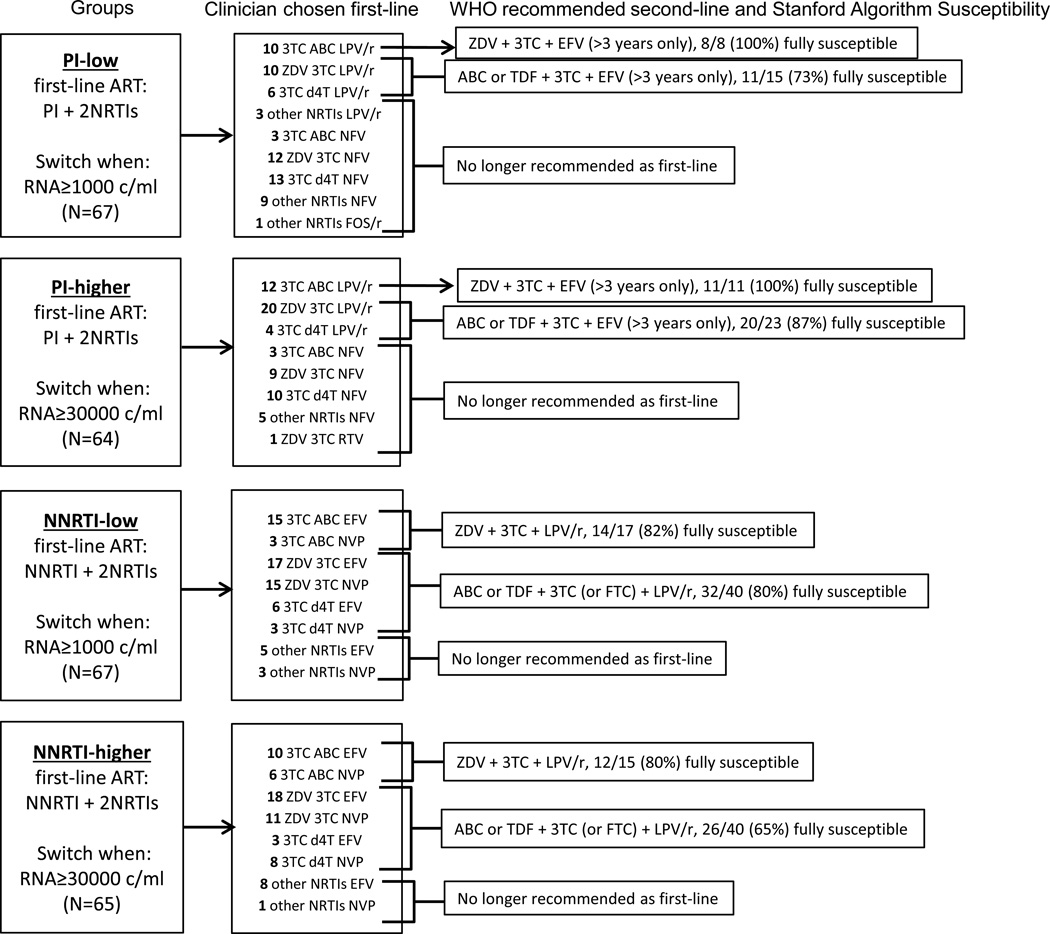
Susceptibility to Potential Second-line ART
Figure 3 displays susceptibility to potential second-line regimens. All children on first-line abacavir+lamivudine with lopinavir/ritonavir were fully susceptible to WHO recommended second-line lamivudine+zidovudine with efavirenz in PI-low and PI-higher. Eleven (73%) in PI-low and 20 (87%) in PI-higher were fully susceptible to WHO recommended second-line after first-line lamivudine+zidovudine or stavudine with lopinavir/ritonavir. After first-line abacavir+lamivudine with an NNRTI, 14 (82%) in NNRTI-low and 12 (80%) in NNRTI-higher were fully susceptible to second-line lamivudine+zidovudine with lopinavir/ritonavir. After first-line lamivudine+zidovudine or stavudine with an NNRTI, 32 (80%) in NNRTI-low and 26 (65%) in NNRTI-higher were fully susceptible to second-line lamivudine+abacavir or tenofovir with lopinavir/ritonavir. This likely reflects accumulation of thymidine analogue mutations (TAMs) on zidovudine or stavudine.
Figure 3.
