Abstract
As a major component of the epidermal tissue, a primary keratinocyte has served as an essential tool not only for the study of pathogenesis of skin-related diseases but also for the assessment of potential toxicities of various chemicals used in cosmetics. However, its short lifespan in ex vivo setting has been a great hurdle for many practical applications. Therefore, a number of immortalization attempts have been made with success to overcome this limitation. In order to understand the immortalization process of a primary keratinocyte, several key biological phenomena governing its lifespan will be reviewed first. Then, various immortalization methods for the establishment of stable keratinocyte cell lines will be explained. Finally, its application to a three-dimensional skin culture system will be described.
Keywords: Primary keratinocyte, Lifespan, Senescence, Immortalization, Skin research, Three-dimensional skin culture
INTRODUCTION
Skin plays an important role as a first-line of defense against chemicals as well as infectious attacks from outer environments. Skin mainly consists of dermal and epidermal tissues. Epidermis, the outermost layer of the skin, is composed of a basal layer, which is located at the bottom of epidermis and a suprabasal layer, which is located at the upper part of epidermis. Depending on differentiation and stratification status, a suprabasal layer is further divided into three different sub-layers. They include spinous, granular, and cornified layers (Fig. 1). While other cell types such as melanocytes and Langerhans cells are also present, keratinocytes are by far the major component of the epidermal tissue (more than 95%). Only those keratinocytes located at the basal layer of the epidermis are able to maintain a stem cell-like property, thus supporting a continuous cell division. Once they start a differentiation program, they gradually exit from a cell cycle and enter into a terminally-differentiated state (Fig. 1). Due to their important roles in skin biology, keratinocytes have served as an essential tool not only for the study of pathogenesis of skin-related diseases but also for the assessment of potential toxicities of various chemicals used in cosmetics. However, their short lifespan in ex vivo setting has been a daunting barrier for many skin-related applications. In order to overcome this limitation, many attempts to immortalize primary keratinocytes have been made with success. As a result, established stable keratinocyte cell lines have been widely applied to study various aspects of skin biology as well as to be used as toxicity assessment tools in cosmetic industry.
Fig. 1.
Schematic diagram of normal keratinocytes in different layers of the epidermal tissue. A direction and levels of epithelial differentiation are indicated with an arrow.
In this review paper, we would like to explain several key biological phenomena governing a lifespan of primary keratinocytes including replicative potential and cellular senescence to understand their immortalization process at molecular levels. Then, we would like to describe and compare various aspects of several immortalization methods, which have been used frequently to establish stable keratinocyte cell lines. Finally, we would like to conclude this review by discussing on how to apply either normal or immortalized keratinocytes to the establishment of a three-dimensional skin culture.
REPLICATIVE POTENTIAL AND CELLULAR SENESCENCE
Unlike germline and stem cells, somatic cells have a limited lifespan. They grow slowly and stop cell division when cultured in vitro for a certain period of time. Leonard Hayflick first described this finite replicative potential of normal cells in culture a half century ago (Hayflick, 1965). Therefore, this phenomenon has been often referred to as the “Hayflick limit” (Ohtani et al., 2009). Typical human primary keratinocytes possess an in vitro lifespan of around 15–20 population doublings (PDs) in serum-free and chemically defined media (Stoppler et al., 1997; Kiyono et al., 1998). When normal cells encounter the so-called “Hayflick limit”, they enter a viable state of permanent quiescence, which is often termed as cellular senescence (Hayflick and Moorhead, 1961). Continuous replication of typical primary human cells appears to be prevented by two discrete events: mortality stage 1 (M1) and mortality stage 2 (M2) (Fig. 2). M1 and M2 are often designated as “replicative senescence” and “cellular crisis” stages, respectively (Dimri et al., 1995; Coates, 2002; Cong et al., 2002). Cells entering replicative senescence first cease to respond to exogenous mitogenic stimuli and acquire increased cellular adhesion to the extracellular matrix while losing cell-cell contacts. They also shows the enlarged and flattened cell morphology, the increased lysosomal biogenesis (Shelton et al., 1999; Serrano and Blasco, 2001; Narita et al., 2003; Ben-Porath and Weinberg, 2004, 2005), the development of multiple nuclei (Stewart and Weinberg, 2002), and the formation of heterochromatic foci (Fridman and Tainsky, 2008). In addition to prolonged in vitro culture of primary cells, various types of cellular stresses including telomere erosion, DNA damage, overexpression of tumor suppressor genes or oncogenes, oxidative stress, continuous mitogenic stimuli, and a variety of chemicals can also induce senescence (Drayton and Peters, 2002; Ben-Porath and Weinberg, 2005). Despite different nature of each stress, replicative and stress-induced senescence seem to share at least some similar molecular pathways to execute their final outcome, which is a blockage of cell division.
Fig. 2.
