Abstract
Acetylshikonin, a natural naphthoquinone derivative compound, has been used for treatment of inflammation and cancer. In the present study, we have investigated whether acetylshikonin could regulate the NF-κB signaling pathway, thereby leading to suppression of tumorigenesis. We observed that acetylshikonin significantly reduced proliferation of several cancer cell lines, including human pancreatic PANC-1 cancer cells. In addition, acetylshikonin inhibited phorbol 12-myristate 13-acetate (PMA) or tumor necrosis-α (TNF-α)-induced NF-κB reporter activity. Proteome cytokine array and real-time RT-PCR results illustrated that acetylshikonin inhibition of PMA-induced production of cytokines was mediated at the transcriptional level and it was associated with suppression of NF-κB activity and matrix metalloprotenases. Finally, we observed that an exposure of acetylshikonin significantly inhibited the anchorage-independent growth of PANC-1 cells. Together, our results indicate that acetylshikonin could serve as a promising therapeutic agent for future treatment of pancreatic cancer.
Keywords: Acetylshikonin, Phorbol 12-myristate 13-acetate, Tumor necrosis-α, NF-κB, Matrix metalloproteinase, Pancreatic cancer
INTRODUCTION
More than 250,000 individuals are annually diagnosed with pancreatic cancer in the world (Raimondi et al., 2009). In addition, the number of patients newly diagnosed with pancreatic cancer is continuously increasing. It is known that patients with pancreatic cancer poorly respond to most conventional chemotherapeutic agents with only 4% survival rate (Raimondi et al., 2009; Bosetti et al., 2012). Although a number of small molecule kinase inhibitors have been developed to target pancreatic cancer, their clinical efficacy is still limited largely due to significant side effects (El Fitori et al., 2007; Moser et al., 2008; Butler et al., 2015).
Acetylshikonin is a natural naphthoquinone derivative compound, abundantly found in the root of traditional Chinese me dical herb Lithospermum erythrorhizon. Previous studies have demonstrated that acetylshikonin exhibits significant cytotoxic effects on various types of cancer cells by affecting diverse cellular processes, such as proliferation, metastasis and the efflux of chemotherapeutic agents (Calonghi et al., 2007; Wiench et al., 2012). In accordance with these facts, we have obtained preliminary evidence that acetylshikonin suppression of proliferation and metastasis is associated with NF-κB in human pancreatic PANC1 cancer cells. NF-κB is a transcription factor that can be activated by many divergent stimuli, including cytokines, lipopolysaccharide and cellular stress such as UV, chemotherapy and radiation (Pahl, 1999; Lee et al., 2007) (Hsu et al., 2013). Because conventional target genes of NF-κB are closely associated with survival and suppression of apoptosis (Duan et al., 2007; Liu et al., 2013; Tafani et al., 2013; Chien et al., 2014), we presumed that suppression of NF-κB could account, at least in part, for the anti-carcinogenic effects of acetylshikonin. Here, we disclose our efforts that led us to conclude that acetylshikonin inhibits NF-κB to suppress proliferation and metastasis of human pancreatic PANC1 cancer cells.
MATERIALS AND METHODS
Chemicals and antibodies
Acetyshikonin (purity >98%) was purchased from Biopurify phytochemicals Ltd (Chengdu, China). CellTiter-Glo® Lumine scent Cell Viability Assay and Proteome Profiler Human Cytokine Array were purchased from Promega Corporation (Madison, WI, USA) and R&D systems (Minneapolis, MN, USA), respectively. DMEM and fetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA, USA). Phorbol 12-myristate 13-acetate (PMA) and β-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Polyclonal antibodies against total NF-κB (p65), phospho-specific p65 (Ser536), total IκBα, phospho-specific IκBα (Ser32), MMP-2 and MMP-9 were obtained from Cell Signaling Technology (Beverly, MA, USA). All other chemicals used in our experiments are molecular biology grade.