Second-line antiretroviral therapy (ART) options. Children are displayed in 4 groups defined by the class of ART initiated as first-line (PI-based versus NNRTI-based) and their randomized switch threshold (low=1000c/ml versus higher=30000c/ml). The clinician chosen initial first-line ART is displayed along with current WHO recommended second-line and the susceptibility to this potential second-line regimen by the Stanford algorithm. The proportion of children susceptible to the potential second-line options assumed that children not meeting requirements for resistance testing were susceptible and excludes children with unavailable resistance results. Second-line containing EFV is only recommended for children >3 years and has been noted on the figure. PI=protease inhibitor, NNRTI=non-nucleoside reverse transcriptase inhibitor, NRTI=nucleoside reverse transcriptase inhibitor, 3TC=lamivudine, FTC=emtricitabine, d4T=stavudine, ZDV=zidovudine, ABC=abacavir, ddI=didanosine, TDF=tenofovir, LPV/r=lopinavir/ritonavir, FOS/r=fosamprenavir/ritonavir, RTV=high-dose ritonavir, NFV=nelfinavir, NVP=nevirapine, EFV=efavirenz
Second-line Response
Sixty children switched to second-line during PENPACT-1 (20 PI-low, 8 PI-higher, 17 NNRTI-low, 15 NNRTI-higher). Five switched before reaching the ‘1000 criteria’ (3 low-threshold, 2 higher-threshold) and 55 (34 low-threshold, 21 higher-threshold) after they were met; 18 of the 21 in the higher-threshold arm waited until the ‘30000 criteria’ were met, but 3 did not. The proportion <400c/ml by 24 weeks on second-line was 79% in PI-low, 63% PI-higher, 64% NNRTI-low and 100% NNRTI-higher (Cox regression P=0.10). Of 46/60 children with resistance data on first-line, 18 (39%) had no NRTI mutations, 22 (48%) 1-2 NRTI mutations and 6 (13%) ≥3 NRTI mutations (all 6 were in NNRTI-higher). Of those without NRTI resistance, 93% suppressed to <400c/ml by 24 weeks on second-line, whereas for those with 1-2 NRTI mutations, 65% suppressed, and those with ≥3 NRTI mutations, 100% suppressed (Cox regression P=0.64). The Supplemental list provides a detailed description of these children.
Discussion
Throughout the world children continue to initiate both NNRTI and PI-based first-line regimens in national treatment programs. Our long-term trial including a wide age range of children starting first-line PIs and NNRTIs provided a unique opportunity to study development of HIV-1 drug resistance and gain insight into resistance consequences of different ART switching strategies. Overall and as predicted, we found that children starting NNRTIs accumulated more HIV-1 drug resistance than those starting PIs. In particular, children switching later on NNRTIs accumulated more NRTI mutations, whereas on PIs, NRTI mutations did not accumulate over the time taken to reach a 30000c/ml threshold. Children taking the currently recommended lopinavir/ritonavir selected even fewer mutations than those on the un-boosted PI, nelfinavir, which is no longer recommended. Furthermore, in this study, before tenofovir was available in children (now approved by the FDA for children >2 years), there was a resistance benefit for children prescribed first-line abacavir+lamivudine compared to lamivudine+zidovudine or stavudine, but randomized evidence to verify this finding is required.
Over approximately 5 years on ART, suppression on first-line regimens was good with only around one third of children ever reaching the ‘1000 criteria’ for switch. Of those, the observed time from 1000 to 30000 criteria was slightly longer for NNRTIs compared to PIs, but time from ‘1000 criteria’ to switch was longer for PIs than NNRTIs. This suggests children failing on NNRTIs spent a slightly longer time with RNA between 1000 and 30000c/ml with prompt switch once the ‘30000 criteria’ were met. In contrast, children on PIs spent a slightly shorter time with RNA between 1000 and 30000c/ml but the treating clinician had a tendency to wait to switch after the ‘30000 criteria’ were met; possibly due to assessment of adequate adherence before switching to an NNRTI-based second-line. Despite this tendency to wait to switch on PIs, which hypothetically would result in more resistance mutations, we still saw less resistance accumulate in PI-higher compared to NNRTI-higher. These data are consistent with clinical trials and observational studies in adults, reporting fewer NRTI mutations on PIs than NNRTIs [8, 9], as well as additional reports on accumulation of NRTI resistance on NNRTIs [10–12].
As well as the overall protective effect of PIs, we saw fewer NRTI and PI resistance mutations for children on the boosted PI lopinavir/ritonavir compared to other PIs (mainly un-boosted nelfinavir). This is consistent with a systematic review of drug resistance after first-line failure in children [13], which observed that the type of PI affected development of resistance. In particular, on nelfinavir, where adequate plasma concentrations are seldom reached in children [14], D30N and N88S, specific nelfinavir mutations, were frequently reported. The review did not describe NRTI mutations by PI exposure, but our data suggest PI choice is likely to be important as lopinavir/ritonavir appeared particularly protective against accumulation of NRTI mutations. This is supported by CHER trial data [15] where only 11/331 (3%) children initiating lopinavir/ritonavir based first-line developed NRTI mutations, 10 of which developed M184V alone and no TAMs were seen.
