More than doubling the national mean, Saskatchewan has the highest incidence of HIV in Canada. The progression of HIV is characterized by the decline in CD4+ T cells over time and can lead to immunological AIDS. Clinicians in Saskatoon, Saskatchewan, have observed a more rapid progression to AIDS in the recent years. The goal of this retrospective longitudinal cohort study was to investigate the rate of CD4+ cell depletion, as well as to determine the effects of multiple clinical and social factors that may contribute to an accelerated progression of HIV to AIDS in this population.
Keywords: CD4+ count, First Nations, HCV, HIV, IDU, Métis, Rapid progression
Abstract
OBJECTIVE:
To assess the impact of clinical and social factors unique to HIV-infected adults in Saskatoon, Saskatchewan, regarding the rate of CD4+ count change, and to identify factors associated with a risk of CD4+ count decline.
METHODS:
A retrospective longitudinal cohort study from medical chart reviews at two clinics was conducted in Saskatoon. Univariate and multivariate linear mixed effects models were used to assess the impact of selected factors on CD4+ count change.
RESULTS:
Four hundred eleven HIV-infected patients were identified from January 1, 2003 to November 30, 2011. Two hundred eighteen (53%) were male, mean (± SD) age was 35.6 ±10.1 years, 257 (70.8%) were First Nations or Métis, 312 (80.2%) were hepatitis C virus (HCV) coinfected and 300 (73.3%) had a history of injection drug use (IDU). In univariate models, age, ethnicity, HCV, IDU, antiretroviral therapy and social assistance were significant. Using ethnicity, HCV and IDU, three multivariate models (models 1, 2, 3) were built due to high correlation. First Nations or Métis ethnicity, HCV coinfection and a history of IDU were associated with significantly lower CD4+ counts in multivariate models. Older age and social assistance were associated with significantly lower CD4+ counts in models 1 and 3. Age was marginally significant in model 2 (P=0.055). Not prescribed antiretroviral therapy was associated with a significantly negative CD4+ count slope in all multivariate models.
CONCLUSION:
The unique epidemiology of this HIV-infected population may be contributing to CD4+ count change. Increased attention and resources focused on this high-risk population are needed to prevent disease progression and to improve overall health and quality of life.
Abstract
OBJECTIF :
Évaluer les répercussions des facteurs cliniques et sociaux propres aux adultes infectés par le VIH de Saskatoon, en Saskatchewan, sur le taux de modifications de la numération de CD4+ et déterminer les facteurs associés à un risque de diminution de la numération de CD4+.
MÉTHODOLOGIE :
Les chercheurs ont réalisé une étude de cohorte longitudinale rétrospective des dossiers médicaux de deux cliniques de Saskatoon. Ils ont utilisé les modèles linéaires à effets mixtes univariés et multivariés pour évaluer les répercussions de certains facteurs associés aux modifications de la numération de CD4+.
RÉSULTATS :
Les chercheurs ont repéré 411 patients infectés par le VIH entre le 1er janvier 2003 et le 30 novembre 2011. Deux cent dix-huit d’entre eux (53 %) étaient de sexe masculin et avaient un âge moyen (± ÉT) de 35,6 ans ±10,1 ans, 257 (70,8 %) étaient Métis ou originaires des Premières nations, 312 (80,2 %) étaient co-infectés par le virus de l’hépatite C (VHC) et 300 (73,3 %) avaient des antécédents de consommation de drogues par injection (CDI). Dans les modèles univariés, l’âge, l’ethnie, le VHC, la CDI, l’antirétrovirothérapie et l’aide sociale étaient déterminants. À l’aide de l’ethnie, du VHC et de la CDI, les chercheurs ont formé trois modèles multivariés (modèles 1, 2, 3) en raison de leur forte corrélation. Le fait d’être Métis ou originaire des Premières nations, d’être co-infecté par le VHC et d’avoir des antécédents de CDI s’associait à des numérations de CD4+ beaucoup plus faibles dans les modèles multivariés. Le fait d’être plus âgé et de recevoir de l’aide sociale s’associait à une numération beaucoup plus faible de CD4+ dans les modèles 1 et 3. L’âge était légèrement significatif dans le modèle 2 (P=0,055). Dans tous les modèles multivariés, l’antirétrovirothérapie ne s’associait jamais à une pente négative de la numération de CD4+.