Step-wise transformation of normal primary keratinocytes into immortalized ones via bypass of mortality stage 1 (M1, replicative senescence) and 2 (M2, cellular crisis). Typical cellular changes, which are associated with M1 and M2, are also described.
ROLE OF TELOMERES IN CELLULAR SENESCENCE
Telomeres are special structures at the ends of mammalian chromosomes that ‘cap’ the chromosomes and provide a protective function such as preventing end-to-end chromosomal fusions (Greider and Blackburn, 1996). Due to the inability of a typical DNA polymerase to perform complete replication of DNA ends, cell loses around 50–200 base pairs of telomeric DNA during each round of cell division. This unprotected chromosomal DNA end seems to release a signal to the cell to induce replicative senescence. Since critical telomere shortening is recognized as a double-stranded DNA break (d’Adda di Fagagna et al., 2003), this unfavorable genetic alternation eventually triggers a classical DNA damage response involving a number of protein which sense and repair genetic defects (Fridman and Tainsky, 2008). However, unrepairable severe terminal telomere shortening eventually leads to cellular crisis, a final anti-proliferative state characterized by massive cell death. Therefore, telomeres seems to play an essential role of ‘division-counting clocks’ in determining the lifespan of cells (Cong et al., 2002; Harley, 2002).
IMMORTALIZATION OF PRIMARY KERATINOCYTES
A small number of cells within the population may acquire the ability to escape from cellular crisis and form an immortalized cell line. Different kinds of primary cells are able to avoid senescence and become immortal through a variety of cellular events including telomere length stabilization, epigenetic gene silencing by selective promoter methylation, oxidative DNA damage, inactivation of cell cycle regulatory genes, overexpression of a cellular or viral oncogenes (Fig. 3) (Berube et al., 1998; Bringold and Serrano, 2000; Lundberg et al., 2000; Neumeister et al., 2002; Itahana et al., 2003). p16INK4A, pRb, p14ARF, p53, and p21CIP1 are representative cell cycle regulatory genes, which are frequently inactivated for immortalization. Overexpression of cellular oncogenes such as c-MYC and Bmi-1 is also shown to be associated with cellular immortalization. In addition, T antigen from simian virus 40 (SV40), E6 and E7 from human papillomavirus (HPV), and E1A and E1B from adenovirus are well-characterized viral oncogenes which induce immortalization of host cells.
Fig. 3.
p16INK4A/pRb and p14ARF/p53 pathways which governs replicative senescence and cellular crisis. ↓ and ⊥ indicate activation and inhibition of the downstream target, respectively. Cell cycle blockers (p16INK4A, p14ARF, and p21CIP1) are colored in purple. Tumor suppressors (pRb and p53) are colored in red. An activator (CDKs) or an inhibitor (MDM2) of tumor suppressors is colored in green. S phase-inducing transcription factors (E2Fs) are colored in black.
Cells that have a lifespan of 20–50 passages under in vitro culture conditions are mostly blast cells, such as fibroblasts and retinoblasts. Cells that have a lifespan of less than 10 passages under in vitro culture conditions are typically epithelial cells, such as breast and ovarian epithelial cells and keratinocytes. In many epithelial cells, epidermal growth factor (EGF) has been shown to be able to increase their lifespan to 10–20 passages before senescence. In general, drug selection is not necessary for primary cells that have less than 10 PDs as the immortalization process will select for the clones capable of growing indefinitely. In case of human keratinocytes, spontaneous immortalization rarely occurs, and thus, following immortalized keratinocyte cell lines including NM1 (Baden et al., 1987), HaCaT (Boukamp et al., 1988), and NIKS (Allen-Hoffmann et al., 2000) remain exceptions. Ideally immortalized keratinocytes are those that are not only capable of extended proliferation, but also possess identical or at least a similar genotype and phenotype to their parental epidermal tissue. Nevertheless, immortalized keratinocyte cell lines turn out to have several undesirable genetic abnormalities, such as mutations in p53 (Lehman et al., 1993) or aneuploidy isochromosomes (Allen-Hoffmann et al., 2000). In spite of these genetic defects, immortalized keratinocytes seem to maintain some aspects of normal keratinocytes, since they do not grow over one another in cell culture (contact inhibition), do not form colonies in soft agar (anchorage-dependent growth), do not form tumors when injected into immune-deficient rodents, and depend on exogenous growth factors for their growth (Cowling and Cole, 2007; De Filippis et al., 2008; Stepanenko and Kavsan, 2012). These normal cell-like properties of immortalized keratinocytes enable them to be used as a substitute for primary keratinocytes in various skin research fields.