To our knowledge, PENPACT-1 is the only trial of randomized criteria for switching from first to second-line ART based on RNA thresholds. There has been one small short-term trial where highly ART experienced adults were randomized to immediate or deferred switch [16], and two trials comparing monitoring strategies with resistance data by arm [17, 18]. In the small ART experienced trial [18], median time to meeting criteria was >60 weeks suggesting a similar delayed switch time to our study. A trend towards more new mutations in the deferred-switch compared to the immediate-switch arm was observed, with most new mutations to NRTIs, particularly TAMs. However, particular drugs or drug classes could not be assessed. The first monitoring trial [17] compared CD4 to RNA monitoring and revealed no difference in a future drug options score between arms. However more patients in the CD4 arm, which had a longer duration >400 c/ml, had ≥3 NRTI mutations. The only two patients who developed TAMs were in the CD4 arm. These data are consistent with our study, showing a low barrier to development of lamivudine and NNRTI resistance, which is similar in the two arms, but documentation of TAM accumulation in the arm with longer duration of virologic failure. The other monitoring trial [18], compared laboratory and clinical monitoring to clinical monitoring alone, and showed a similar number of patients with major mutations in the clinical monitoring alone versus the laboratory and clinical monitoring arm. The authors noted that switching occurred late after first detectable RNA in the laboratory and clinical monitoring arm, which may account for the fact no differences were seen.
Since the advent of triple therapy two randomized trials [19, 20] on NRTI backbones in children suggest abacavir+lamivudine has similar or better efficacy compared to other NRTI combinations. Our data add to the current weight of evidence that prescribing an NRTI backbone of abacavir+lamivudine first-line followed by zidovudine in second-line has some resistance advantages, as previously described in the PENTA-5 trial [21]. For children prescribed abacavir+lamivudine in combination with lopinavir/ritonavir, we did not detect any resistance, and in combination with NNRTIs we only saw 5 children with NRTI resistance. The abacavir specific mutations do not affect susceptibility to second-line zidovudine and there is evidence that K65R induces hyper-susceptibility to zidovudine [22]. Conversely, using zidovudine (or stavudine) first-line results in accumulation of TAMs such that the efficacy of abacavir second-line is reduced. Overall accumulation of NRTI resistance on lamivudine+zidovudine or stavudine was greater than on abacavir+lamivudine with TAMs occurring in 10 children (including 1 on lopinavir/ritonavir), suggesting abacavir+lamivudine has beneficial resistance properties when prescribed first-line with lopinavir/ritonavir or NNRTIs. More than 2 TAMs accumulated for 4 children on lamivudine+zidovudine or stavudine and 5 children developed mutations from the TAM type 1 pathway which is known to have a negative impact on response to abacavir [22]. Additionally, the M184V mutation, present in nearly all children developing resistance on first-line, increases susceptibility to thymidine analogues (zidovudine and stavudine) but causes low-level resistance to abacavir [22]. Therefore, these data support a resistance benefit of prescribing abacavir+lamivudine first-line in settings where children may spend longer with high RNAs due to limited laboratory monitoring or unavailable second-line regimens.
Our data on efficacy of second-line are limited by the small number of children switching by trial end. However, the data suggests a similar (or maybe better) suppression rate after failing in the NNRTI-higher arm. The effect of NRTI resistance prior to switch on second-line efficacy revealed a consistent pattern, suggesting children who had developed 1-2 NRTI mutations on first-line did worst and those with ≥3 mutations best. In our data there was no evidence that clinicians selected more potent second-line regimens for children known to be failing with extensive resistance, so one could hypothesize that a boosted PI with partially effective NRTIs is sufficiently potent to suppress virus at least until week 24 of second-line. Alternatively, it may be that adherence more than drug resistance influences second-line success; two studies in adults [23, 24] with resistance tests prior to switch from an NNRTI to PI based ART found either no association of NRTI resistance with the success of second-line or paradoxically a higher suppression rate in those with resistance. The authors of the second study found evidence from pharmacokinetic drug levels that it was indeed adherence rather than drug resistance that influenced second-line success.
Conclusions
This study confirms the protective effect of a boosted PI against accumulation of HIV-1 drug resistance mutations, as reported in adult studies. Analysis of non-randomized NRTI backbones suggests abacavir+lamivudine results in fewer resistance mutations than lamivudine+zidovudine or stavudine, whether prescribed with an NNRTI or lopinavir/ritonavir. Overall, these data support WHO 2013 pediatric guidelines [1] recommending abacavir+lamivudine as the first-line NRTI backbone with an NNRTI, and provide reassurance that despite the possibility of considerable time spent on first-line with detectable viremia (where HIV-1 RNA monitoring is not available), response to second-line with a boosted PI and zidovudine+lamivudine is expected to be good.