CONCLUSION :
L’épidémiologie unique de cette population infectée par le VIH contribue peut-être à une modification de la numération de CD4+. Il faudra se pencher sur ces patients à haut risque et y injecter plus de ressources pour prévenir l’évolution de leur maladie et améliorer leur santé et leur qualité de vie globales.
The HIV epidemic in Saskatchewan has been growing at an alarming rate since 2003 (1,2). At the present time, the incidence of HIV in Saskatchewan is the highest in Canada (19.6 per 100,000 in 2011), at more than double the national average (7.6 per 100,000) (3). Importantly, the highest recorded incidences have occurred in the most recent years (23.8 per 100,000 in 2009; 20.3 in 2010 and 19.6 in 2011), indicating that the epidemic in this province is not approaching resolution (3).
The epidemiology of HIV in Saskatchewan is unique to Canada in that female sex and/or individuals self-identifying as being of First Nations and Métis ethnicity are over-represented compared with other HIV-infected populations across Canada (4–6). The unfortunate prevalence of low socioeconomic status among First Nations and Métis communities in Canada poses barriers to achieving optimum health. Such challenges include poverty and the associated housing insecurity and malnutrition, in addition to increased rates of incarceration, injection drug use (IDU) and mental illness. Another unique characteristic of the HIV epidemic in this province is that IDU is the predominant mode of transmission, in contrast to men who have sex with men, which has remained the most commonly reported exposure nationally, since the beginning of the epidemic in Canada (1,2). Given the high prevalence of IDU, this population is also afflicted with high rates of coinfection with hepatitis C virus (HCV), which has been shown to be 10 times more transmissible through IDU than HIV (7–9).
Immune deficiency in AIDS is caused by the virally mediated destruction of CD4+ T cells (10,11). HIV disease progression is characterized by the progressive decline in CD4+ count over time (11–15). The monitoring of CD4+ cell counts over time provides a surrogate measure of HIV disease progression (10). CD4+ cell counts are used by clinicians in determining when to initiate antiretroviral therapy (ART) and other prophylactic therapies in the treatment of opportunistic infections, as well as being commonly used as an end point in clinical studies (10,11,16,17). In the present study, we used CD4+ cell count to evaluate HIV disease progression.
Clinicians in Saskatoon, Saskatchewan caring for HIV-infected individuals have anecdotally reported the observation of a more rapid than expected progression to immunological AIDS (CD4+ count <200 cells/µL) in recent years. The aim of the present study was to examine this phenomenon of rapid progression from HIV infection to immunological AIDS.
The HIV-infected population of Saskatchewan is afflicted with a multitude of health-compromising conditions; this population is understudied and is showing evidence of rapid progression to AIDS. The objectives of the present study were to estimate the rate of CD4+ cell depletion among HIV-infected adults in the city of Saskatoon, and to determine the effects of the following clinical and social factors regarding CD4+ cell count changes: age at diagnosis; sex; ethnicity; HCV coinfection; history of IDU; ART; incarceration during follow-up; engagement in case management services; receipt of social assistance; and presence of a sexually transmitted infection (STI).
METHODS
Setting and population
The present study was a retrospective longitudinal cohort anaylsis of HIV-infected patients followed at two clinics in Saskatoon, specializing in the care of this population: the Positive Living Program at Royal University Hospital and the Westside Community Clinic (WSCC).
Data collection
Data were abstracted from patient charts. Inclusion criteria included a new HIV diagnosis of patients ≥18 years of age between January 1, 2003 and November 30, 2011. Patient data abstracted from medical charts included demographics, social history, clinical variables, laboratory data and ART. First CD4+ count and viral load measurement within six months of HIV diagnosis were considered to be baseline measurements.
