Abstract
Colon cancer is considered as the precarious forms of cancer in many developed countries, with few to no symptoms; the tumor is often diagnosed in the later stages of cancer. Monoterpenes are a major part of plant essential oils found largely in fruits, vegetables and herbs. The cellular and molecular activities show therapeutic progression that may reduce the risk of developing cancer by modulating the factors responsible for colon carcinogenesis. Colon cancer was induced with DMH with a dose of (20 mg/Kg/body weight) for 15 weeks by subcutaneous injection once in a week. Myrtenal treatment was started with (230 mg/Kg/body weight) by intragastric administration, one week prior to DMH induction and continued till the experimental period of 30 weeks. The Invivo results exhibit the elevated antioxidant and lipid peroxidation levels in DMH treated animals. The Histopathological analysis of colon tissues well supported the biochemical alterations and inevitably proves the protective role of Myrtenal. Treatment with myrtenal to cancer bearing animals resulted in a remarkable increase in the inherent antioxidants and excellent modulation in the morphological and physiological nature of the colon tissue. It is thus concluded that myrtenal exhibits excellent free radical scavenging activity and anticancer activity through the suppression of colon carcinoma in Wistar albino rats.
Keywords: Myrtenal, DMH, Colon cancer, Antioxidants, Monoterpenes
INTRODUCTION
Colon cancer is considered as the precarious forms of cancer in many developed countries, with few to no symptoms; the tumor is often diagnosed in the later stages of cancer (De Salvo et al., 2004) and has a potential to spread to distinct parts of the body including liver, lung, ovaries and other gastrointestinal organs (Donaldson, 2004). It arises predominantly by environmental genotoxic and non-genotoxic carcinogens triggering free radical mediated damage to the normal cells, (Marks and Fürstenberger, 2000) producing reactive oxygen species (ROS), that bind to react with and attack several cellular components, forming reactive oxygen metabolites (Pham-Huy et al., 2008). The release of free radicals from leukocytes and activated macrophages has been shown to generate peroynitrite (ONOO−) in vivo, a powerful ROS with low specificity causing inflammation (Marnett, 2000). As the link between cancers with inflammation appears to be causal, and inflammation mediated angiogenesis is a well established paradigm which is most likely due to deregulated interactions between the host mucosal epithelium and microbes, leading to a hyper-active immune system (Macarthur et al., 2004). In this juncture excessive generation of reactive oxygen species can cause oxidative damage to biomolecules resulting in mutagenesis, carcinogenesis and lipid peroxidation (LPO). Free radicals induced LPO has been implicated in neoplastic transformation (Tas et al., 2005). Hence the products of lipid peroxidation may be decomposed to alkoxyl and peroxyl free radicals that oxidize other cell components, resulting in the change of enzyme activity or the generation of mediators that cause further cell damage.
Lipid peroxidation has gained more importance nowadays because of its involvement in pathogenesis of many diseases like cancer, diabetes and also aging (Halliwell, 1994). To cope with the injury potential of ROS mediated LPO, cells are equipped with effective antioxidant systems (enzymatic and non-enzymatic) that make them mobile free radical scavengers, providing antioxidant protection not only to themselves but also to other tissues and organs in the body (Arbos et al., 2008). Supporting the antioxidant enzymes the detoxification system in our body, which includes a wide spectrum of phase I and II drug metabolizing enzymes, that can compete with activating enzymes by eliminating reactive electrophiles via conjugation, rendering them more water soluble and more readily excretable from the cell and the body (Jiang et al., 2003). Consumption of natural antioxidants in fruits and vegetables, favors the xenobiotic metabolizing enzymes to act against cardiovascular disease and cancers (Duthie et al., 2000). Hence extracts of plant parts are extensively explored for different bioactivities (Kumari and Kakkar, 2008). Epidemiological studies have demonstrated that colon cancer development is closely associated with dietary habits and lifestyle, with food in high fat and carbohydrates could promote the tumor growth by inflammation (Pisani et al., 2002).