Supplementary Material
Acknowledgments
Source of Funding: The PENPACT-1 trial was sponsored jointly by the Paediatric European Network for Treatment of AIDS (PENTA) Foundation, Agence Nationale de Recherche sur le Sida et les hepatites virales (ANRS) and the Pediatric AIDS Clinical Trials Group (PACTG), subsequently the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT). Overall support for PACTG/IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
PENTA is a coordinated action of the European Commission/European Union, supported by the seventh framework programme (FP7/2007-2013) under the Eurocoord grant agreement number 260694, the sixth framework contract number LSHP-CT-2006-018865, the fifth framework programme contract number QLK2-CT-2000-00150, and by the PENTA Foundation. UK clinical sites were supported by a grant from the MRC; those in Italy by a grant from the Istituto Superiore di Sanita – Progetto Terapia Antivirale 2004, 2005. GSK and BMS provided drugs in Romania. The trial was coordinated by four trials centers: the Medical Research Council (MRC) Clinical Trials Unit at University College London, London, UK (with support from the MRC); INSERM SC10-US019, Paris, France (supported by ANRS); Frontier Science, Buffalo, NY, USA; and Westat, Maryland, USA (supported by NICHD).
We thank all the children, families and staff from the centers participating in the PENPACT-1 (PENTA 9/PACTG 390) trial PENPACT-1 Protocol Team: PACTG/IMPAACT/NICHD: P Brouwers, D Costello, E Ferguson, S Fiscus, J Hodge, M Hughes, C Jennings, A Melvin (Co-Chair), R McKinney (Co-Chair), L Mofenson, M Warshaw, E Smith, S Spector, E Stiehm, M Toye, R Yogev. PENTA: JP Aboulker, A Babiker, H Castro, A Compagnucci, C Giaquinto, J Darbyshire, M Debré, DM Gibb, L Harper, L Harrison, G Tudor-Williams (Co-Chair), Y Saidi, AS Walker. DSMB: B Brody, C Hill, P Lepage, J Modlin, A Poziak, M Rein (Chair 2002–2003), M Robb (Chair 2004–2009), T Fleming, S Vella, KM Kim. Clinical Sites Argentina: : Hospital de Pediatria Dr JP Garrahan, Buenos Aires: R Bologna, D Mecikovsky, N Pineda, L Sen (L), A Mangano (L), S Marino (L), C Galvez (L); Laboratorio Fundai: G Deluchi (L). Austria: Universitätsklinik für Kinder und Jugendheilkunde, Graz: B Zöhrer, W Zenz, E. Daghofer, K Pfurtscheller, B Pabst (L). Bahamas: Princess Margaret Hospital: MP Gomez, P McNeil, M Jervis, I Whyms, D Kwolfe, S Scott (P). Brazil: University of Sao Paulo at Ribeirao Preto: MM Mussi-Pinhata, ML Issac, MC Cervi, BVM Negrini, TC Matsubara, C BSS de Souza (L), JC Gabaldi (P); Institute of Pediatrics (IPPMG), Federal University of Rio de Janeiro: RH Oliveira, MC Sapia, T Abreu, L Evangelista , A Pala, I Fernandes, I Farias, M de F Melo (L), H Carreira (P), LM Lira (P); Instituto de Infectologia Emilio Ribas, Sao Paolo: M della Negra, W Queiroz, YC Lian; DP Pacola; Fleury Laboratories; Federal University of Minas Gerais, Belo Horizonte: J Pinto, F Ferreira, F Kakehasi, L Martins, A Diniz, V Lobato, M Diniz, C Hill (L), S Cleto (L), S Costa (P), J Romeiro (P). France: Hôpital d'enfants Armand Trousseau, Paris: C Dollfus, MD Tabone, MF Courcoux, G Vaudre A Dehée (L), A Schnuriger (L), N Le Gueyades (P), C De Bortoli (P); CHU Hôtel Dieu, Nantes: F Méchinaud, V Reliquet, J Arias (L), A Rodallec (L), E André (L), I Falconi (P), A Le Pelletier (P); Hôpital de l'Archet II, Nice, F Monpoux, J Cottalorda (L), S Mellul (L); Hôpital Jean Verdier, Bondy: E Lachassinne; Laboratoire de virologie-Hôpital Necker Enfants Malades, Paris: J Galimand (L), C Rouzioux (L), ML Chaix (L), Z Benabadji (P), M Pourrat (P); Hôpital Cochin Port-Royal- Saint Vincent de Paul, Paris: G Firtion, D Rivaux, M Denon, N Boudjoudi, F Nganzali, A Krivine (L), JF Méritet (L), G Delommois (L), C Norgeux (L), C Guérin (P); Hôpital Louis Mourier, Colombes: C Floch, L Marty, H Hichou (L), V Tournier (P); Hôpital Robert Debré, Paris, A Faye, I Le Moal, M Sellier (P), L Dehache (P); Laboratoire de virologie-Hôpital Bichat Claude Bernard-Paris: F Damond (L), J Leleu (L), D Beniken (L), G Alexandre-Castor (L) Germany: Universitäts - Kinderklinik