Data analyses
Patient characteristics at HIV diagnosis (baseline) and during follow-up (time dependent) were summarized using descriptive statistics. A χ2 test was used to assess associations between categorical variables. Pearson correlation analysis was performed. Independent variables considered included sex, age, ethnicity, HCV seropositivity, coinfection with an STI, IDU, history of incarceration, history of receipt of social assistance, case management (a program of intensive social work at the WSCC) and ART. Before fitting mixed effect models on CD4+ count outcome, the CD4+ count of an individual was summarized in three-month intervals for the first three years and in six-month intervals for the remainder of the study time. If a subject underwent more than one measurement in a given interval, the mean was used. This interval was selected because this is the standard clinical follow-up timeline for patients observed in the two clinics. The interval was increased to six months after the three-year period because there were far fewer patients followed for >3 years compared with patients followed for ≤3 years. CD4+ count was then modelled longitudinally by fitting mixed effects models with random intercept a nd random slope (18). A linear regression of CD4+ count change according to months since diagnosis among 10 randomly selected patients, as well as a linear regression of mean CD4+ count according to months since diagnosis was created to ensure the assumption of a robustly linear change in CD4+ count over time was not violated. Interactions among covariates were examined. Variables were identified as significant using a 0.05 α level. All analyses were performed using SAS version 9.2 (SAS Institute, USA).
The present study was approved by the University of Saskatchewan Ethics Review Board, the Saskatoon Health Region and the WSCC.
RESULTS
A total of 411 patients who had at least one CD4+ recorded met the study inclusion criteria. A total of 2555 CD4+ counts were recorded among all patients (Table 1). One hundred eighty-eight patients were followed at the Positive Living Program, 122 at the WSCC and 101 were followed at both sites. The mean (± SD) age at diagnosis was 35.7±10.3 years and 53% of patients were men. Two hundred fifty-seven (70.8%) patients self-identified as being of First Nations or Métis ethnicity. The most commonly reported exposure category was IDU (75.5%), followed by heterosexual contact (14.2%) and men having sex with men (6.5%). Fifty-four (13.1%) patients were case management clients, 146 (35.5%) had a record of receiving social assistance, 134 (32.6%) acquired an STI throughout the course of their care, 312 (80.2%) were HCV seropositive, 300 (73.3%) had a history of IDU, 118 (28.7%) had a history of incarceration and 257 (62.5%) were ever prescribed ART. The mean baseline CD4+ count was 379±233 cells/µL and the mean baseline log viral load was 4.3±1 copies/mL. The mean follow-up time for all patients was 32.5 months.
TABLE 1.
Baseline and time-dependent patient characteristics according to ethnicity (n=411)
| Variables | Total sample (n=411) | First Nations or Métis (n=257, 70.8%) | Others* (n=106, 29.2%) |
|---|---|---|---|
| Follow-up time, months, median (IQR) | 27.6 (31.9) | 27.6 (29.9) | 32.6 (37.7) |
| Variable at baseline | |||
| Age at enrollment, years, mean ± SD | 35.7±10.3 | 33.7±9.1 | 41±11.5 |
| Exposure category† | |||
| MSM | 21 (6.5) | 1(0.5) | 20 (22.0) |
| MSM/IDU | 12 (3.7) | 5 (2.3) | 6 (6.6) |
| IDU | 244 (75.5) | 193 (89.8) | 36 (39.5) |
| Heterosexual contact | 46 (14.2) | 16 (7.4) | 29 (31.9) |
| Site | |||
| PLP | 188 (45.7) | 100 (38.9) | 80 (75.5) |
| WSCC | 122 (29.7) | 84 (32.7) | 7 (6.6) |
| Both | 101 (24.6) | 73 (28.4) | 19 (17.9) |
| CD4+ count, cells/µL | |||
| n, mean ± SD | 300, 379±233 | 181, 369±218 | 87, 376±236 |
| Missing, n | 111 | 76 | 19 |
| CD4+ count, cells/µL | |||
| <200 | 68 (22.7) | 39 (21.6) | 21 (24.1) |
| 200–350 | 88 (29.3) | 60 (33.1) | 22 (25.3) |
| >350 | 144 (48.0) | 82 (45.3) | 44 (50.6) |
| Log10 of viral load, copies/mL | |||
| n, mean ± SD | 276, 4.3±1.0 | 167, 4.2±1.0 | 85, 4.4±1.0 |
| Missing, n | 135 | 90 | 21 |
| HCV antibody positive | 312 (80.2) | 227 (92.6) | 47 (46.5) |
| Missing, n | 22 | 12 | 5 |
| History of IDU | 300 (73.3) | 222 (87.1) | 43 (40.6) |
| Missing, n | 2 | 2 | |
| STI | 134 (32.6) | 86 (33.5) | 38 (35.8) |
| Time-dependent variables | |||
| Incarcerated during follow-up | 118 (28.7) | 89 (34.6) | 22 (20.7) |
| Ever use of social assistance | 146 (35.5) | 110 (42.8) | 25 (23.6) |
| Ever use of ART | 257 (62.5) | 167 (65.0) | 70 (66.0) |
| ART naïve | 154 (37.5) | 90 (35.0) | 36 (34.0) |
| Case management | 54 (13.1) | 45 (17.5) | 4 (3.8) |
| Clinical AIDS | 30 (7.3) | 18 (7.0) | 12 (11.3) |
| All-cause mortality | 29 (7.1) | 20 (7.8) | 8 (7.5) |
Data presented as n (%) unless otherwise indicated.