The incidence rate of colon cancer is lower in Asian countries where the diet is predominantly rich in vegetables and fruits (Haggar and Boushey, 2009). Thus, cancer prevention has tended to diet-based intervention, considering safety and economics, while synthetic anticancer drugs prolong survival, but they often have adverse effects and off target actions. Based on this, Nutraceuticals and Phytochemicals have been investigated for colon cancer therapeutics (Belkaid et al., 2006). In this connection Monoterpenes a naturally occurring plant hydrocarbon composed of two isoprene units widely distributed in plant flora and are best known for occurrence as essential oils (Meinschein, 1969). Monoterpenes have been shown to have remarkable biological activities such as antioxidant, chemotherapeutic and chemopreventive effects in different models of cancer (Bishayee et al., 2011). Myrtenal an essential oil of the monoterpenes family present in cumin, pepper, mint, eucalyptus is used as a food additive for flavor (Strehle et al., 2006). In contrast, many studies have postulated its various biological activities such as anti-malarial, anti-plasmodial, anti-radicular, cyclooxygenase-inhibitor, gonadotrophic, hypocholesterolemic and immunostimulant effects (Vibha et al., 2009). In this aspect the oxidation of free radicals by myrtenal stabilizes the cellular integrity and maintains homeostasis (Ziajka and Pasiuk-Bronikowska, 2005). Exploring the various benefits of myrtenal the study comprised its active role in the colon cancer treatment since there is a paucity of information regarding its role as a chemotherapeutic agent, especially in DMH induced colon carcinogenesis. Hence, the present investigation was undertaken to evaluate the anticancer potential of myrtenal against experimental colon carcinoma (CRC) in Male Wistar Albino rats.
MATERIALS AND METHODS
Reagents
1, 2-Dimethylhydrazine (DMH), Myrtenal was purchased from Sigma Chemical Company, St. Louis, MO, USA. All the other chemicals used in this study were of analytical grade available commercially.
Experimental animals
Experiments were carried out with 5 weeks old male Wistar rats procured from the Central Animal House Facility, Dr. A.L.M. Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai-600113. They were maintained in the controlled environment conditions of temperature and humidity on alternative 12 h light/dark cycle, noise level maintained below 85 dB and had unrestricted access to standard diet consisting of 24% protein, 4.5% fat and 4% fiber. The experiment was sanctioned and approved by the Institutional Animal Ethical Committee (IAEC No.01/13/2013).
Experimental Design
The experimental animals were divided into four groups, each group comprising six animals.
Group I: Control animals fed with standard diet and pure drinking water.
Group II: Animals were administered with 20 mg/kg body weight of DMH, in 1 mM EDTA, pH adjusted to 6.5 with 1 mM NaOH and subcutaneously injected once in a week for 15 weeks.
Group III: Animals were treated with Myrtenal (230 mg/Kg b.wt.) with corn oil as vehicle for 30 weeks by intragastric administration. Myrtenal treatment was started 1 week prior to the first dose of 20 mg/kg body weight of DMH (as in group II) and continued till end of the experimental period.
Group IV: Animals were treated with Myrtenal (230 mg/Kg b.wt.) for 30 weeks by intragastric administration to assess the cytotoxicity if any, induced by Myrtenal, and rats were referred as drug control.
After the end of the experimental period, the rats were fasted overnight and anesthetized using diethyl ether and sacrificed by cervical decapitation. A portion of the colon was used for histopathological studies and remaining tissue was homogenized in 0.1 M Tris-HCl buffer pH 7.4 and centrifuged, the supernatant was used for biochemical studies.
Colon analysis
Colons were excised from experimental rats, and were blotted dry and opened longitudinally, with the inner surface examined for visible macroscopic lesions. Tumor weight was determined for the colons. Immediately following sacrifice, colons were removed and washed with ice-cold saline, and colon homogenates (10%) were prepared in ice cold TBS (Tris 50 mM and NaCl 150 mM; pH 7.2) then centrifuged at 10,000 g for 10 min at 4°C and were stored as aliquots at or below −20°C for subsequent assays.
Biochemical analysis
The protein content was estimated by the method of Lowry et al. (1951) (Lowry et al., 1951) using bovine serum albumin as standard. The nucleic acids were extracted by the method of Schneider (1957) (Schneider, 1957). DNA was estimated by the method of Burton (1956) (Brown and Caston, 1962). RNA was estimated by the method of Rawal et al. (1977) (Rawal et al., 1977).
Lipid peroxidation and antioxidant assay
The macromolecular damage such as LPO was estimated by the method of Ohkawa et al., (1979) (Ohkawa et al., 1979) For lipid peroxidation (MDA) analysis, butylated hydroxytoluene (BHT) was added to colon homogenates at 1% final concentration to prevent further oxidation during sample storage (a week at ± 20°C). MDA production in the colon was measured according to the method of Yagi, (Ohkawa et al., 1979) in which MDA forms a pink colored complex with thiobarbituric acid with maximum absorbance at 535 nm. CAT activity was measured by determining the decomposition of H2O2 as described by Sinha, (1972) (Sinha, 1972) SOD activity was estimated by the method of Marklund and Marklund (1974) (Marklund and Marklund, 1974). GPx activity was measured according to the method of Rotruck et al. (1973) (Rotruck et al., 1973). GSH level in the colon was determined by the method of Moron et al., (1979) (Moron et al., 1979). GST was estimated by the method of Habig (1981) (Habig et al., 1974). GR was estimated by the method of (Staal et al., 1969). Vitamin E was estimated by the method of Desai (1984) (Desai, 1984). The levels of phase I enzymes (Omura and Sato, 1964) (CytochromeP450, Cytochrome b5, NADPH Cytochrome ‘C’ reductase) and phase II enzymes (Habig et al., 1974) (Glutathione-S-Transferase (GST) and UDP-Glucuronyl transferase) in liver tissue homogenate were determined.