Cell culture
Human pancreatic PANC-1 cancer cells, together with other cell lines used in the present study were purchased from American Type Culture Collection (Manassas, VA, USA). These cells were cytogenetically examined and authenticated before storage in the nitrogen tank. PANC-1 cells were maintained in DMEM media, containing penicillin and streptomycin (100 units/ml) and 10% FBS at 37°C in 5% CO2 incubator.
CellTiter-Glo® luminescent cell viability assay
PANC-1 cells were plated in 96-well plates (1×104 cells/well). After 24 h, cells were treated with appropriate concentrations of acetylshikonin (DMSO as a vehicle) for indicated times and the cell viability was determined using the CellTiter-Glo® luminescent cell viability kit according to the manufacturer’s instructions. It should be stated that this method is based on the measurement of ATP by the luciferase reaction in cells, whose production is proportional to the number of viable cells. The rate of cell proliferation inhibition was calculated by the following formula: cell proliferation inhibition rate = (relative luminescence of the experimental group/relative luminescence of the control group)×100. The resulting luminescence, which indirectly reflects the cell viability was measured by LuBi luminometer (Berthold TEC GmbH & Co., Oak Ridge, TN, USA). All experiments were repeated at least three times and the average value together with standard deviations were depicted.
Measurement of luciferase activity
HEK293 cells permanently transfected with NF-κB-luciferase construct were seeded at 2×104 cells in 96-well plate and maintained in DMEM media containing 5% FBS for 24 h. HEK293 cells were then exposed to PMA alone or in combination with acetylshikonin for 24 h. After cell lysis, the resulting luciferase activity was measured by luminometer. All experiments were repeated at least three times and the average values together with standard deviations were depicted.
RNA isolation and real-time quantitative PCR
PANC-1 cells (5×105 cells/well) were seeded into 6-well plates and exposed to PMA (20 ng/ml) alone or in combination with acetylshikonin (1, 10 and 30 μM) for 24 h. Total RNA was then isolated, using Aurum total RNA mini kit (Bio-Rad, Hercules, CA, USA). First-strand cDNA was synthesized using 1 μg of total RNA with Moloney murine leukemia virus reverse transcriptase (MMLV-RTase) (Bio-Rad, Hercules, CA, USA). The synthesized cDNA was amplified with IL-2, IL-4, IL-8, IL-12, IL-13, TNF-α and GAPDH PCR primers, using GeneAmp PCR 9700 thermocycler (Thermo Fisher Scientific, Waltham, MA, USA). PCR products were analyzed by 1% agarose gel using 1X TAE buffer. Relative mRNA levels were quantified using myECL imager analysis software (Thermo Fisher Scientific, Waltham, MA, USA). The quantitative gene expression levels of cytokines were measured, using SYBR Green reagents (Bio-Rad, Hercules, CA, USA) on CFX96 Touch PCR system (Bio-Rad, Hercules, CA, USA). Relative target gene expression was determined after normalization with the levels of GAPDH. Results were calculated from two independent experiments with triplicates in an individual experiment. The primer pairs for RT-PCR were as follows: GAPDH forward 5′-TGGCAAATTCCATGGCAC-3′, reverse 5′-CCATGGTGGTGAAGACGC-3′; IL-2 forward 5′-CCAAAGAGTCATCAGAAGAGG-3′, reverse 5′-GCACTTCCTCCAGAGGTTTGAG-3′; IL-4 forward 5′-ACTTTGAACAGCCTCACAGAG-3′, reverse 5′-TTGGAGGCAGCAAAGATGTC-3′; IL-8 forward 5′-AGATATTGCACGGGAGAA-3′, reverse 5′-AACTAGGATTGTTAGTTC-3′; IL-12 forward 5′-AGAGCTGAGCCCAGTCATCAC-3′, reverse 5′-TCCAGACGCGCCACTG A-3′; IL-13 forward 5′-TGAGGAGCTGGTCAACATCA-3′, reverse 5′-AGGTTGATGCTCCATACCAT-3′; TNF-α forward 5′-CAAGCCTGTAGCCCATGTTGTAGC-3′, reverse 5′-ATCCCAAAGTAGACCTGCCCAGAC-3′.