48 were missing;
88 were missing. ART Antiretroviral therapy; HCV Hepatitis C virus, IDU Injection drug use; IQR Interquartile range; MSM Men who have sex with men; PLP Positive Living Program; STI Sexually transmitted infection; WSCC Westside Community Clinic
HCV coinfection, First Nations or Métis ethnicity, and IDU were highly correlated (Figure 1). Proportion of HCV coinfection is significantly higher in injection drug users compared with individuals who did not have a history of IDU (98.6% versus 25.8%; P<0.0001). Individuals self-identifying as of First Nations or Métis ethnicity experienced higher odds of having a history of IDU (OR 9.86 [95% CI 5.78 to 16.79]; P<0.0001) and HCV coinfection (OR 14.49 [95% CI 7.80 to 6.91]; P<0.0001). Time to ART initiation was not significantly different between First Nations or Métis and other ethnicity (P=0.43, log-rank test, Figure 2).
Figure 1).
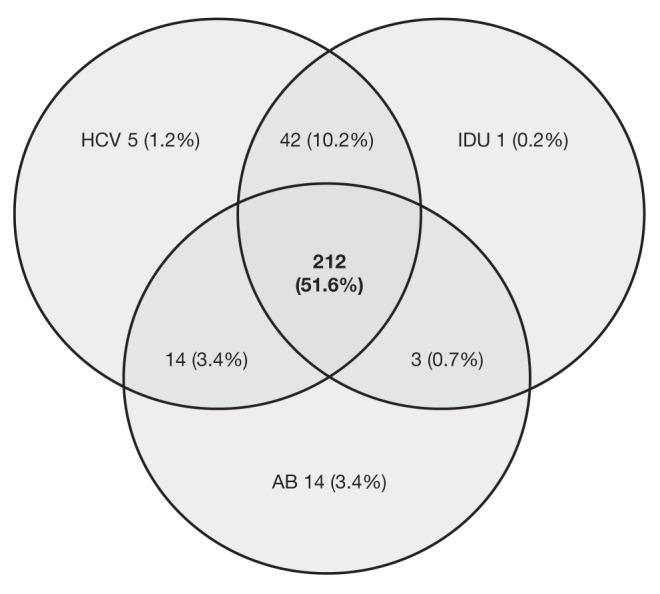
High correlation among patients coinfected with hepatitis C virus (HCV), injection drug use (IDU) and self-identifying as First Nations or Métis ethnicity (AB). (Note: 53 [12.9%] patients did not possess any of these cofactors and 67 [16.3%] had missing information regarding at least one of these cofactors)
Figure 2).
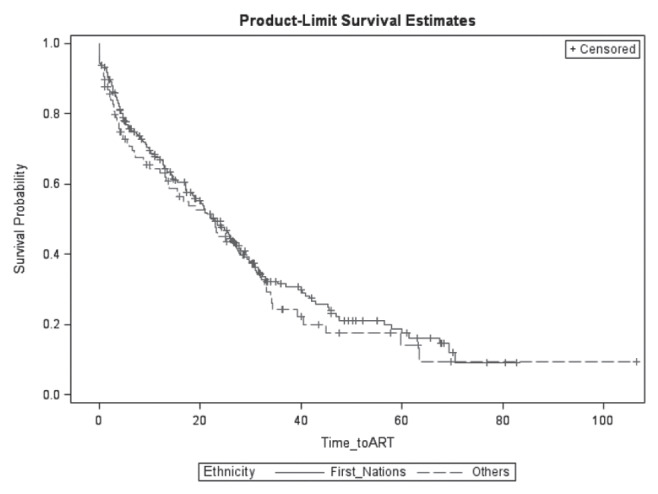
Time to antiretroviral therapy (ART) initiation among First Nations patients compared with patients of other ethnicities
In the univariate mixed effects models, ethnicity, social assistance, HCV coinfection, history of IDU and ever use of ART were significant. Sex, age, incarceration, case management and STI coinfection were not significant (results are not shown for the univariate analysis).