Histopathology
Fresh colon tissue specimens were fixed in buffered formalin for 48 h, followed by dehydration in ascending grades of alcohol, cleared in benzene and was embedded in paraffin wax. Paraffin block 0.5 μm thick sections were double stained with haematoxylin and eosin, and were analyzed using an optical microscope.
Statistical analysis
Values are expressed as mean ± S. D. The results were statistically evaluated using one-way analysis of variance (ANOVA) by SPSS 10.0 student version followed by Tukey’s multiple comparison method to compare means of different groups. The mean difference is significant at the 0.05 levels.
RESULTS
The effect of Myrtenal on body weight and tumor weight of control and experimental animals is presented in Table 1. The body weights were found to be significantly decreased in group II DMH induced cancer bearing animals when compared with group I control animals. On the contrary, the administration of Myrtenal increased the body weight in group III animals when compared to group II animals. However, no significant changes were observed in Myrtenal alone treated group IV animals when compared to group I control animals. The tumor was macroscopically visible in group II cancer bearing animals compared to group I control animals. In myrtenal treated group III animals the tumors were under developed and had a significant tumor weight reduction compared to colon cancer bearing animals. Control and myrtenal treated animals showed no tumor development expressing the nontoxic property of myrtenal. The changes in tumor weight and bodyweight evidence the cytotoxic effect of DMH and its intermediates in the experimental animals with pathophysiological alterations. In this juncture myrtenal treatment have reverted the altered levels to near normal and this might be due to its inhibitory activity against the colon carcinogen and its metabolic degradation in the liver.
Table 1.
Effect of body weight and tumor weight of control and experimental rats
| Animals | Initial body weight (g) | Final body weight (g) | Tumor weight (mg) |
|---|---|---|---|
| Group I | 135 ± 3.7 | 227 ± 2.1 | - |
| Group II | 132 ± 1.7 | 184 ± 3.5a* | 260 ± 0.21 |
| Group III | 136 ± 2.1 | 205 ± 4.1b* | 132 ± 0.14b* |
| Group IV | 137 ± 2.0 | 231 ± 4.7ns | - |
Group I control animals express normal body weight at the initial and final experimental period. Group II cancer bearing animals show decreased final body weight compared to group I animals. Group III myrtenal treated animals express gradual increase in the final body weight compared to group II animals. No significant changes observed in group IV myrtenal alone treated animals. Values represent mean ± S.D. of six rats from each group.
Group II, III, IV compared with group I,
Group III compared with group II,
p<0.05,
not significant.
ROS generation
ROS clearly possess the capacity to behave in a sporadic and destructive fashion. The high rates of lipid peroxidation and the different forms of DNA base lesions that result in genomic instability, such as strand breaks, base modifications and DNA-protein cross linkages, have been found in the majority of neoplastic tissues. ROS-mediated DNA damage plays an essential role in the initiation of carcinogenesis, as well as in malignant transformation (Nair et al., 2007). In this study the ROS level was seen to increase in DMH treated group II rats compared to group I control. Myrtenal treated group III rats showed significant decrease in ROS levels Compared to group II rats. Group 4 myrtenal alone treated rats showed no changes. The DNA-reactive aldehyde can damage DNA either by reacting directly with DNA bases, or by generating more reactive bifunctional intermediates, which form exocyclic DNA adducts. Of these, 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA), acrolein, and crotonaldehyde have been most intensively studied with respect to their chemical and biological interactions with nucleic acid bases. The ability of these reactive electrophiles to modify DNA bases, yielding promutagenic lesions, is considered to contribute in the mutagenic and carcinogenic effects associated with oxidative stress-induced LPO, HNE and MDA which have increasingly been implicated in carcinogenesis (Zarkovic, 2003).