Proteome profiler human cytokine array
PANC-1 cells (5×105 cells/well) were seeded into 6-well plates. After appropriate treatments, cell media (300 μl) were thoroughly suspended in a 1.25 ml of Array Buffer 5, incubated at room temperature for 15 min, and centrifuged for 5 min at 5,000 g. The resulting supernatant (1 ml each) was added to a cocktail of biotinylated antibodies and incubated at room temperature for 1 h. The sample-antibody mixture was incubated at 4°C overnight. Following a washing step, 3 ml of a 1:1000 dilution of secondary antibody conjugated with streptavidin-HRP was added to each membrane and incubated at room temperature for 1 h. For detection, the membranes were probed with chemiluminescence (ECL) detection kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Western blot analysis
After appropriate treatments, cells were disrupted on ice for 30 min in cell lysis buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM b-glycerophosphate, 1 mM sodium vanadate, 1 mg/ml leupeptin and 1 mM phenylmethylsulfonyl fluoride (PMSF). After centrifugation at 20,817 g for 15 min, the supernatant was harvested and the protein concentration was determined. The total cellular protein extracts were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes in 20 mM Tris-HCl (pH 8.0), containing 150 mM glycine and 20% (v/v) methanol. Membranes were blocked with 5% nonfat dry milk in 1x TBS containing 0.05% Tween 20 (TBS-T buffer) and incubated with appropriate antibodies. Blots were washed three times in 1x TBS-T buffer, followed by the incubation with the appropriate HRP-linked IgG. Hybridized proteins were visualized using the enhanced chemiluminescence (ECL) detection system.
Anchorage-independent growth assay (soft agar assay)
The assay was done in 6-well plates with a bottom layer containing 0.5% agar in DMEM media containing 10% FBS. This layer was overlaid with a low-melting-point layer of 1 ml of 0.3% agar (containing 10% fetal bovine serum), mixed with 1 ml of a suspension of 800 cells and appropriate concentrations of acetylshikonin. After 2 week, plates were stained with 0.2% crystal violet and the colonies with diameter greater than 0.2 mm were counted under the light microscope.
RESULTS
Acetylshikonin inhibits the growth of PANC-1 cells
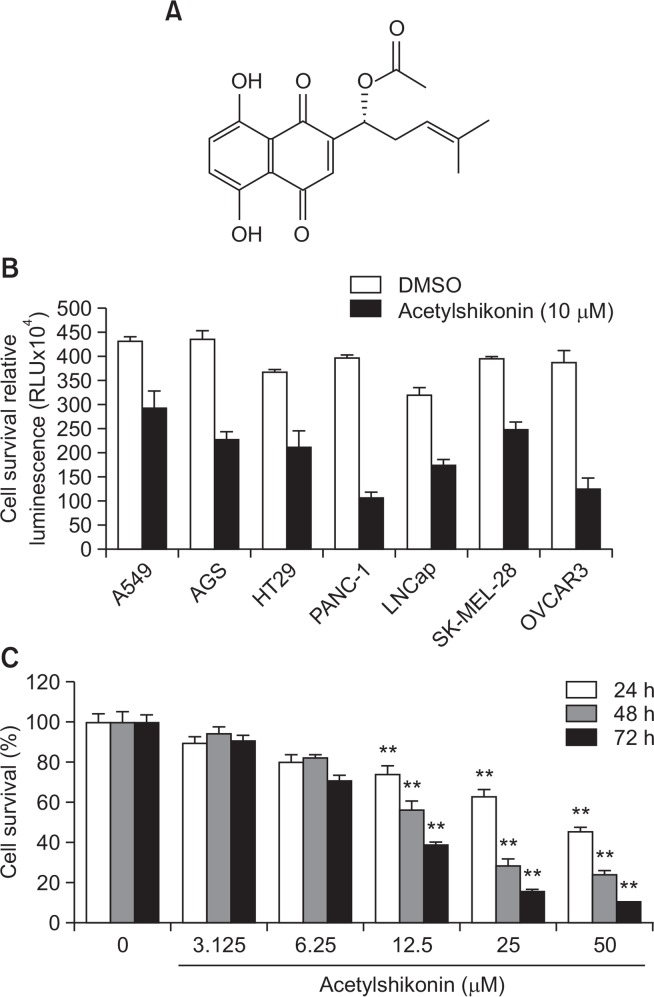
We have exposed 10 μM acetylshikonin (Fig. 1A) to various cancer cell lines, such as A549 cells (lung cancer), HT-29 cells (colon cancer), PANC-1 cells (pancreatic cancer), LNCaP-FGC cells (prostate cancer), SK-MEL-28 cells (melanoma) and OVCAR3 cells (ovarian cancer) for 72 h and assessed the resulting growth-inhibitory effects by CellTiter-Glo® luminescent cell viability assay. As a result, we observed that acetylshikonin exhibited the strongest growth-inhibitory effects on PANC-1 cells among tested cancer cell lines (Fig. 1B). Supporting this observation, acetylshikonin exhibited significant inhibitory effects on the growth of PANC-1 cells in time- and dose-dependent manners (Fig. 1C).