All significant covariates from the univariate mixed effects models analysis were included in the multivariate models. Although age was not univariately significant, it was considered to be a potential confounder and added in the multivariate models. Due to the significant correlation among ethnicity, HCV infection and IDU, the following three separate multivariate mixed effects models were built.
Model 1: In the first model incorporating ethnicity (Table 2), the estimated mean regression coefficient of time for the ART-naïve group was −27.18 (95% CI −44.95 to −9.41), suggesting a significant decrease in CD4+ count at a rate of 27.18 cells/µL per year among ART-naïve patients after controlling for other covariates. CD4+ count remained almost unchanged among ART recipients (−27.18+27.94=0.76 cells/µL per year). The rate of CD4+ count change was significantly different between these two groups (27.94 cells/µL per year, P=0.008). First Nations ethnicity (P=0.027), receipt of social assistance (P=0.008) and higher age at diagnosis (P=0.001) were significant predictors of lower CD4+ count.
Model 2: In the second model incorporating HCV coinfection (Table 3), CD4+ count significantly decreased at a rate of 33.72 cells/µL per year among ART-naive patients (P=0.0001). CD4+ count was not changed among ART-exposed patients (−33.72+37.17=3.45 cells/µL per year). However, the rate of CD4+ count change was significantly different between these two groups (37.17 cells/µL per year, P=0.0003). HCV antibody positivity (P=0.003) and older age at diagnosis (P=0.055) were associated with lower CD4+ counts.
Model 3: In the third model incorporating a history of IDU (Table 4), CD4+ count significantly decreased at a rate of 32.41 cells/µL per year among ART-naive patients after controlling for other covariates (P=0.0002). CD4+ count remained almost unchanged among ART recipients (−32.41+34.08=1.67 cells/µL per year). The rate of CD4+ count change was significantly different between these two groups (34.08 cells/µL per year, P=0.0007). A history of IDU (P=0.042), receipt of social assistance (P=0.042) and increasing age at diagnosis (P=0.026) were significant predictors of lower CD4+ counts.
TABLE 2.
Multivariate mixed effects model (model 1) containing First Nations or Métis ethnicity (n=363)
| Covariate | β ± SE | 95% CI | P |
|---|---|---|---|
| Intercept | 618.50±45.63 | 528.77 to 708.24 | <0.0001 |
| Time (in years) | −27.18±9.03 | −44.95 to −9.41 | 0.002 |
| Time*ART (yes) | 27.94±10.52 | 7.31 to 48.57 | 0.008 |
| First Nations or Métis | −47.20±21.32 | −89.03 to −5.37 | 0.027 |
| Social assistance (yes) | −50.85±19.24 | −88.59 to −13.10 | 0.008 |
| Age at diagnosis | −2.93±0.93 | −4.76 to −1.10 | 0.001 |
ART Antiretroviral therapy
TABLE 3.
Multivariate mixed effects model (model 2) containing hepatitis C virus (HCV) coinfection (n=389)
| Covariate | β ± SE | 95% CI | P |
|---|---|---|---|
| Intercept | 607.17±45.34 | 518.03 to 696.32 | <0.0001 |
| Time (in years) | −33.72±8.69 | −50.82 to −16.62 | 0.0001 |
| Time*ART (yes) | 37.17±10.20 | 17.17 to 57.17 | 0.0003 |
| HCV (yes) | −67.24±23.28 | −112.91 to −21.58 | 0.003 |
| Social assistance (yes) | −33.57±19.40 | −71.63 to 4.49 | 0.08 |
| Age at diagnosis | −1.72±0.90 | −3.49 to 0.04 | 0.055 |
ART Antiretroviral therapy
TABLE 4.