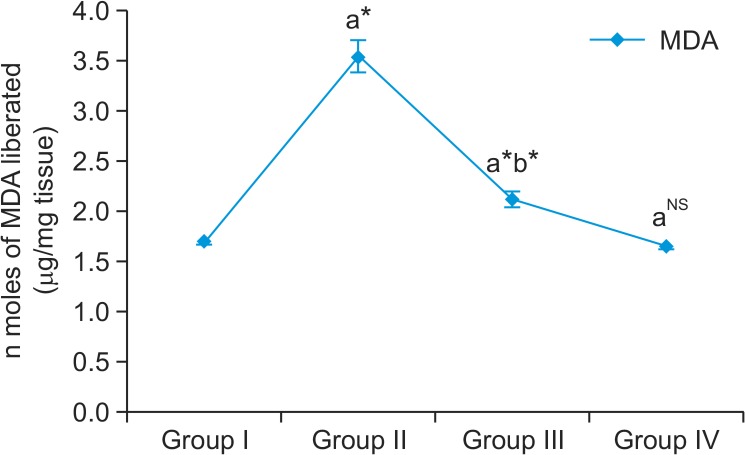
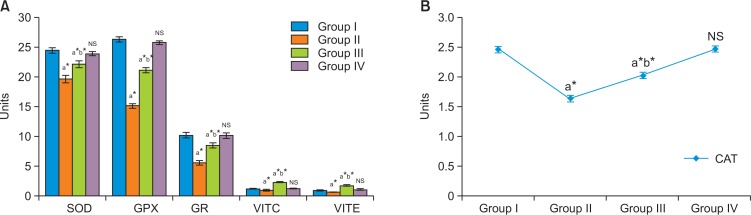
In our study, MDA levels were found to be significantly increased in group II cancer bearing animals compared to group I control animals (Fig. 1). Myrtenal administration significantly reduced the peroxidation reaction in group III animals compared with group II cancer bearing animals. However, no significant changes were observed in group IV myrtenal alone treated animals. Enzymic and non-enzymic antioxidant levels are shown in Fig. 2 with a significant reduction of SOD, CAT, GPX, and GR activities in DMH alone-treated group II animals as compared to the control. But on myrtenal supplementation in group III the decreased levels were significantly elevated as compared to the DMH-treated group II (Fig. 2). The concentrations of α-tocopherol and vitamin C were reduced in DMH treated group II cancer bearing animals as compared to group I control animals. But on Myrtenal supplementation, the concentration of α-tocopherol and vitamin C were significantly increased (p<0.05) as compared to the DMH-treated group II animals. No significant changes were observed in group IV myrtenal alone treated animals.
Fig. 1.
Effect of myrtenal on MDA levels in colon tissue of control and experimental animals. Group I control animals express normal levels of MDA, a biomarker for lipid peroxidation. Group II DMH induced animals show increased MDA levels as a result of lipid peroxidation and enhanced cell division by DNA methylating adducts by DMH. Group III Myrtenal treated animals express significant reduction in the levels of MDA as a result of gradual regeneration of the colon cells. Group IV Myrtenal alone treated animals posses no significant changes expressing no cytotoxic effect on the experimental animals. Results are expressed as mean ± S.D for six animals. aGroup II, III & IV compared with Group I, bGroup III compared with Group II,*p<0.05, NSNot significant.
Fig. 2.
(A, B) Effect of myrtenal on the enzymic & non-enzymic antioxidants levels in colon tissue of control and experimental animals. The diagram represents the antioxidant levels in control and experimental animals. Group I shows the normal levels of antioxidants in control animals. Group II DMH induced animals express decreased antioxidant levels as a result of excess free radicals in the microenvironment of the cells leading to oxidative stree related malignancy. Group III myrtenal treated animals exhibit cellular integrity by reverting the metabolic pathways and control enzymatic reactions causing significant increase in the levels of antioxidants. Myrtenal alone treated group IV animals show no significant changes in addition express profound antioxidant levels compared to group I control animals. Results are expressed as mean ± S.D for six animals. aGroup II, III & IV compared with Group I. bGroup III compared with Group II. *p<0.05, NSNot significant. Units: SOD=units/mg protein; CAT=μ mole of H2O2 consumed/mg protein/min; GR=μ mole of NADPH oxidized/min/mg protein; GPx=μ mole of glutathione oxidized/mg protein/min; GSH, Vit-C=mg/gm of wet tissue Vit-E=mg/gm of wet tissue.