Fig. 1.
Effects of acetylshikonin on the viability of PANC-1 cells. (A) The chemical structure of acetylshikonin. (B) Various cancer cells were exposed to a single concentration (10 μM) of acetylshikonin for 72 h and the resulting cell viability was assessed. (C) PANC-1 cells were exposed to various concentrations of acetylshikonin for 24 h, 48 h and 72 h. Values are expressed as means ± SD. The asterisk(s) indicate a significant statistical significance (**p<0.01).
Acetylshikonin inhibits PMA or TNF-α-induced NF-κB transcriptional activity
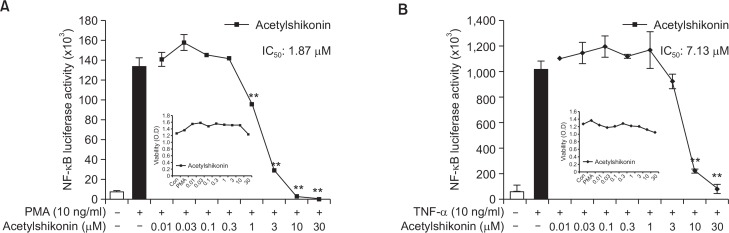
Next, we examined the effect of acetylshikonin on PMA or TNF-α-induced NF-κB promoter luciferase activity in HEK293 NF-κB-luciferase cells. As a result, we found that acetylshikonin significantly inhibited the NF-κB luciferase activation, induced by PMA (Fig. 2A) or TNF-α (Fig. 2B). The IC50 value for the inhibition of NF-κB luciferase activation by PMA and TNF-α was calculated to be 1.87 μM (Fig. 2A) and 7.13 μM (Fig. 2B), respectively. It is notable that acetylshikonin did not affect the growth of PANC-1 cells at these concentrations and that we decided to proceed subsequent experiments with PMA rather than TNF-α, since IC50 value for PMA was much lower (Fig. 2A and 2B).
Fig. 2.
Effects of acetylshikonin on PMA or TNF-α-induced NF-κB reporter activity. (A) HEK293 cells, stably transfected with NF-κB-luciferase vector were incubated with PMA (10 ng/ml) alone or in combination with acetylshikonin (0.01, 0.03, 0.1, 0.3, 1, 3, 10 or 30 μM) for 24 h and the NF-κB transcriptional activity was measured. (B) Measurement of TNF-α-induced NF-κB transcriptional activity. HEK293 cells, stably transfected with NF-κB-luciferase vector were exposed to various concentrations of acetylshikonin for 24 h and the luciferase activity was measured. The asterisk(s) indicate a significant statistical significance (**p<0.01).