Multivariate mixed effects model (model 3) containing injection drug use (IDU) (n=409)
| Covariate | β±SE | 95% CI | P |
|---|---|---|---|
| Intercept | 590.62±42.69 | 506.70 to 674.54 | <0.0001 |
| Time (in years) | −32.41±8.47 | −49.07 to −15.74 | 0.0002 |
| Time*ART (yes) | 34.08±9.99 | 14.48 to 53.68 | 0.0007 |
| IDU (yes) | −42.77±21.02 | −84.01 to −1.54 | 0.042 |
| Social assistance (yes) | −38.92±19.16 | −76.51 to −1.33 | 0.042 |
| Age at diagnosis | −1.97±0.89 | −3.70 to −0.23 | 0.026 |
ART Antiretroviral therapy
DISCUSSION
The present study was a retrospective longitudinal cohort analysis of 411 HIV-positive patients diagnosed between January 1, 2003 and November 30, 2011 and followed by physicians at the Positive Living Program and WSCC in Saskatoon, Saskatchewan. We investigated CD4+ changes over time to explore HIV disease progression to immunological AIDS. The objective of the present study was to identify clinical and social factors associated with rates of change in CD4+ counts.
HIV and HCV are both transmitted through IDU (19–22). There is an increased frequency of IDU among incarcerated populations, because patients with more extreme addictions may be at an increased likelihood to incur criminal charges (23,24). IDU and incarceration have been found to be associated with a lower uptake of and adherence to ART (25–29). Additionally, the stress of the prison environment has been found to contribute to a faster rate of decline in CD4+ count (13). The tracking of patients through the prison systems in Saskatchewan has been identified as a barrier to ensuring continuity of care among incarcerated HIV-positive individuals, in particular in ensuring a continual supply of ART. This reduced ability to track a patient’s incarceration, duration and release also likely led to an under-representation of the true prevalence of incarceration in the study population. Therefore, the negative impact of incarceration regarding HIV disease progression may be underestimated in the present study.
A longitudunal mixed-effects model found self-identifying as being of First Nations or Métis ethnicity, HCV coinfection, a history of IDU, older age and receipt of social assistance to be associated with significantly lower CD4+ counts in multivariate models. Not being exposed to ART was significantly associated with a negative CD4+ count slope in multivariate mixed effects models.
The mixed effects models have the potential to be developed into clinical tools. These clinical tools may predict the CD4+ counts of patients at follow-up visits and, therefore, potentially be helpful in the identification of patients at risk for rapid progression. By identifying these patients early in the clinical course of HIV disease progression, interventions to mitigate risk factors for rapid progression may be initiated; for example, increased social support to mitigate the effects of low socioeconomic status such as poor nutrition. Such increased social support has recently been introduced at the WSCC in the form of case management service. Associations between engagement in case management services and improvements in overall health among HIV-infected individuals have been previously reported (30–32). Although we did not observe a significant association with increased CD4+ counts and involvement with case management services, this finding was likely due to dataset limitations.
Socioeconomic disadvantages, which are unfortunately prevalent among First Nations and Métis communities, HCV coinfection, a history of IDU, receipt of social assistance and older age at diagnosis were associated with lower CD4+ counts. CD4+ count significantly decreased over time among patients who were never prescribed ART. The present study highlights the urgent need for both clinical, as well as social, interventions to address the HIV epidemic in Saskatchewan.
The findings of the present study may contribute to improvements in clinical care in that these findings may aid clinicians in the identification of HIV-infected patients who may be at risk for rapid progression. The identification of cofactors that can be implicated in contributing to rapid progression to AIDS and/or death has the potential to slow disease progression if the impacts of such cofactors can be mitigated. Earlier and more aggressive ART may be beneficial to patients who can be identified as higher risk for rapid progression to AIDS.
Strengths of the present study include the unique epidemiology of the study population; namely, an over-representation of individuals of First Nations or Métis ethnicity and therefore, an increased prevalence of social and economic disadvantage that unfortunately exists among this population; a high prevalence of HCV coinfection; and a high prevalence of IDU. Another strength is that the follow-up period of the present study incorporates the course of the emergence of the dramatic increase in incidence of HIV in Saskatchewan.