Düsseldorf: J Neubert, T Niehues, HJ Laws, K Huck, S Gudowius, (H Loeffler), S Bellert(L), A Ortwin (L); Universitäts - Kinderkliniken, Munich: G Notheis, U Wintergerst, F Hoffman, (A Werthmann, S Seyboldt, L Schneider, B Bucholz); Charité – Medizische Fakultät der Humboldt-Universität zu Berlin: C Feiterna-Sperling, C Peiser, R Nickel, T Schmitz, T Piening, C Müller (L); Kinder- und Jugendklinik, Universität Rostock: G Warncke, M Wigger, R Neubauer. Ireland: : Our Lady's Hospital for Sick Children, Dublin: K Butler, AL Chang, T Belger, A Menon, M O'Connell, L Barrett, A Rochford, M Goode, E Hayes, S McDonagy, A Walsh, A Doyle, J Fanning (P), M O’Connor (P), M Byrne (L), N O’Sullivan (L), E Hyland (L). Italy: : Clinica Pediatrica, Ospedale L Sacco, Milan: V Giacomet, A Viganò, GV Zuccotti, D Trabattoni (L), A Berzi (L); Clinica Pediatrica, Università di Brescia: R Badolato, F Schumacher, V Bennato, M Brusati, A Sorlini, E Spinelli, M Filisetti, C Bertulli; Clinica Pediatrica, Università di Padova: O Rampon, C Giaquinto, M Zanchetta (L); Ospedale S. Chiara, Trento: A Mazza, G Stringari, G Rossetti (L); Ospedale del Bambino Gesù, Rome: S Bernardi, A Martino, G Castelli Gattinara, P Palma, G Pontrelli, H Tchidjou, A.Furcas, C. Frillici, A. Mazzei, A Zoccano (P), C Concato (L). Romania: : Spitalul Clinic de Boli Infectioase Victor Babes, Bucharest: D Duiculescu, C Oprea, G Tardei (L), F Abaab (P); Institutul de Boli Infectioase Matei Bals, Bucharest: M Mardarescu, R Draghicenoiu, D Otelea (L), L Alecsandru (P); Clinic Municipal, Constanta: R Matusa, S Rugina, M Ilie, Silvia Netescu (P). Clinical monitors: C Florea, E Voicu, D Poalelungi, C Belmega, L Vladau, A Chiriac Spain: : Hospital Materno-Infantil 12 de Octubre, Madrid: JT Ramos Amador, MI Gonzalez Tomé, P Rojo Conejo, M Fernandez, R Delgado Garcia (L), JM Ferrari (P); Institute de Salud Carlos III, Madrid: M Garcia Lopez, MJ Mellado Peña, P Martin Fontelos, I Jimenez Nacher (P); Biobanco Gregorio Marañon, Madrid: MA Muñoz Fernandez (L), JL Jimenez (L), A García Torre (L); clinical monitors: M Penin, R Pineiro Perez, I Garcia Mellado. UK: : Bristol Royal Children’s Hospital: A Finn, M LaJeunesse, E Hutchison, J Usher (L), L Ball (P), M Dunn (P); St. George’s Healthcare NHS Trust, London: M Sharland, K Doerholt, S Storey, S Donaghy, C Wells (P), K Buckberry (P), P Rice (P); University Hospital of North Staffordshire: P McMaster, P Butler, C Farmer (L), J Shenton (P), H Haley (P), J Orendi (L), University Hospital Lewisham: J Stroobant, L Navarante, P Archer, C Mazhude, D Scott, R O'Connell, J Wong (L), G Boddy (P); Sheffield Children's Hospital: F Shackley, R Lakshman, J Hobbs, G Ball (L), G Kudesia (L), J Bane (P), D Painter (P); Ealing Hospital NHS Trust: K Sloper, V Shah, A Cheng (P), A Aali (L); King's College Hospital, London: C Ball, S Hawkins, D Nayagam, A Waters, S Doshi (P); Newham University Hospital: S Liebeschuetz, B Sodiende, D Shingadia, S Wong, J Swan (P), Z Shah (P); Royal Devon and Exeter Hospital: A Collinson, C Hayes, J King (L), K 0'Connor (L); Imperial College Healthcare NHS Trust, London: G Tudor-Williams, H Lyall, K Fidler, S Walters, C Foster, D Hadamache, C Newbould, C Monrose, S Campbell, S Yeung, J Cohen, N Martinez-Allier, (G Tatum, A Gordon), S Kaye (L), D Muir (L), D Patel (P); Great Ormond Street Hospital: V Novelli, D Gibb, D Shingadia, K Moshal, J Lambert, N Klein, J Flynn, L Farrelly, M Clapson, L Spencer, M Depala (P); Institute of Child Health, London: M Jacobsen (L); John Radcliffe Hospital, Oxford: S Segal, A Pollard, S Yeadon, Y Peng (L), T