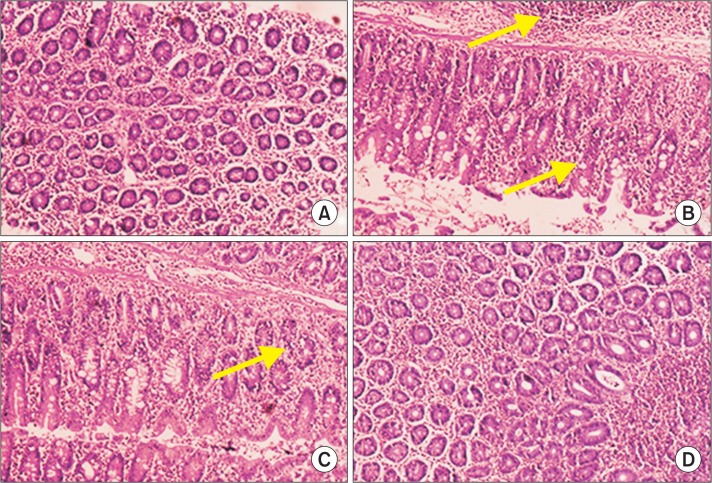
In group II colon cancer bearing animals Phase I enzyme levels were elevated compared to group I control. The activities of cytochrome P450 and cytochrome b5 were significantly decreased in myrtenal treated group III animals compared to group II cancer bearing animals in (Fig. 3). No significant changes were observed between group IV myrtenal alone treated animals and group I control animals. Phase II biotransformation enzymes projected decreased levels in group II cancer bearing animals compared to group I control animals. Group III myrtenal treated animals showed elevated levels of GST and UDP-GT compared to group II animals (Fig. 3). Group IV myrtenal alone treated animals and group I control animals showed no significant difference. Histopathological analysis of colons from rats treated with DMH in group II (Fig. 4B) showed differentiated signs of adenoma, with thickened epithelium and enlarged nuclei, DMH-induced colon tumor nearly restored to normal histoarchitecture, with a slight loss of nuclear polarity being evident following myrtenal treatment (Fig. 4C). Normal architecture with clear morphology was observed in (Fig. 4A) control animals and in (Fig. 4D) Myrtenal alone treated animals.
Fig. 3.
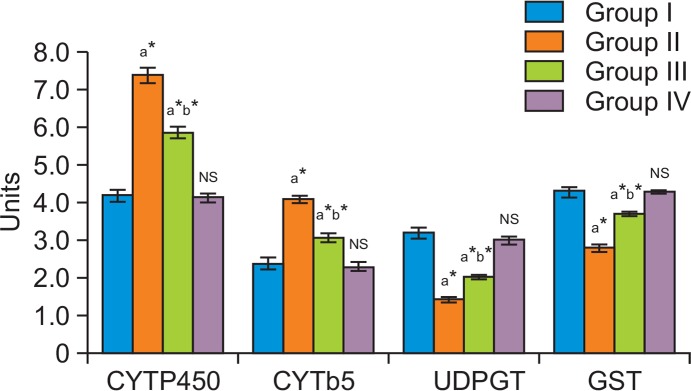
Effect of myrtenal on Phase I & Phase II enzyme levels in liver of control and experimental animals. The levels of xenobiotic metabolizing enzymes in the liver of control and experimental animals are presented in the bar diagram. Liver metabolizing enzyme levels were found normal in group I control animals. Group II DMH induced animals express increased levels of phase I enzymes and decreased phase II enzymes. Myrtenal treated group III animals exhibit reversion of altered levels of phase I and phase II biotrans-formation enzymes to near normal compared to group II DMH induced animals. No significant changes were observed in group IV myrtenal alone treated animals. Results are expressed as mean ± S.D for six animals. aGroup II, III & IV compared with Group I. bGroup III compared with Group II.*p<0.05, NSNot Significant. Units: Cytochrome P450-n moles/mg microsomal protein/min; Cytoch rome b5-n moles/mg microsomal protein/min; GST- n moles/mg microsomal protein/min; UDP-GT- n moles/mg microsomal protein/min.
Fig. 4.
Histopathological analysis of control and experimental animals. (A) shows normal architecture of control animals with effective staining in nucleus and cytoplasm. (B) express distorted nuclei and enlarged size of nuclei with closely packed glands causing infiltration of cancer cells with invasive adenoma. Villus and goblet cells of simple coloumnar epithelium show profound staining with reference to cellular damage and diffusion into lymphatic system. (C) show regeneration of villus and epithelial linings with respect to myrtenal treatment and decreased infiltration of cancer cells into the connective tissue. (D) show normal morphology of experimental animals with myrtenal supplementation alone showing no toxicity by cellular damage.
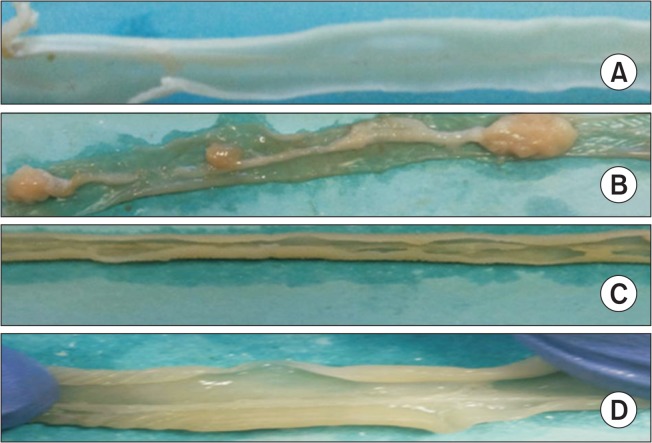
Fig. 5 shows the presence of tumors in the colon of control and experimental animals. Control animals show normal colonic structure and epithelial linings invariably throughout the colon. DMH induced animals exhibit multiple colon tumors with possible macroscopic lesions and shrinkage of the epithelial linings. Histopathological analysis further supports the presence of lesions. Myrtenal treated group III animals have formed lesser macroscopic lesion compared to group II DMH treated animals. The effect of DMH on group III animals expresses condensed epithelial linings of the colon. Myrtenal alone treated group IV animals posses normal structure of the colon.