Limitations of the present study include those of all retrospective data sets, in that we were limited to information that was previously recorded in patient medical records, which may not always have included all of the variables of interest. For example, absolute lymphocyte count, leukocyte count, and information regarding alcohol use and abuse among patients were not available. Alcoholism has known effects on CD4+ counts in HIV-infected patients (33,34). Another limitation of this dataset was an unknown date of seroconversion among many patients, which poses a challenge to an accurate depiction of the entire clinical course of HIV. We were able to arrive at a more accurate depiction of the effects of selected cofactors on the clinical course of HIV through the use of multiple analyses and comparisons of the results of these analyses.
Future studies should include the creation of a prospective cohort database, ideally enrolling at-risk individuals while seronegative, to establish date of seroconversion and, therefore, enable a more accurate and complete depiction of the individual and population-level clinical course of HIV infection. In addition, studies with a longer follow-up period would also allow for a more comprehensive picture of the course of HIV among Saskatchewan patients. Finally, a study examining social factors contributing to the HIV epidemic in Saskatchewan and, in particular, the contribution of social factors to the phenomenon of a rapid progression to AIDS would be of particularly importance to this unique population.
SUMMARY
The HIV-infected population of Saskatoon is characterized according to unique social and clinical factors, which may conspire to contribute to an accelerated progression to AIDS. The variables of HCV coinfection, First Nations or Métis ethnicity and IDU were highly correlated. Ethnicity, receipt of social assistance, older age at diagnosis, HCV coinfection, a history of IDU and ever use of ART were significantly associated with lower CD4+ counts. Patients presenting as HIV positive with one or more of these cofactors can be considered to be at risk for accelerated progression to AIDS. The early identification by clinicians of patients with these risk factors and the implementations of targeted interventions to mitigate the negative health effects of these cofactors may contribute to improved health and quality of life for this HIV-infected population.
Acknowledgments
The authors acknowledge the Saskatoon Health Region, the Positive Living Program, the Westside Community Clinic and the thesis committee for their assistance and support of this project.
REFERENCES
- 1.Saskatchewan Ministry of Health, Population Health Branch HIV Strategy for Saskatchewan 2010–2013
- 2.Public Health Agency of Canada HIV and AIDS in Canada. Surveillance Report to December 31, 2009. Surveillance and Risk Assessment Division, Centre for Communicable Diseases and Infection Control. 2010.
- 3.Public Health Agency of Canada At a Glance – HIV and AIDS in Canada: Surveillance Report to December 31st, 2011–2012
- 4.Disease Prevention Unit, Population Health Branch, Saskatchewan Ministry of Health HIV and AIDS in Saskatchewan 2010. Nov 30, 2011.
- 5.Public Health Agency of Canada HIV/AIDS Epi Updates – July 2010, Chapter 1: National HIV Prevalence and Incidence Estimates in Canada for 2008. 2011. < www.phac-aspc.gc.ca/aids-sida/publication/epi/2010/1-eng.php#note5> (Accessed March 12, 2012).
- 6.HIV in Saskatchewan Panel Discussion. Feb 14, 2011.
- 7.Montaner JSG, Le T, Hogg R, et al. The changing spectrum of AIDS index diseases in Canada. AIDS. 1994;8:693. doi: 10.1097/00002030-199405000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman DC, Semaan S. Associations between herpes simplex virus type 2 and HCV with HIV among injecting drug users in New York City: The current importance of sexual transmission of HIV. Am J Public Health. 2011;101:1277. doi: 10.2105/AJPH.2011.300130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Des Jarlais D, Semaan S. HIV and other sexually transmitted infections in injection drug users and crack cocaine smokers. In: Holmes KK, Spanling PF, Stamm WE, et al., editors. Sexually Transmitted Diseases. 4th edn. New York: McGraw Hill; 2008. pp. 237–55. [Google Scholar]
- 10.Seligmann M, Pinching AJ, Rosen FS, et al. Immunology of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Ann Intern Med. 1987;107:234–42. doi: 10.7326/0003-4819-107-2-234. [DOI] [PubMed] [Google Scholar]
- 11.McCune JM. The dynamics of CD4 T-cell depletion in HIV disease. Nature. 2001;410:974–9. doi: 10.1038/35073648. [DOI] [PubMed] [Google Scholar]