CRITICAL PATHWAYS FOR PREVENTION OF IMMORTALIZATION
Cellular senescence pathways are believed to have multiple layers of regulation with additional redundancy to prevent spontaneous cellular immortalization (Smith and Pereira-Smith, 1996). Senescent cells express activated forms of DNA damage response proteins, which are the central activators of p53 in response to DNA damage (d’Adda di Fagagna et al., 2003; Herbig et al., 2004). At replicative senescence, signaling by shortened telomeres results in activation of the p53 and pRb pathways. Stress imposed by inadequate culture conditions also induces senescence due to accumulation of p16INK4A. Growth in different media, or on a layer of feeder cells, has been reported to delay p16INK4A induction and consequently prevent the onset of cellular crisis (Herbert et al., 2002).
Then, what kinds of proteins and which molecular pathways play key roles in induction of senescence and prevention of immortalization? According to results from several proteomics-based studies, genes in the cell cycle control, interferon induction, insulin growth factor pathway, MAP kinase pathway, and oxidative stress pathway were identified as critical regulators of senescence and immortalization (Fridman et al., 2006; Fridman and Tainsky, 2008). In addition to immortalization, perturbation of p53, pRb, protein phosphate 2A, telomerase, Raf, and Ral guanine nucleotide exchange factor (Ral-GEF) pathways are also found to be required for the full tumorigenic conversion of normal human cells (Rangarajan et al., 2004).
p16INK4A/pRb AND p14ARF/p53 PATHWAYS
Immortalization induced by a number of oncogenic viruses is intimately associated with inactivation of cell cycle checkpoint proteins such as p16INK4A, p14ARF, and p21CIP1. SV40 T antigen, HPV E6 or E7, and adenovirus E1A or E1B are well-characterized viral oncogenic proteins, whose transforming activities depend on disruption of pRb and p53 tumor suppressor proteins. This virus-induced inactivation of tumor suppressor proteins leads to restoration of telomerase activity, resulting in telomere ends stabilization. There is also a link between pRb inactivation, cell aneuploidy, and chromosome instability. The signaling pathways activated by various cellular stresses are also funneled to the pRb and p53 proteins. Therefore, pRb and p53 can be regarded as two central players governing replicative senescence (Fig. 3). pRb is found at senescence in its active, hypophosphorylated form, in which it binds to the E2F protein family members to repress their transcriptional activation of several target genes involved in G1/S phase transition (Narita et al., 2003). Growth-suppressive activity of pRb is apparently maintained independently of p53 (Beausejour et al., 2003). Therefore, p53 can induce senescence of human cells through a pathway independent of the pRb family (Smogorzewska and de Lange, 2002).
p16INK4A is an inhibitor of cyclin D/cyclin-dependent kinase (CDK) 4, 6 complexes. p16INK4A is solely responsible for the induction of an early, stress-induced, senescence stage in keratinocytes (Foster et al., 1998; Kiyono et al., 1998; Rheinwald et al., 2002). Of note, p16INK4A is one of the most frequently inactivated genes in human tumors (Rocco and Sidransky, 2001). While p53 and p21CIP1 act to initiate the senescence response, p16INK4A seems to act to maintain this state. The p16INK4A response is found to be more enhanced in human cells than in mouse cells, and provides an additional safety layer to prevent tumor development. Similarly, a constitutively active p16-insensitive CDK4 mutant was shown to overcome replicative senescence (Ramirez et al., 2003).
The p14ARF activates p53 by sequestering MDM2 (Mouse double minute 2 homolog), an E3 ubiquitin ligase, in the nucleolus, thereby preventing MDM2-mediated targeting of p53 for proteolytic degradation (Fig. 3). Mouse embryo fibroblasts may preferentially rely on the p14ARF/p53 pathway (Carnero et al., 2000), whereas human keratinocytes employ the p16INK4A/pRb pathway to enforce the senescence program (Kiyono et al., 1998; Munro et al., 1999). Therefore, regardless of species and tissue tropisms, the integrity of both the p16INK4A/pRb and p14ARF/p53 pathways appears to be essential for oncogene-induced senescence (Serrano et al., 1997; Palmero et al., 1998).
HOW TO IMMORTALIZE KERATINOCYTES
Primary cells can be immortalized artificially by different kinds of methods including overexpression of telomerase, inactivation of cell cycle regulatory genes, overexpression of viral oncogenes, and inhibition of a specific host kinase. We would like to explain and compare various kinds of immortalization methods with a special focus on keratinocyte immortalization.
Overexpression of telomerase
Human telomerase is comprised of two core components: a human telomerase RNA component (hTERC) and a protein catalytic subunit (human telomerase reverse transcriptase, hTERT). Human foreskin keratinocytes (HFKs) grown on fibroblast feeders maintain elevated telomerase activity and lower levels of p16INK4A for 60 PDs before senescing at 81 PDs. In most primary human cells, hTERT and telomerase activity are either absent or present at levels that are insufficient for telomere maintenance (Kim et al., 1994; Shay and Bacchetti, 1997; Masutomi et al., 2003). In addition, its expression is restricted to the early stages of embryonic development, and in the adult, to rare cells of the blood, skin and digestive tract. Therefore, overexpression of hTERT has been envisioned as an attractive approach to extend lifespan of normal cells.