Dong (L), Y Peng (L), K Jeffries (L), M Snelling (P), Nottingham University Hospitals: A Smyth, J Smith; Chelsea and Westminster Hospital, London: B Ward; UCLH, London: E Jungmann; Doncaster Royal Infirmary: C Ryan, K Swaby; Health Protection Agency, London: A Buckton (L); Health Protection Agency, Birmingham: E Smidt (L). USA: Harlem Hospital Center: EJ Abrams, S Champion, AD Fernandez, D Calo, L Garrovillo, K Swaminathan, T Alford, M Frere, Columbia University laboratories, J Navarra (P, Town Total Health); NYU School of Medicine: W Borkowsky, S Deygoo, T Hastings, S Akleh, T Ilmet (L); Seattle Children's Hospital: A Melvin, K Mohan, G Bowen; University of South Florida: PJ Emmanuel, J Lujan-Zimmerman, C Rodriguez, S Johnson, A Marion, C Graisbery, D Casey, G Lewis; All Children's Hospital laboratories; Oregon Health and Science University: J Guzman-Cottrill, R Croteau; San Juan City Hospital: M Acevedo-Flores, M Gonzalez, L Angeli; L Fabregas, Lab 053, P Valentin (P); SUNY- Upstate Medical University-Syracuse: L Weiner, KA Contello, W Holz, M Butler; SUNY, Health Science Center at Stonybrook: S Nachman, MA. Kelly, DM. Ferraro, UNC Retrovirology Lab; Howard University Hospital: S Rana, C Reed, E Yeagley, A Malheiro, J Roa; LAC and USC Medical Center: M Neely, A Kovacs, L Spencer, J Homans, Y Rodriguez Lozano, Maternal Child Virology Research Laboratory, Investigational Drug Service; South Florida Childrens Diagnostic & Treatment Center: A Puga, G Talero, R Sellers; Broward General Medical Center, University of Miami (L); University College of Florida College of Medicine- Gainsville: R Lawrence; University of Rochester Pediatrics: GA. Weinberg, B Murante, S Laverty; Miller Children's Hospital Long Beach: A Deveikis, J Batra, T Chen, D Michalik, J Deville, K Elkins, S Marks, J Jackson Alvarez, J Palm, I Fineanganofo (L), M Keuth (L), L Deveikis (L), W Tomosada (P); Tulane University New Orleans: R Van Dyke, T Alchediak, M Silio, C Borne, S Bradford, S Eloby-Childress (L), K Nguyen (P); University of Florida/Jacksonville: MH. Rathore, A Alvarez; A Mirza, S Mahmoudi, M Burke; University of Puerto Rico: IL Febo, L Lugo, R Santos; Children’s Hospital Los Angeles: JA Church, T Dunaway, C Rodier; St. Jude/UTHSC: P Flynn, N Patel, S DiScenza, M Donohoe; WNE Maternal Pediatric Adolescent AIDS: K Luzuriaga, D Picard; Texas Children's Hospital: M Kline, ME Paul, WT Shearer, C McMullen-Jackson; Children’s Memorial Hospital, Chicago: R Yogev, E Chadwick, E Cagwin, K Kabat; New Jersey Medical School: A Dieudonne, P Palumbo, J Johnson; Robert Wood Johnson Medical School, New Brunswick : S Gaur, L Cerracchio; Columbia IMPAACT: M Foca, A Jurgrau, S Vasquez Bonilla, G Silva; Babies’ Hospital, Columbia/Presbyterian Medical Center, New York (A Gershon); University of Massachusetts Medical Center, Worcester (J Sullivan); UCLA Medical Center, Los Angeles (Y Bryson); Children’s Hospital, Seattle: L Frenkel; UNC-Chapel Hill Virology Lab: S Fiscus (L), J Nelson (L). Trials Units/Support: INSERM SC10-US019 Paris, France: : JP Aboulker, A Compagnucci, G Hadjou, S Léonardo, Y Riault, Y Saïdi; MRC Clinical Trials Unit at University College London, UK: : A Babiker, L Buck, JH Darbyshire, L Farrelly, S Forcat, DM Gibb, H Castro, L Harper, L Harrison, J Horton, D Johnson, C Taylor, AS Walker; Westat/NICHD: D Collins, S Buskirk, P Kamara, C Nesel, M Johnson, A Ferreira; Frontier Science: : J Hodge, J Tutko, H Sprenger; IMPAACT SDAC: : M. Hughes, M. Warshaw, P. Britto, C. Powell, L Harrison; NIAID: : R DerSimonian, E Handelsman; PENTA Steering Committee: JP Aboulker, J Ananworanich, A Babiker, E Belfrage, S Bernardi, S Blanche, AB Bohlin, R Bologna, D Burger, K Butler, G Castelli-Gattinara, H Castro, P Clayden, A Compagnucci, T Cressey, JH Darbyshire, M Debré, R De Groot, M Della Negra, A De Rossi, D Duicelescu (deceased), A Faye, V Giacomet, C Giaquinto (Chair), DM Gibb, I Grosch-Wörner, M Hainault, N Klein, M Lallemant, J Levy, H Lyall, M Marczynska, L Marques, M Mardarescu, MJ Mellado Pena, D Nadal, E Nastouli, L Naver, T Niehues, A Noquera, C Peckham, D Pillay, J Popieska, JT Ramos Amador, P Rojo Conejo, L Rosado, R Rosso (deceased), C Rudin, Y Saïdi, H Scherpbier, M Sharland, M Stevanovic, C Thorne, PA Tovo, G Tudor-Williams, A Turkova, N Valerius, A Volokha, AS Walker, S Welch, U Wintergerst