Acetylshikonin inhibits PMA-induced production of cytokines in PANC-1 cells
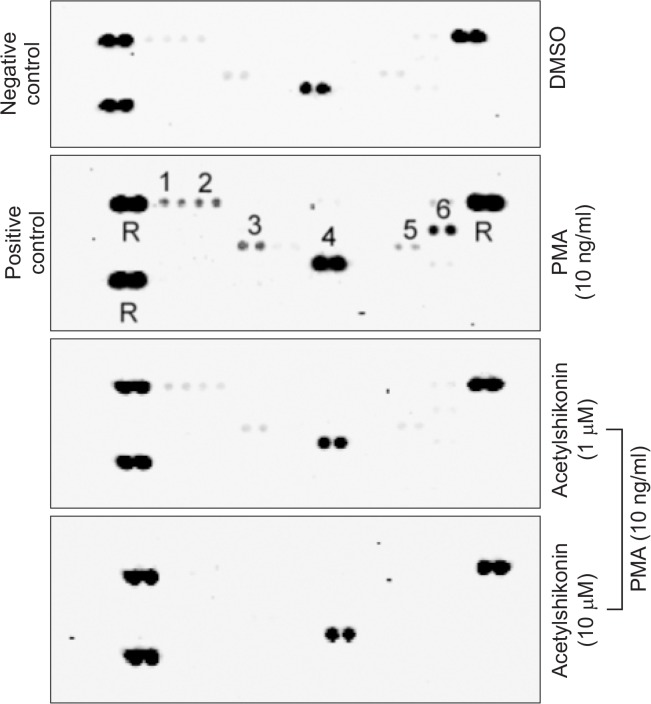
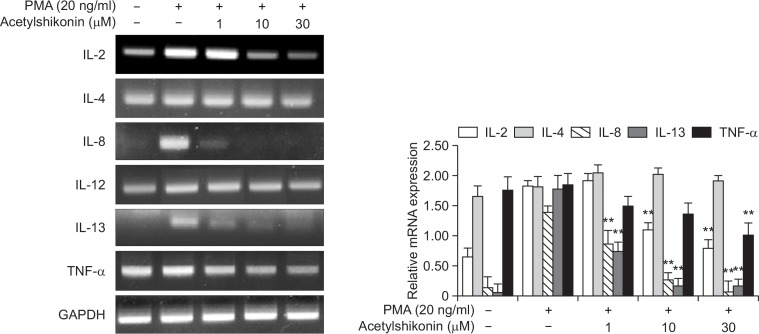
A previous study illustrated that the activation of NF-κB signaling pathway by PMA is responsible for the production of a variety of cellular cytokines (Im et al., 2014). By conducting the human proteome cytokine array, we observed that that PMA increased the production of a number of cytokines, such as C5/C5a, CD40 ligand, IL-13, MIF, IL-23 and IL-8 and that acetylshikonin inhibited PMA-induced production of these cytokines in HEK293 cells (Fig. 3). To examine whether acetylshikonin suppression of PMA-induced production of these cytokines could be transcriptionally regulated, we conducted both conventional and real-time reverse transcription-polymerase chain reaction (RT-PCR). As a result, we observed that an exposure of acetylshikonin to PANC-1 cells suppressed PMA-induced production of a number of cytokine mRNAs (IL-2, IL-4, IL-8, IL-12, IL-13 and TNF-α) (Fig. 4). Together, these results illustrate that acetylshikonin inhibition of PMA-induced production of cytokines is mediated by modulating the transcription.
Fig. 3.
Levels of human cytokines released in the medium of PMA-treated HEK293 cells. HEK293 cells were exposed to PMA (10 ng/ml) alone or in combination with acetylshikonin and the media was collected to examine the level of released cytokines, using the human proteome cytokine array kit. The intensity were determined by pixel densities using myECL imager analysis software (Themo Fisher Scientific, Waltham, MA) (Membrane spot No : 1.C5/C5a., 2. CD40 Ligand., 3. IL-13., 4. MIF., 5. IL-23., 6. IL-8., R. Reference).
Fig. 4.
Effect of acetylshikonin on PMA-induced mRNA expression. PANC-1 cells were exposed to PMA alone or in combination with acetylshikonin for 24 h. The mRNA levels of amplified genes by RT-PCR were determined on 1 % agarose gel, in which GAPDH was used as a control for normalization (Left panel). Real-time RT-PCR data are depicted as means ± standard deviation (SD) of three independent experiments (Right panel). The asterisk(s) indicate a significant statistical significance (**p<0.01).