Telomerase may assist in immortalization bypassing two separate events including replicative senescence and cellular crisis (Cukusic et al., 2008). Ectopic expression of hTERT was shown to restore telomere length in fibroblasts and several other cell types and allows early passage cultures of cells to circumvent senescence and become immortalized (Bodnar et al., 1998; Vaziri and Benchimol, 1998). In some cases, like foreskin fibroblasts and retinal pigment epithelial cells, telomerase activity was sufficient for complete immortalization (Bodnar et al., 1998). However, most of normal human fibroblast lines cannot be immortalized by ectopic expression of hTERT alone (Kiyono et al., 1998; Jarrard et al., 1999). Introduction of hTERT sometimes induce apoptosis in primary epithelial cells and other cells that have a lifespan of less than 10 passages. In many cases, other uncharacterized factors need to be supplemented together for full immortalization (Kiyono et al., 1998). This was also true for primary keratinocytes since telomerase expression alone was not sufficient for their efficient immortalization. The ectopic expression of hTERT in keratinocytes, mammary epithelial cells, and other cell types restores telomerase enzymatic function and telomere lengths but still does not allow these cells to bypass senescence (Kiyono et al., 1998; Dickson et al., 2000). This indicates the existence of a cell type-specific regulation of the cellular senescence program for each type of cells (Kiyono et al., 1998; Dickson et al., 2000). Therefore, immortalizing capabilities of telomerase seem to be only limited to a subset of cell types.
Nevertheless, an hTERT-induced immortalization process possesses some advantages. (Herbert et al. 2002). The enzyme telomerase can immortalize cells without causing cancer-associated changes or altering phenotypic properties of normal primary cells (Jiang et al., 1999; Morales et al., 1999; Ouellette et al., 2000). These unfavorable cancer-like changes may include a loss of contact inhibition, reduced growth factor requirements, inhibition of differentiation, genomic instability, aneuploidy, as well as disruptions of cell cycle checkpoints. Primary human cells immortalized with hTERT alone tend to have a relatively ‘normal’ phenotype. Primary human cells, which were immortalized by hTERT, remain diploid, differentiated, contact-inhibited, non-tumorigenic and anchorage-dependent. In addition, they are genomically stable, possess functional cell cycle checkpoints, and express functional p53, pRb, and p16INK4A (Jiang et al., 1999; Morales et al., 1999; Yang et al., 1999; Ouellette et al., 2000; Yudoh et al., 2001; Herbert et al., 2002; Ramirez et al., 2003; Lee et al., 2004). Thanks to these desirable properties of hTERT-induc1989ed immortalization, this method has been combined with a number of other immortalization approaches described below.
Inactivation of p16INK4A and p14ARF
Since replicative senescence occurs after approximately 20 PDs via activation of the p16INK4A/pRb pathway, p16INK4A function must be disrupted in order to achieve immortalization (Kiyono et al., 1998; Dickson et al., 2000). Importance of inactivation of p16INK4A/pRb pathway for immortalization was further evidenced by prevention of replicative senescence by using HPV E7, which targets pRb for degradation (Foster and Galloway, 1996). Spontaneous reduction in p16INK4A expression due to promoter methylation has been also documented to expedite cellular immortalization. (Foster et al., 1998; Kiyono et al., 1998; Wong et al., 1999). In addition, inactivation of p14ARF also seems to assist immortalization of primary human keratinocytes by maintaining cells in the stem cell compartment (Maurelli et al., 2006) since p14ARF binds directly to and sequesters MDM2, resulting in inhibition of the capacity of MDM2 to induce degradation of the p53 tumor suppressor protein (Fig. 3).
Retroviral transduction of HPV E6 and E7
A number of high risk type HPVs play an etiological role in development of most of cervical cancers. Among HPV proteins, E6 and E7 are regarded as central players for the oncogenic conversion of host keratinocytes through their specific inactivation of p53 and pRb tumor suppressor genes via their accelerated ubiquitin-mediated degradation. HPV E7 is able to immortalize human keratinocytes at a very low efficiency when cultured in serum-free synthetic medium (Klingelhutz and Roman, 2012). Abrogation of pRb function by HPV E7 blocks p16INK4A-mediated senescence (Fig. 3). Human corneal cells were also immortalized by using the HPV16 E6/E7 oncogenes for ophthalmologic issues (Schulz et al., 2013). Use of HPV E6 and E7 turned out to have advantage over other immortalization methods since tissue-innate features have been shown to be better preserved in the descending cell lines, which were immortalized by HPV E6 and E7 than those by other methods (Durst et al., 1987; Hawley-Nelson et al., 1989; Flores et al., 1999). In addition, human mammary epithelial cells (HMECs), which were immortalized by HPV E6 and E7, are shown to stay at pre-neoplastic stage. Therefore, they do not grow in an anchorage-independent manner or produce tumors when implanted in immune-deficient mice (Wazer et al., 1995; Ratsch et al., 2001).