Abbreviations
- ‘1000 criteria’
not achieving HIV-1 RNA <1000c/ml by week 24 of first-line, confirmed rebound ≥1000c/ml thereafter or CDC stage C event
- ‘30000 criteria’
not achieving HIV-1 RNA <30000c/ml by week 24 of first-line, confirmed rebound ≥30000c/ml thereafter or CDC stage C event
- ART
antiretroviral therapy
- CDC
Center for Disease Control and Prevention
- higher-threshold arm
randomized to switch at the ‘30000 criteria’
- IAS
International AIDS Society-USA
- low-threshold arm
randomized to switch at the ‘1000 criteria’
- MTCT
mother to child transmission
- NRTI
nucleos(t)ide reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- TAMs
thymidine analogue mutations
- WHO
world health organization
Footnotes
Data were presented at the Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, March 3-6, 2014
Conflicts of Interest: SF received an honorarium from Roche Molecular Systems for serving on a scientific advisory board. For the remaining authors, none were declared.
References
- 1.World Health Organisation. Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection; Recommendations for a Public Health Approach. 2013 Jun; [PubMed]
- 2.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Available at: http://aidsinfo.nih.gov/guidelines.
- 3.PENTA Steering Committee. PENTA 2009 Guidelines for the use of antiretroviral therapy in paediatric HIV-1 infection. HIV Med. 2009;10:591–613. doi: 10.1111/j.1468-1293.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.PENPACT-1 (PENTA 9/PACTG 390) Study Team. Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, Gibb DM, Harper L, Harrison L, Hughes M, McKinney R, Melvin A, Mofenson L, Saidi Y, Smith ME, Tudor-Williams G, Walker AS. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11(4):273–283. doi: 10.1016/S1473-3099(10)70313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson VA, Brun-Vezinet, Clotet B, et al. Update of the drug resistance mutations in HIV-1: December 2009. Top HIV Med. 2009;17:138–145. [PubMed] [Google Scholar]
- 6.Pillay D, Green H, Matthias R, et al. Estimating HIV-1 Drug Resistance in antiretroviral-treated individuals in the United Kingdom. J Infect Dis. 2005;192:967–973. doi: 10.1086/432763. [DOI] [PubMed] [Google Scholar]
- 7.Stanford University HIV Drug Resistance Database. Available at: http://hivdb/stanford.edu/
- 8.Hill A, McBride A, Sawyer AW, et al. Resistance at Virological Failure Using Boosted Protease Inhibitor Versus Nonnucleoside Reverse Transcriptase Inhibitors As First-Line Antiretroviral Therapy – Implications for Sustained Efficacy of ART in resource-Limited Settings. JID. 2013;207(Suppl 2) doi: 10.1093/infdis/jit112. [DOI] [PubMed] [Google Scholar]
- 9.Scherrer AU, Boni J, Yerly S, et al. Long-Lasting Protection of Activity of Nucleoside Reverse Transcriptase Inhibitors and Protease Inhibitors (PIs) by Boosted PI Containing Regimens. PLOS One. 2012;7(11):e50307. doi: 10.1371/journal.pone.0050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cozzi-Lepri A, Phillips A, Ruiz L, et al. Evolution of drug resistance in HIV-infected patients remaining on a virologically failing combination antiretroviral therapy regimen. AIDS. 2007;21:721–732. doi: 10.1097/QAD.0b013e3280141fdf. [DOI] [PubMed] [Google Scholar]