- 12.Rowland-Jones S, Pinheiro S, Kaul R. New insights into host factors in HIV-1 pathogenesis. Cell. 2001;104:473. doi: 10.1016/s0092-8674(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffin MM, Ryan JG, Briscoe VS, Shadle KM. Effects of incarceration on HIV-infected individuals. J Natl Med Assoc. 1996;88:639. [PMC free article] [PubMed] [Google Scholar]
- 14.Anastos K, Gange SJ, Lau B, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr. 2000;24:218. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Lyles RH, Muñoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. J Infect Dis. 2000;181:872. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- 16.Maini M, Gilson R, Chavda N, et al. Reference ranges and sources of variability of CD4 counts in HIV-seronegative women and men. Genitourin Med. 1996;72:27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsouska C, Bernard N. Markers Predicting progression of human immunodeficiency virus-related disease. Clin Microbiol Rev. 1994;7:14–28. doi: 10.1128/cmr.7.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982:963–74. [PubMed] [Google Scholar]
- 19.Lewden C, Thiébaut R, Boufassa F, et al. Comparison of early CD4 T-cell count in HIV-1 seroconverters in Cote d’Ivoire and France: The ANRS PRIMO-CI and SEROCO cohorts. J Acquir Immune Defic Syndr. 2010;53:260. doi: 10.1097/QAI.0b013e3181b84260. [DOI] [PubMed] [Google Scholar]
- 20.Ledergerber B, Egger M, Erard V, et al. AIDS-related opportunistic illnesses occurring after initiation of potent antiretroviral therapy. JAMA. 1999;282:2220–6. doi: 10.1001/jama.282.23.2220. [DOI] [PubMed] [Google Scholar]
- 21.Staples JC, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): The effect of coinfection on survival. Clin Infect Dis. 1999;29:150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 22.Dorrucci M, Pezzotii P, Phillips A, Lepri A, Rezza G. Coinfection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. J Infect Dis. 1995;172:1503–8. doi: 10.1093/infdis/172.6.1503. [DOI] [PubMed] [Google Scholar]
- 23.Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. Human immunodeficiency virus in correctional facilities: A review. Clin Infect Dis. 2002;35:305–12. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- 24.Freudenberg N. Jail, prisons, and the health of urban populations: A review of the impact of the correctional system on community health. J Urban Health. 2001;78:214–35. doi: 10.1093/jurban/78.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrin I, Geskus R, Bhaskaran K, et al. Gender differences in HIV progression to AIDS and death in industrialized countries: Slower disease progression following HIV seroconversion in women. Am J Epidemiol. 2008;168:532. doi: 10.1093/aje/kwn179. [DOI] [PubMed] [Google Scholar]
- 26.Pezzotti P, Phillips AN, Dorrucci M, et al. Category of exposure to HIV and age in the progression to AIDS: Longitudinal study of 1199 people with known dates of seroconversion. BMJ. 1996;313:583–6. doi: 10.1136/bmj.313.7057.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin LJ, Houston S, Yasui Y, Wild TC, Saunders LD. All-cause and HIV-related mortality rates among HIV-infected patients after initiating highly active antiretroviral therapy: The impact of Aboriginal ethnicity and injection drug use. Can J Public Health. 2011;102:90–6. doi: 10.1007/BF03404154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palepu A, Tyndall MW, Joy R, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: The role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–94. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S, Moore RD, Graham NMH. Potential factors affecting adherence with HIV therapy. AIDS. 1997;11:1665. doi: 10.1097/00002030-199714000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 31.Katz MH, Cunningham WE, Fleishman JA, et al. Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected persons. Ann Intern Med. 2001;135(Part 1):557–65. doi: 10.7326/0003-4819-135-8_part_1-200110160-00006. [DOI] [PubMed] [Google Scholar]
- 32.Fleishman JA, Mor V, Piette J. AIDS case management: The client’s perspective. Health Serve Res. 1991;26:447. [PMC free article] [PubMed] [Google Scholar]
- 33.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46:194–9. doi: 10.1097/QAI.0b013e318142aabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miguez MJ, Shor-Posner G, Morales G, Rodriguez A, Burbano X. HIV treatment in drug abusers: Impact of alcohol use. Addict Biol. 2003;8:337. doi: 10.1080/1355621031000069855. [DOI] [PubMed] [Google Scholar]