In regards to a potential linkage to immortalization by hTERT, HPV E6 protein, via direct binding, was shown to increase c-MYC efficiency in activating the hTERT promoter. Therefore, replicative senescence can be bypassed by HPV E6 or induction of telomerase activity (Shay et al., 1993; Kiyono et al., 1998). HPV E6 protein was also able to induce telomerase activity through its transcriptional activation of the hTERT promoter via p53-independent regulatory mechanisms (Klingelhutz et al., 1996; Veldman et al., 2001). In this regard, wild-type hTERT protein can functionally replace the HPV E6 protein, which cooperates with the HPV E7 protein in the immortalization of primary keratinocytes (Miller et al., 2013). Interestingly, HPV E7 protein and hTERT proteins defective for telomere maintenance were also able to cooperate to immortalize human keratinocytes (Miller et al., 2013). This suggests that the uncharacterized function of hTERT, which is not required for telomerase maintenance, plays a critical role in hTERT-induced immortalization.
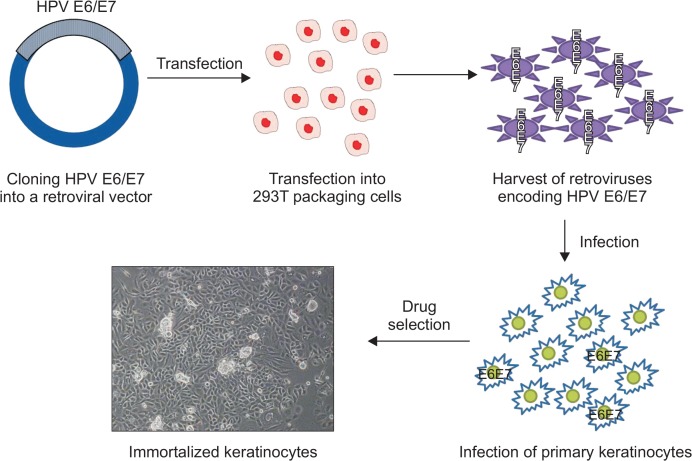
For an efficient immortalization of primary keratinocytes, overexpression of HPV E6/E7 by retroviral transduction has been a method of choice so far thanks to its capacity to preserve normal cell-like properties in immortalized cells. A schematic diagram on how to use a retroviral transduction to induce immortalization of target cells was shown in Fig. 4. First, cDNA encoding HPV E6/E7 is cloned into a retroviral vector. Then, this cloned retroviral vector is transfected into 293T packaging cells together with other retroviral envelope or packaging vectors, followed by harvesting of retroviruses, which are encoding HPV E6/E7, in the supernatant. Then, primary keratinocytes, which are typically isolated from human foreskin tissues, are infected with these harvested retroviruses. Through a drug selection, immortalized keratinocytes, which are stably expressing HPV E6/E7, are isolated and propagated.
Fig. 4.
Schematic diagram on how to immortalize primary keratinocytes by a retroviral transduction of HPV E6 and E7 oncogenes.
Various immortalization methods in combination with telomerase overexpression
Efficient immortalization of primary human cells was achieved by p16INK4A-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT (Haga et al., 2007). This suggests that both inactivation of p16INK4A and expression of telomerase were required to immortalize keratinocytes (Kiyono et al., 1998; Dickson et al., 2000; Lundberg et al., 2000). As explained previously, catalytically inactive hTERT was also able to cooperate with HPV E7 in mediating bypass of the senescence blockade and induction of cellular immortalization. This indicates that a telomerase-independent activity of hTERT can collaborate with E7 in the immortalization of primary human cells. Additional inactivation of the p16INK4A/pRb pathway by E7 or down-regulation of p16INK4A expression by promoter methylation was shown to be required for efficient immortalization (Foster and Galloway, 1996; Foster et al., 1998; Kiyono et al., 1998; Itahana et al., 2003). These data further emphasize fundamental roles of the intact p16INK4A/pRb and p14ARF/p53 pathways in preventing immortalization of cells by enforcing replicative senescence as well as cellular crisis.