- 11.Barth RE, Aikten SC, Tempelman H, et al. Accumulation of drug reistance and loss if therapeutic options precede commonly used criteria for treatment failure in HIV-1 subtype-C-infected patients. Antiviral Therapy. 2012;17:377–386. doi: 10.3851/IMP2010. [DOI] [PubMed] [Google Scholar]
- 12.Sigaloff KCE, Ramatsebe T, Viana R, et al. Accumulation of HIV Drug resistance Mutations in Patients Failing First-Line Antiretroviral Treatment in South Africa. AIDS Research and Human Retroviruses. 2012;28(2):171–175. doi: 10.1089/aid.2011.0136. [DOI] [PubMed] [Google Scholar]
- 13.Sigaloff KCE, Calis JCJ, Geelen Sp, et al. HIV-1-resistance-associated mutations after failure of first-line antiretroviral treatment among children in resource-poor regions: a systematic review. Lancet Infect Dis. 2011;11:769–779. doi: 10.1016/S1473-3099(11)70141-4. [DOI] [PubMed] [Google Scholar]
- 14.Bergshoeff AS, Fraaij PL, van Rossum AM, et al. Pharmacokinetics of nelfinavir in children: influencing factors and dose implications. Antivir Ther. 2003;8:215–222. [PubMed] [Google Scholar]
- 15.Violari A, Cotton M, Otwombe K, et al. Does early initiation of ART in infants affect virological and resistance outcomes? Data from the CHER trial after 6 years of follow-up. Journal of the International AIDS Society. 2012;15(Suppl 4):18085. [Google Scholar]
- 16.Riddler SA, Jiang H, Tenorio A, et al. A randomized study of antiviral medication switch at lower- versus higher-switch threshold: AIDS Clinical Trials Group Study A5115. Antivir Ther. 2007;12:531–541. doi: 10.1177/135965350701200415. [DOI] [PubMed] [Google Scholar]
- 17.Jourdain G, Le Coeur S, Ngo-Giang-Huong N, et al. Switching HIV Treatment in Adults Based on CD4 Count Versus Viral Load Monitoring: A Randomized, Non-Inferiority Trial in Thailand. PLOS medicine. 2013;10(8):e1001494. doi: 10.1371/journal.pmed.1001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurent C, Kouanfack C, Laborde-Balen G, et al. Monitoring of HIV viral loads, CD4 cell counts, and clinical assessment versus clinical monitoring alone for antiretroviral therapy in rural district hospitals in Cameroon (Stratal ANRS 12110/ESTHER): a randomized non-inferiority trial. Lancet Infect Dis. 2011;11:825–833. doi: 10.1016/S1473-3099(11)70168-2. [DOI] [PubMed] [Google Scholar]
- 19.Paediatric European Network for Treatment of AIDS (PENTA) Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet. 2002;359:733–740. doi: 10.1016/S0140-6736(02)07874-1. [DOI] [PubMed] [Google Scholar]
- 20.Musiime V, Kekitiinwa A, Mulenga A, et al. CHAPAS 3: A randomized trial comparing stavudine vs zidovudine vs abacavir as NRTI backbone in NNRTI-based first-line ART in 478 HIV-infected children in Uganda and Zambia. Reviews in Antiviral Therapy & Infectious Disease. 2014;6 Abstract O_21. [Google Scholar]
- 21.Gibb DM, Walker AS, Kaye S, et al. Evolution of antiretroviral phenotypic and genotypic drug resistance in antiretroviral-naïve HIV-1-infected children treated with abacavir/lamivudine, zidovudine/lamivudine or abacavir/zidovudine, with or without nelfinavir (the PENTA 5 trial) Antiviral Therapy. 2002;7:293–303. [PubMed] [Google Scholar]
- 22.Stanford University HIV Drug Resistance Database, NRTI Resistance Notes. Available at: http://hivdb.stanford.edu/DR/NRTIResiNote.html.
- 23.Waters L, Bansi L, Asboe D, et al. Second-line protease inhibitor-based antiretroviral therapy after non-nuceloside reverse transcriptase inhibitor failure: the effect of nucleoside backbone. Antriviral Therapy. 2013;18:213–219. doi: 10.3851/imp2329. [DOI] [PubMed] [Google Scholar]
- 24.Johnston V, Cohen K, Wiesner L, et al. Viral Suppression Following Switch to Second-line Antiretroviral Therapy: Associations With Nucleoside Reverse Transcriptase Inhibitor Resistance and Subtherapeutic Drug Concentrations Prior to Switch. JID. 2014;209:711–720. doi: 10.1093/infdis/jit411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.