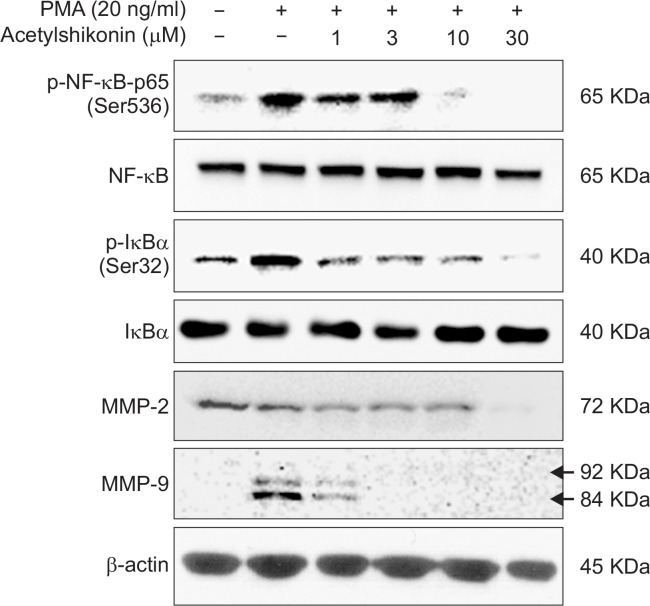
Acetylshikonin inhibited PMA-induced MMP-9 expression in the PANC-1 cells
It is known that matrix metalloproteinase-9 (MMP-9) plays an important role in the invasion and metastasis of cancer cells and that NF-κB transcriptionally regulates the expression of MMP-9 in response to a variety of stimuli. Base on the observation that acetylshikonin inhibits PMA-induced NF-κB activation in PANC-1 cells (Fig. 2A), we further investigated how acetylshikonin could affect the expression of MMP-9. To this end, we exposed PANC-1 cells to PMA alone or in combination with various concentrations of acetylshikonin and conducted Western blot analyses. As a result, we observed that acetylshikonin suppressed PMA-induced phosphorylation of p65 and IκBα, but did not affect their endogenous levels (Fig. 5). In addition, we found that acetylshikonin inhibited the endogenous expression of MMP-2 and MMP-9 (Fig. 5). Together, these results imply that acetylshikonin suppression of NF-κB might be responsible for downregulation of MMP-2 and MMP-9.
Fig. 5.
Effects of acetylshikonin treatment on expression of NF-κB-p65, p-IκBα, MMP-2 and MMP-9. PANC-1 cells were exposed to various concentrations of acetylshikonin and Western blot analysis was conducted as described in the Materials and Methods.
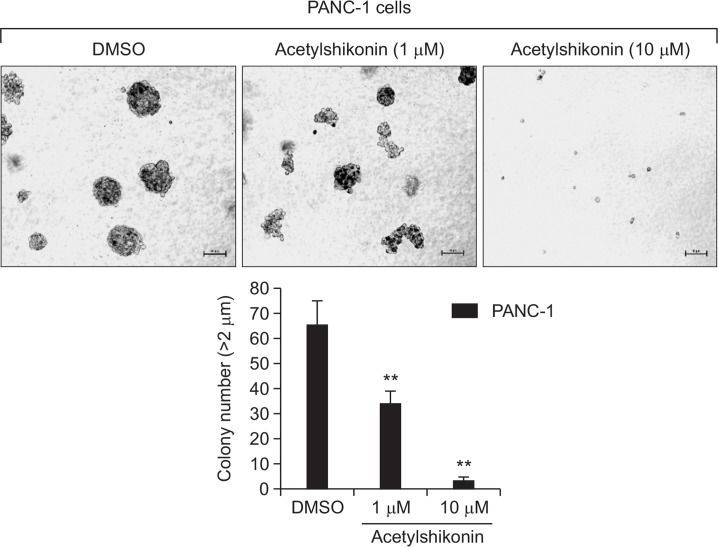
Acetylshikonin suppressed anchorage-independent cell growth of PANC-1 cells
To explore the anti-tumorigenic effects of acetylshikonin on PANC-1 cells, we conducted the soft-agar assays. Our results show that the growth of PANC-1 cells in a soft agar could be significantly inhibited by treatment of acetylshikonin in a dose-dependent manner (Fig. 6), suggesting that acetylshikonin possess significant inhibitory effects on the anchorage-independent growth of PANC-1 cells.