Use of Rho kinase inhibitor
In order to achieve efficient immortalization of primary cells, a pharmacological approach using small molecules has been an attractive alternative with much convenience compared with a genetic manipulation. In this regard, identification of a certain Rho kinase (ROCK) inhibitor as a potent immortalization inducer was a highly anticipated and appreciated discovery. Treatment with an ROCK inhibitor, Y-27632 was shown to greatly increase long-term proliferation of primary human keratinocytes and, unexpectedly, enabled them to efficiently bypass senescence and become immortal without detectable cell crisis. Co-culture with fibroblasts was necessary to immortalization by Y-27632. Keratinocytes that had been previously cultured with the ROCK inhibitor retained their capacity to differentiate normally into a stratified epithelial tissue. Keratinocytes, which were immortalized by an ROCK inhibitor, displayed characteristics typical of primary keratinocytes. They had a normal karyotype and an intact DNA damage response. They were able to differentiate into a stratified epithelium. In this case, culture with feeder fibroblasts and exposure to a Rho kinase inhibitor were required for efficient immortalization of keratinocytes (Chapman et al., 2010). This suggests that the ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells toward immortalization (Liu et al., 2012).
IMMORTALIZATION OF KERATIONCYTES BY RETROVIRAL TRANSDUCTION APPLICATION TO A THREE-DIMENSIONAL SKIN CULTURE SYSTEM
In order to grow differentiating epithelial tissues that mimic many important morphological and biochemical aspects of a normal skin, a three-dimensional skin culture system was introduced (Ozbun and Patterson, 2014). This technique is often called an organotypic raft culture due to its apparent floating nature with growing keratinocytes on top of a dermal equivalent, which consist of fibroblasts and a collagen lattice (Fig. 5A). Epithelial stratification and differentiation is induced when this keratinocyte-dermal matrix is placed at the air-liquid interface. Organotypic raft culture promotes stratification and full differentiation of keratinocytes, which are evidenced by gradual loss of nuclei upon differentiation and expression of various keratinocyte differentiation markers in each distinct epidermal layer (Fig. 5B). Several types of modified three-dimensional skin culture systems were developed by different companies and their commercialized products have been used to test irritancy and corrosiveness of cosmetic materials. However, due to the limited supply of primary keratinocytes necessary for generation of organotypic raft cultures, prices of these commercialized products are relatively high. Therefore, immortalized keratinocytes will be valuable tools as a substitute for normal primary keratinocytes in mass production of three-dimensional skin cultures with a consistent quality.
Fig. 5.
(A) Schematic diagram of an organotypic raft culture system. (B) A cross-sectional view of the organotypic raft culture grown with primary keratinocytes after a hematoxylin and eosin staining. A direction and levels of epithelial differentiation are indicated with an arrow in both (A) and (B).
CONCLUSION
In order to understand the immortalization process of primary keratinocytes, we first reviewed several key biological phenomena governing their lifespan. Then, we explained various immortalization methods for the establishment of stable keratinocyte cell lines. Finally, we described application of immortalized keratinocytes to a three-dimensional skin culture system. Better understanding of cellular senescence at molecular level will enable us to immortalize various kinds of primary cells with ease. This unlimited supply of immortalized cells with normal cell-like properties will be essential not only for regenerative medicine but also for the economic development of a three-dimensional skin culture system.
Acknowledgments
This work was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HI13C1046) and the grant (13172MFDS987) from the Ministry of Food and Drug Safety. This work was also supported by the GRRC program of Gyeonggi province [(GRRC-DONGGUK2014-A01), Development of therapeutics for hepatitis C].
REFERENCES
- Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O’Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114:444–455. doi: 10.1046/j.1523-1747.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- Baden HP, Kubilus J, Kvedar JC, Steinberg ML, Wolman SR. Isolation and characterization of a spontaneously arising long-lived line of human keratinocytes (NM 1) In Vitro Cell Dev Biol. 1987;23:205–213. doi: 10.1007/BF02623581. [DOI] [PubMed] [Google Scholar]
- Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. When cells get stressed: an integrative view of cellular senescence. J Clin Invest. 2004;113:8–13. doi: 10.1172/JCI200420663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Berube NG, Smith JR, Pereira-Smith OM. The genetics of cellular senescence. Am J Hum Genet. 1998;62:1015–1019. doi: 10.1086/301848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringold F, Serrano M. Tumor suppressors and oncogenes in cellular senescence. Exp Gerontol. 2000;35:317–329. doi: 10.1016/S0531-5565(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Carnero A, Hudson JD, Price CM, Beach DH. p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat Cell Biol. 2000;2:148–155. doi: 10.1038/35004020. [DOI] [PubMed] [Google Scholar]
- Chapman S, Liu X, Meyers C, Schlegel R, McBride AA. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates PJ. Markers of senescence? J Pathol. 2002;196:371–373. doi: 10.1002/path.1073. [DOI] [PubMed] [Google Scholar]
- Cong YS, Wright WE, Shay JW. Human telomerase and its regulation. Microbiol Mol Biol Rev. 2002;66:407–425. doi: 10.1128/MMBR.66.3.407-425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling VH, Cole MD. E-cadherin repression contributes to c-Myc-induced epithelial cell transformation. Oncogene. 2007;26:3582–3586. doi: 10.1038/sj.onc.1210132. [DOI] [PubMed] [Google Scholar]
- Cukusic A, Skrobot Vidacek N, Sopta M, Rubelj I. Telomerase regulation at the crossroads of cell fate. Cytogenet Genome Res. 2008;122:263–272. doi: 10.1159/000167812. [DOI] [PubMed] [Google Scholar]
- d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- De Filippis L, Ferrari D, Rota Nodari L, Amati B, Snyder E, Vescovi AL. Immortalization of human neural stem cells with the c-myc mutant T58A. PLoS One. 2008;3:e3310. doi: 10.1371/journal.pone.0003310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton S, Peters G. Immortalisation and transformation revisited. Curr Opin Genet Dev. 2002;12:98–104. doi: 10.1016/S0959-437X(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Durst M, Dzarlieva-Petrusevska RT, Boukamp P, Fusenig NE, Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1:251–256. [PubMed] [Google Scholar]
- Flores ER, Allen-Hoffmann BL, Lee D, Sattler CA, Lambert PF. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology. 1999;262:344–354. doi: 10.1006/viro.1999.9868. [DOI] [PubMed] [Google Scholar]
- Foster SA, Galloway DA. Human papillomavirus type 16 E7 alleviates a proliferation block in early passage human mammary epithelial cells. Oncogene. 1996;12:1773–1779. [PubMed] [Google Scholar]
- Foster SA, Wong DJ, Barrett MT, Galloway DA. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol. 1998;18:1793–1801. doi: 10.1128/mcb.18.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–5987. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman AL, Tang L, Kulaeva OI, Ye B, Li Q, Nahhas F, Roberts PC, Land SJ, Abrams J, Tainsky MA. Expression profiling identifies three pathways altered in cellular immortalization: interferon, cell cycle, and cytoskeleton. J Gerontol A Biol Sci Med Sci. 2006;61:879–889. doi: 10.1093/gerona/61.9.879. [DOI] [PubMed] [Google Scholar]
- Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996;274:92–97. doi: 10.1038/scientificamerican0296-92. [DOI] [PubMed] [Google Scholar]
- Haga K, Ohno S, Yugawa T, Narisawa-Saito M, Fujita M, Sakamoto M, Galloway DA, Kiyono T. Efficient immortalization of primary human cells by p16INK4a-specific short hairpin RNA or Bmi-1, combined with introduction of hTERT. Cancer Sci. 2007;98:147–154. doi: 10.1111/j.1349-7006.2006.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB. Telomerase is not an oncogene. Oncogene. 2002;21:494–502. doi: 10.1038/sj.onc.1205076. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Herbert BS, Wright WE, Shay JW. p16(INK4a) inactivation is not required to immortalize human mammary epithelial cells. Oncogene. 2002;21:7897–7900. doi: 10.1038/sj.onc.1205902. [DOI] [PubMed] [Google Scholar]
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Mol Cell. 2004;14:501–513. doi: 10.1016/S1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, Van Lohuizen M, Band V, Campisi J, Dimri GP. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23:389–401. doi: 10.1128/MCB.23.1.389-401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard DF, Sarkar S, Shi Y, Yeager TR, Magrane G, Kinoshita H, Nassif N, Meisner L, Newton MA, Waldman FM, Reznikoff CA. p16/pRb pathway alterations are required for bypassing senescence in human prostate epithelial cells. Cancer Res. 1999;59:2957–2964. [PubMed] [Google Scholar]
- Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380:79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology. 2012;424:77–98. doi: 10.1016/j.virol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology. 2004;45:33–38. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman TA, Modali R, Boukamp P, Stanek J, Bennett WP, Welsh JA, Metcalf RA, Stampfer MR, Fusenig N, Rogan EM, et al. p53 mutations in human immortalized epithelial cell lines. Carcinogenesis. 1993;14:833–839. doi: 10.1093/carcin/14.5.833. [DOI] [PubMed] [Google Scholar]
- Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/S0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, Weinberg RA, Stewart SA, Hahn WC. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/S0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- Maurelli R, Zambruno G, Guerra L, Abbruzzese C, Dimri G, Gellini M, Bondanza S, Dellambra E. Inactivation of p16INK4a (inhibitor of cyclin-dependent kinase 4A) immortalizes primary human keratinocytes by maintaining cells in the stem cell compartment. FASEB J. 2006;20:1516–1518. doi: 10.1096/fj.05-4480fje. [DOI] [PubMed] [Google Scholar]