Fig. 5.
Shows the presence of tumor incidence in control and experimental animals. (A) shows normal colon from group I control animals. (B) shows the presence of tumors in group II DMH induced colon cancer bearing animals with inflammatory reaction on the colon epithelial cells. (C) shows the shuttle expression of colon tumors in group III myrtenal treated animals compared to group II colon cancer bearing animals. (D) shows normal colon structure of myrtenal alone treated animals compared to group I control animals.
DISCUSSION
ROS are generated biologically under normal cellular metabolism of oxygen to perform in the stimulation of signaling pathways in plant and animal cells with response to the intra- and extracellular environmental conditions (Jabs, 1999). But increased ROS levels seen in DHM induced animal reports the imbalance between the generation and elimination, leading to oxidative stress related malignancy characterized by the functional decline of the cellular antioxidants during the primary defense (Ahmad et al., 2000). In this connection Giannoni et al. have demonstrated that (ROS) mediated oxidative stress are characteristic for both fibrosis and malignancy leading to cancer-associated fibroblasts (CAFs) (Giannoni et al., 2011). Hence the primary target was to maintain homeostasis and prevent oxidative stress related pathological conditions in the cells by controlling the excess ROS production. Thus ROS-eliminating strategies have been developed with the change in lifestyle and dietary supplementation of antioxidants in the prevention and treatment of a variety of diseases. In this context, terpenes are found beneficial because of their potential protective role in the pathogenesis of multiple diseases associated with oxidative stress. Members of this class of chemicals have carbon structures which can be decomposed into C5H8 residues based on the number of carbons in the molecule, as monoterpenes. They contribute in stabilizing cellular integrity by reverting the metabolic pathways and control, enzymatic reactions (Bicas et al., 2009).
Research on dietary products has so far showed that monoterpenes such as myrtenal biologically perform as a potent antioxidant and is well known to cure digestive ailments by regeneration of distorted cells to active morphology and resolve cellular abnormality in cancer condition (Milan et al., 2008). This regressing nature of myrtenal of the existing tumor cells further prevents them from the attack of free radicals such as superoxide anion and nitric oxide by scavenging the OH radical (Babu et al., 2012). Peroxide-mediated oxidations of organic compounds by transition metals produce superoxide radical leading to secondary reactions and accumulate free radicals throughout the system by electro-Fenton reaction, of which superoxide anion existing in the unconjugated form prolongs its activity by targeting DNA and causes cell death. Our study revealed that the superoxide anion scavenging activity of myrtenal in vitro with DPPH and ABTS in a dose dependant manner had depleted the free radicals by quenching the active intermediates (Casado et al., 2005). Hence external supply of antioxidants by natural compounds protects damage by nitric oxide radical coupled reaction with superoxide anion related imbalance causing damage of biomolecules, with potential impact on the key cellular components including DNA, proteins, and lipids. Thus the nitric oxide scavenging activity of myrtenal by its competitive binding directly against oxygen prevents peroynitrite radical formation which may damage lipids, proteins and nucleic acids by its anticarcinogenic property (Joshi et al., 2005).
Antioxidants act as reducing agents to get oxidized and prevent excess free radical production, but under malignant condition the levels of antioxidants provided is insufficient to overplay the emerging free radicals all over, which may be also due to DMH metabolism in the liver to release more free radicals. Although cells are equipped with a vast array of enzymatic antioxidants such as SOD family members (Mn, Cu and Zn SOD) which catalyze the dismutation of superoxide to O2 and hydrogen peroxide (H2O2), to water by glutathione peroxidase (Martin et al., 2010). CAT plays an important role in the preservation of its antioxidant ability and the balance between the production and destruction of ROS in organisms. On the contrary, GPx and CAT could also be inactivated by superoxide radical released by DMH. So the optimal protection of cells could be achieved only when an appropriate balance between the activities of these enzymes is maintained (Mao et al., 1993). Glutathione S-transferases (GSTs) catalyze the conjugation of glutathione (GSH) with different species of electrophilic compound to detoxify and protect cells against reactive oxygen metabolites (Laughlin et al., 1998).