Fig. 6.
Inhibitory effect of acetylshikonin on the anchorage-independent colony formation of PANC-1 human pancreatic cancer cells. Anchorage-independent colony formation assay of PANC-1 cells was conducted according to the protocol in the Materials and Methods. Representative figures were photographed (Upper Panel) and the average colony number (Lower Panel) was calculated from three independent experiments. Colony sizes were calculated using the Image J software program. The asterisk(s) indicate a significant statistical significance (**p<0.01).
DISCUSSION
Although the exact molecular mechanisms underlying the anti-tumorigenic effects are largely unknown, regular con sumption of fruit and vegetables is known to lower the incidence of cancer in various tissues in human. Follow-up studies have demonstrated that selected naturally-occurring compounds found in vegetables, fruits, plant extracts, and herbs exhibit significant anti-carcinogenic activities as well (Nishino et al., 2000) (Abdulla and Gruber, 2000; Reddy et al., 2003). In line with this notion, the anti-carcinogenic effects of acetylshikonin have been previously demonstrated by other colleagues (Zeng et al., 2009; Moon et al., 2014). The main finding of our study is that acetylshikonin inhibits NF-κB-dependent transcription and the resulting expression of NF-κB target genes. Therefore, whether acetylshikonin inhibition of NF-κB might account, at least in part, for its anti-carcinogenic activity, e.g. the inhibition of cancer growth and metastasis, needs to be further addressed in the future experiment.
In the present study, we have observed that acetylshikonin inhibition of NF-κB is mediated by suppression of NF-κB phosphorylation and MMP-9 expression (Fig. 5). As a consequence, acetylshikonin inhibited the production of selected cellular cytokines in PANC-1 cells in response to PMA treatment (Fig. 3). Intriguingly, we observed that the expression of MMP-2 was inducible by an exposure to PMA in PANC-1 cells (Fig. 5) and the reason for this phenomenon is unclear at present. In addition, we observed that acetylshikonin dramatically suppressed PMA-induced MMP-9 expression, presumably by the inhibition of PMA-induced NF-κB-p65 and its upstream IκBα phosphorylation (Fig. 5). Based on the these results, we are tempted to speculate that acetylshikonin significantly inhibits the growth or invasion of PANC-1 human pancreatic cancer cells by suppression of PMA-induced NF-κB-dependent production of cytokines or MMP-9 expression. Collectively, our study provides evidence that acetylshikonin could be harnessed as a potential future therapeutic agent against human pancreatic cancer.
Acknowledgments
We thank to Dr. Tae Hoon Kang (KOTMIN, Gyeongsan, Korea) for 1st screening providing purified natural compounds.
Footnotes
CONFLICTS OF INTEREST
Authors declare no competing financial interests.