- Miller J, Dakic A, Chen R, Palechor-Ceron N, Dai Y, Kallakury B, Schlegel R, Liu X. HPV16 E7 protein and hTERT proteins defective for telomere maintenance cooperate to immortalize human keratinocytes. PLoS Pathog. 2013;9:e1003284. doi: 10.1371/journal.ppat.1003284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115–118. doi: 10.1038/5063. [DOI] [PubMed] [Google Scholar]
- Munro J, Stott FJ, Vousden KH, Peters G, Parkinson EK. Role of the alternative INK4A proteins in human keratinocyte senescence: evidence for the specific inactivation of p16INK4A upon immortalization. Cancer Res. 1999;59:2516–2521. [PubMed] [Google Scholar]
- Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003;113:703–716. doi: 10.1016/S0092-8674(03)00401-X. [DOI] [PubMed] [Google Scholar]
- Neumeister P, Albanese C, Balent B, Greally J, Pestell RG. Senescence and epigenetic dysregulation in cancer. Int J Biochem Cell Biol. 2002;34:1475–1490. doi: 10.1016/S1357-2725(02)00079-1. [DOI] [PubMed] [Google Scholar]
- Ohtani N, Mann DJ, Hara E. Cellular senescence: its role in tumor suppression and aging. Cancer Sci. 2009;100:792–797. doi: 10.1111/j.1349-7006.2009.01123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette MM, McDaniel LD, Wright WE, Shay JW, Schultz RA. The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum Mol Genet. 2000;9:403–411. doi: 10.1093/hmg/9.3.403. [DOI] [PubMed] [Google Scholar]
- Ozbun MA, Patterson NA. Using organotypic (raft) epithelial tissue cultures for the biosynthesis and isolation of infectious human papillomaviruses. Curr Protoc Microbiol. 2014;34:14B.3.1–14B.3.18. doi: 10.1002/9780471729259.mc14b03s34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- Ramirez RD, Herbert BS, Vaughan MB, Zou Y, Gandia K, Morales CP, Wright WE, Shay JW. Bypass of telomere-dependent replicative senescence (M1) upon overexpression of Cdk4 in normal human epithelial cells. Oncogene. 2003;22:433–444. doi: 10.1038/sj.onc.1206046. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ratsch SB, Gao Q, Srinivasan S, Wazer DE, Band V. Multiple genetic changes are required for efficient immortalization of different subtypes of normal human mammary epithelial cells. Radiat Res. 2001;155:143–150. doi: 10.1667/0033-7587(2001)155[0143:MGCARF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 2001;264:42–55. doi: 10.1006/excr.2000.5149. [DOI] [PubMed] [Google Scholar]
- Schulz S, Steinberg T, Beck D, Tomakidi P, Accardi R, Tommasino M, Reinhard T, Eberwein P. Generation and evaluation of a human corneal model cell system for ophthalmologic issues using the HPV16 E6/E7 oncogenes as uniform immortalization platform. Differentiation. 2013;85:161–172. doi: 10.1016/j.diff.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol. 2001;13:748–753. doi: 10.1016/S0955-0674(00)00278-7. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/S0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur. J. Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Shay JW, Wright WE, Brasiskyte D, Van der Haegen BA. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999;9:939–945. doi: 10.1016/S0960-9822(99)80420-5. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. EMBO J. 2002;21:4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanenko AA, Kavsan VM. Immortalization and malignant transformation of eukaryotic Cells. Tsitol Genet. 2012;46:36–75. [PubMed] [Google Scholar]
- Stewart SA, Weinberg RA. Senescence: does it all happen at the ends? Oncogene. 2002;21:627–630. doi: 10.1038/sj.onc.1205062. [DOI] [PubMed] [Google Scholar]
- Stoppler H, Hartmann DP, Sherman L, Schlegel R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J Biol Chem. 1997;272:13332–13337. doi: 10.1074/jbc.272.20.13332. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 1998;8:279–282. doi: 10.1016/S0960-9822(98)70109-5. [DOI] [PubMed] [Google Scholar]
- Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75:4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazer DE, Liu XL, Chu Q, Gao Q, Band V. Immortalization of distinct human mammary epithelial cell types by human papilloma virus 16 E6 or E7. Proc Natl Acad Sci USA. 1995;92:3687–3691. doi: 10.1073/pnas.92.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DJ, Foster SA, Galloway DA, Reid BJ. Progressive region-specific de novo methylation of the p16 CpG island in primary human mammary epithelial cell strains during escape from M(0) growth arrest. Mol Cell Biol. 1999;19:5642–5651. doi: 10.1128/mcb.19.8.5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- Yudoh K, Matsuno H, Nakazawa F, Katayama R, Kimura T. Reconstituting telomerase activity using the telomerase catalytic subunit prevents the telomere shorting and replicative senescence in human osteoblasts. J Bone Miner Res. 2001;16:1453–1464. doi: 10.1359/jbmr.2001.16.8.1453. [DOI] [PubMed] [Google Scholar]