Non-enzymatic antioxidants act against the deleterious effects of the cellular toxicants produced, in which ascorbic acid and alpha tocopherol play a significant role in reducing the oxidative stress. Since these antioxidants work co-operatively, a change in any one of them may break the equilibrium and cause cell damage leading to malignancy (Navarro et al., 1999). The oxidative degradation of lipids alters the membrane fluidity, induce cellular redox imbalance and shut off immune functions leading to lipid peroxidation, which serves as a biomarker of carcinogenesis causing mutagenic and cytotoxic mediated cell death (Suzuki et al., 2011). Elevated levels of MDA, an end product of LPO during carcinogenesis were seen which may be due to DMH, a methylating agent which stimulates cell division and induce colon tumor formation by releasing ROS, which interferes with DNA, in a manner similar to that which occurs in humans.
Chemotherapy of chemically induced tumors with monoterpenes acts in tumor redifferentiation which would appear to be through multiple mechanisms in the chemoprevention and chemotherapy of cancer (Crowell, 1999). It is reported that essential oil, obtained from monoterpenes, is a potential source of xenobiotic enzyme inducers that rapidly regulate the detoxification mechanism and suppress the free radicals related oxidative stress in cancer condition (Nakamura et al., 2003). Histological evidence of colon cancer cells shows the normal architecture in control animals compared to the distorted nuclei and increased number of crypts in induced group. Myrtenal treated animals had shown gradual regeneration, of the mucosal layer provided with a clear evidence of no impact on the myrtenal alone treated animals.
In conclusion, our study revealed that monoterpenes and its derivatives mainly myrtenal have possessed efficient anticarcinogenic and anti inflammatory property against DMH induced colon carcinogenesis in Wistar albino rats.
Acknowledgments
The authors extremely grateful to Dr. R. Venkatakrishna Murali, M.D., Ph.D., Professor and Head, Department of Pharmacology and Environmental Toxicology, Dr. A. L. Mudhaliar Post Graduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai-600 113 for providing the laboratory facilities.
Footnotes
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- Ahmad I, Hamid T, Fatima M, Chand HS, Jain SK, Athar M, Raisuddin S. Induction of hepatic antioxidants in freshwater catfish (Channa punctatus Bloch) is a biomarker of paper mill effluent exposure. Biochim. Biophys. Acta. 2000;1523:37–48. doi: 10.1016/S0304-4165(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Arbos KA, Claro LM, Borges L, Santos CA, Weffort-Santos AM. Human erythrocytes as a system for evaluating the antioxidant capacity of vegetable extracts. Nutr Res. 2008;28:457–463. doi: 10.1016/j.nutres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Babu LH, Perumal S, Balasubramanian MP. Myrtenal, a natural monoterpene, down-regulates TNF-α expression and suppresses carcinogen-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012;369:183–193. doi: 10.1007/s11010-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Belkaid A, Currie J-C, Desgagnés J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicas JL, Dionisio AP, Pastore GM. Bio-oxidation of terpenes: an approach for the flavor industry. Chem Rev. 2009;109:4518–4531. doi: 10.1021/cr800190y. [DOI] [PubMed] [Google Scholar]
- Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci. 2011;16:980–996. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Caston JD. Biochemistry of amphibian development: I. Ribosome and protein synthesis in early development of Rana pipiens. Dev Biol. 1962;5:412–434. doi: 10.1016/0012-1606(62)90022-2. [DOI] [PubMed] [Google Scholar]
- Casado J, Fornaguera J, Galán MI. Mineralization of aromatics in water by sunlight-assisted electro-Fenton technology in a pilot reactor. Environ Sci Technol. 2005;39:1843–1847. doi: 10.1021/es0498787. [DOI] [PubMed] [Google Scholar]
- Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- De Salvo GL, Gava C, Lise M, Pucciarelli S. Curative surgery for obstruction from primary left colorectal carcinoma: primary or staged resection? Cochrane Database Syst Rev. 2004:CD002101. doi: 10.1002/14651858.CD002101.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–147. doi: 10.1016/S0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- Donaldson MS. Nutrition and cancer: a review of the evidence for an anti-cancer diet. Nutr J. 2004;3:19. doi: 10.1186/1475-2891-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthie GG, Duthie SJ, Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- Giannoni E, Bianchini F, Calorini L, Chiarugi P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid Redox Signal. 2011;14:2361–2371. doi: 10.1089/ars.2010.3727. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/S0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Jiang Z-Q, Chen C, Yang B, Hebbar V, Kong A-NT. Differential responses from seven mammalian cell lines to the treatments of detoxifying enzyme inducers. Life Sci. 2003;72:2243–2253. doi: 10.1016/S0024-3205(03)00101-2. [DOI] [PubMed] [Google Scholar]