REFERENCES
- Abdulla M, Gruber P. Role of diet modification in cancer prevention. Biofactors. 2000;12:45–51. doi: 10.1002/biof.5520120108. [DOI] [PubMed] [Google Scholar]
- Bosetti C, Bertuccio P, Negri E, La Vecchia C, Zeegers MP, Boffetta P. Pancreatic cancer: overview of descriptive epidemiology. Mol Carcinog. 2012;51:3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- Butler AM, Scotti Buzhardt ML, Erdogan E, Li S, Inman KS, Fields AP, Murray NR. A small molecule inhibitor of atypical protein kinase C signaling inhibits pancreatic cancer cell transformed growth and invasion. Oncotarget. 2015;6:15297–15310. doi: 10.18632/oncotarget.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calonghi N, Pagnotta E, Parolin C, Mangano C, Bolognesi ML, Melchiorre C, Masotti L. A new EGFR inhibitor induces apoptosis in colon cancer cells. Biochem Biophys Res Commun. 2007;354:409–413. doi: 10.1016/j.bbrc.2006.12.214. [DOI] [PubMed] [Google Scholar]
- Chien W, Lee DH, Zheng Y, Wuensche P, Alvarez R, Wen DL, Aribi AM, Thean SM, Doan NB, Said JW, Koeffler HP. Growth inhibition of pancreatic cancer cells by histone deacetylase inhibitor belinostat through suppression of multiple pathways including HIF, NFkB, and mTOR signaling in vitro and in vivo. Mol Carcinog. 2014;53:722–735. doi: 10.1002/mc.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Friedman J, Nottingham L, Chen Z, Ara G, Van Waes C. Nuclear factor-kappaB p65 small interfering RNA or proteasome inhibitor bortezomib sensitizes head and neck squamous cell carcinomas to classic histone deacetylase inhibitors and novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2007;6:37–50. doi: 10.1158/1535-7163.MCT-05-0285. [DOI] [PubMed] [Google Scholar]
- El Fitori J, Su Y, Buchler P, Ludwig R, Giese NA, Buchler MW, Quentmeier H, Hines OJ, Herr I, Friess H. PKC 412 small-molecule tyrosine kinase inhibitor: single-compound therapy for pancreatic cancer. Cancer. 2007;110:1457–1468. doi: 10.1002/cncr.22931. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Lien JC, Chang CW, Chang CH, Kuo SC, Huang TF. Yuwen02f1 suppresses LPS-induced endotoxemia and adjuvant-induced arthritis primarily through blockade of ROS formation, NFkB and MAPK activation. Biochem Pharmacol. 2013;85:385–395. doi: 10.1016/j.bcp.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Im NK, Jang WJ, Jeong CH, Jeong GS. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-kappaB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food. 2014;17:855–861. doi: 10.1089/jmf.2013.3077. [DOI] [PubMed] [Google Scholar]
- Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19–35. doi: 10.1002/biof.5520290103. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu YP, Yu XJ, Zhang XD, Ming PH, Zhou GB, Huang L. The natural compound magnolol inhibits invasion and exhibits potential in human breast cancer therapy. Sci Rep. 2013;3:3098. doi: 10.1038/srep03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, Koh SS, Malilas W, Cho IR, Kaewpiboon C, Kaowinn S, Lee K, Jhun BH, Choi YW, Chung YH. Acetylshikonin induces apoptosis of hepatitis B virus X protein-expressing human hepatocellular carcinoma cells via endoplasmic reticulum stress. Eur J Pharmacol. 2014;735:132–140. doi: 10.1016/j.ejphar.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J, Schlitt HJ, Geissler EK, Stoeltzing O. Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur. J. Cancer. 2008;44:1577–1586. doi: 10.1016/j.ejca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Okuda M, Khachik F, Narisawa T, Takasuka N, Yano M. Cancer prevention by natural carotenoids. Biofactors. 2000;13:89–94. doi: 10.1002/biof.5520130115. [DOI] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- Reddy L, Odhav B, Bhoola KD. Natural products for cancer prevention: a global perspective. Pharmacol Ther. 2003;99:1–13. doi: 10.1016/S0163-7258(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Tafani M, Pucci B, Russo A, Schito L, Pellegrini L, Perrone GA, Villanova L, Salvatori L, Ravenna L, Petrangeli E, Russo MA. Modulators of HIF1alpha and NFkB in cancer treatment: is it a rational approach for controlling malignant progression? Front Pharmacol. 2013;4:13. doi: 10.3389/fphar.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiench B, Eichhorn T, Paulsen M, Efferth T. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. Evid Based Complement Alternat Med. 2012;2012:726025. doi: 10.1155/2012/726025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Liu G, Zhou LM. Inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 in vitro and in vivo. World J Gastroenterol. 2009;15:1816–1820. doi: 10.3748/wjg.15.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]