- Joshi R, Kumar MS, Satyamoorthy K, Unnikrisnan M, Mukherjee T. Free radical reactions and antioxidant activities of sesamol: pulse radiolytic and biochemical studies. J Agric Food Chem. 2005;53:2696–2703. doi: 10.1021/jf0489769. [DOI] [PubMed] [Google Scholar]
- Kumari A, Kakkar P. Screening of antioxidant potential of selected barks of Indian medicinal plants by multiple in vitro assays. Biomed Environ Sci. 2008;21:24–29. doi: 10.1016/S0895-3988(08)60003-3. [DOI] [PubMed] [Google Scholar]
- Laughlin LT, Bernat BA, Armstrong RN. Mechanistic imperative for the evolution of a metalloglutathione transferase of the vicinal oxygen chelate superfamily. Chem Biol Interact. 1998;111:41–50. doi: 10.1016/S0009-2797(97)00150-6. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Macarthur M, Hold GL, El-Omar EM. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G515–G520. doi: 10.1152/ajpgi.00475.2003. [DOI] [PubMed] [Google Scholar]
- Mao GD, Thomas P, Lopaschuk G, Poznansky M. Superoxide dismutase (SOD)-catalase conjugates. Role of hydrogen peroxide and the Fenton reaction in SOD toxicity. J Biol Chem. 1993;268:416–420. [PubMed] [Google Scholar]
- Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Marks F, Fürstenberger G. Cancer chemoprevention through interruption of multistage carcinogenesis: the lessons learnt by comparing mouse skin carcinogenesis and human large bowel cancer. Eur. J. Cancer. 2000;36:314–329. doi: 10.1016/S0959-8049(99)00318-4. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- Martin RC, Li Y, Liu Q, Barker DF, Doll MA, Hein DW. Manganese superoxide dismutase expression as a function of genotype and lung cancer pathology. Cancer Invest. 2010;28:813–819. doi: 10.3109/07357900903405918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinschein W. Hydrocarbons-saturated, unsaturated and aromatic. In: Eglinton G, editor. Organic geochemistry. Springer; Berlin: 1969. pp. 330–356. [DOI] [Google Scholar]
- Milan KM, Dholakia H, Tiku PK, Vishveshwaraiah P. Enhancement of digestive enzymatic activity by cumin (Cuminum cyminum L.) and role of spent cumin as a bionutrient. Food Chem. 2008;110:678–683. doi: 10.1016/j.foodchem.2008.02.062. [DOI] [Google Scholar]
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Miyamoto M, Murakami A, Ohigashi H, Osawa T, Uchida K. A phase II detoxification enzyme inducer from lemongrass: identification of citral and involvement of electrophilic reaction in the enzyme induction. Biochem Biophys Res Commun. 2003;302:593–600. doi: 10.1016/S0006-291X(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Navarro J, Obrador E, Carretero J, Petschen I, Avino J, Perez P, Estrela JM. Changes in glutathione status and the antioxidant system in blood and in cancer cells associate with tumour growth in vivo. Free Radic Biol Med. 1999;26:410–418. doi: 10.1016/S0891-5849(98)00213-5. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- Pisani P, Bray F, Parkin DM. Estimates of the worldwide prevalence of cancer for 25 sites in the adult population. Int. J. Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- Rawal U, Patel U, Rao G, Desai R. Clinical and biochemical studies on cateractous human lens III. Quantitative study of protein, RNA and DNA. Arogya J Health Sci. 1977;3:69–72. [Google Scholar]
- Rotruck J, Pope A, Ganther H, Swanson A, Hafeman DG, Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Schneider WC. Determination of nucleic acids in tissues by pentose analysis. Methods Enzymol. 1957;3:680–684. doi: 10.1016/S0076-6879(57)03442-4. [DOI] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys. Acta. 1969;185:39–48. doi: 10.1016/0005-2744(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Strehle KR, Rösch P, Berg D, Schulz H, Popp J. Quality control of commercially available essential oils by means of Raman spectroscopy. J Agric Food Chem. 2006;54:7020–7026. doi: 10.1021/jf061258x. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R. Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Tas F, Hansel H, Belce A, Ilvan S, Argon A, Camlica H, Topuz E. Oxidative stress in breast cancer. Med Oncol. 2005;22:11–15. doi: 10.1385/MO:22:1:011. [DOI] [PubMed] [Google Scholar]
- Vibha J, Choudhary K, Singh M, Rathore M, Shekhawat N. A study on pharmacokinetics and therapeutic efficacy of Glycyrrhiza glabra: a miracle medicinal herb. Bot Res Int. 2009;2:157–163. [Google Scholar]
- Zarkovic N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol Aspects Med. 2003;24:281–291. doi: 10.1016/S0098-2997(03)00023-2. [DOI] [PubMed] [Google Scholar]
- Ziajka J, Pasiuk-Bronikowska W. Rate constants for atmospheric trace organics scavenging SO 4− in the Fe-catalysed autoxidation of S (IV) Atmos Environ. 2005;39:1431–1438. doi: 10.1016/j.atmosenv.2004.11.024. [DOI] [Google Scholar]