Abstract
The human neonate and infant are unduly susceptible to infection with a wide variety of microbes. This susceptibility is thought to reflect differences from adults in innate and adaptive immunity, but the nature of these differences is incompletely characterized. The innate immune response directs the subsequent adaptive immune response after integrating information from Toll-like receptors (TLRs) and other environmental sensors. We set out to provide a comprehensive analysis defining differences in response to TLR ligation between human neonates and adults. In response to most TLR ligands, neonatal innate immune cells, including monocytes, conventional and plasmacytoid dendritic cells (cDCs and pDCs, respectively), produced less IL-12p70 and IFN-α (and consequently induced less IFN-γ), moderately less TNF-α, but as much or even more IL-1β, IL-6, IL-23, and IL-10 than adult cells. At the single-cell level, neonatal innate cells generally were less capable of producing multiple cytokines simultaneously, i.e., were less polyfunctional. Overall, our data suggest a robust if not enhanced capacity of the neonate vs. the adult white blood cell TLR-mediated response to support Th17- and Th2-type immunity, which promotes defense against extracellular pathogens, but a reduced capacity to support Th1-type responses, which promote defense against intracellular pathogens.
Introduction
Neonates and infants are unduly susceptible to a wide variety of infections (1). This increased susceptibility is thought to reflect deficiencies in both innate and adaptive immunity compared to adults (2). The innate immune system is central to all immunity because it decodes the nature of microbial threats, and then produces mediators to help provide appropriate immediate protection and determine the quality and magnitude of the subsequent adaptive immune response. However, the extent to which innate immune responses to microbial stimuli differ between adults and neonates, and the contribution of such differences to the neonate’s increased susceptibility, are still only incompletely characterized (2–4).
In general, neonatal cord blood mononuclear cells (CBMCs) have been found to produce less IL-1α, IL-1β, TNF-α, IL-18 and IL-12p70 but equal or greater IL-6 or IL-10 compared to adult cells in response to the TLR4 ligand lipopolysaccharide (LPS) and some other TLR ligands (5–8). However, strikingly different and even contradictory results have been reported. For example, neonatal cells have been reported to produce significantly less, as much, or even more TNF-α as adults (9–12). It is difficult to reconcile these discordant findings because the information was accrued in an incremental fashion, through studies differing in experimental design, assessing either one or only a few cytokines produced in response to a single or only a limited set of TLR ligands (often of uncertain purity), and under conditions in which the contribution of specific cell types was not addressed, or using cells whose phenotype and function were conditioned by prior culture in vitro. A more complete picture of how neonatal and adult innate immune responses differ could be obtained through a comprehensive side-by-side analysis of responses to well-defined ligands for TLRs, done using approaches that allow to the extent possible responses attributable to cDCs, pDCs, and monocytes to be identified using cells studied directly ex vivo. Conducting such a study in a sample set large enough to allow a well-powered statistical analysis, we confirmed and extended findings suggesting that the neonatal as compared to the adult innate immune responses to TLR stimulation were not so much deficient in quantity, but differed in quality.
Methods
TLR stimulation plates
TLR stimulation plates were prepared as described (13). Briefly, deep-96-well (VWR) source plates containing 1.3 μL of various TLR ligands at 10x the desired concentration were prepared using sterile procedures under a laminar air-flow hood. The following TLR ligands were used at the concentrations noted in the figure or table legends: PAM3CSK4 (TLR2/1, EMC microcollections); poly I:C (TLR3, Amersham); 0111:B4 LPS (TLR4, InVivogen); 3M-002 (TLR8, 3M); 3M-003 (TLR7/8, 3M); 3M-013 (TLR7, 3M); CpG (A type, 2336, Coley). For the 6-hr intracellular cytokine staining (ICS) plates, Brefeldin A (BFA, Sigma) was added at a concentration of 100 μg/mL (10x the desired final concentration of 10 μg/mL) to all wells except those wells containing TLR3 and TLR9 ligands. Brefeldin A was not added to the 10x source plates for the plates that were used to get 18-hr supernatants for Luminex and ELISA assays. Source plates were sealed with sterile aluminum plate sealers (USA Scientific), frozen at −80°C and thawed prior to use. Twenty microliters from each well of the source plate was dispensed into each well of recipient 96-well round bottom polystyrene plates (Corning) using the Evolution™ P3 Precision Pipetting Platform (Perkin Elmer) under a laminar airflow hood using sterile procedures. Recipient plates were sealed with sterile aluminum plate sealers and frozen at −80°C until use.
Blood sample processing and in vitro stimulation
All studies were approved by the Institutional Ethics Review Board at both the University of Washington and the University of British Columbia. Blood samples were processed as described (13). Cord blood from healthy, full-term elective Caesarean sections without labor and adult peripheral blood was collected directly into Na-Heparin-containing vacutainers (Becton Dickinson). Neonatal cord blood or adult peripheral blood mononuclear cells (MC) were isolated by density gradient centrifugation; whole blood (WB) was mixed 1:1 with sterile pre-warmed (37°C) RPMI-1640 medium (RPMI, Invitrogen). MC were cultured in RPMI supplemented with 100 units penicillin/ml, 100mg streptomycin/ml (Invitrogen) and 10% human AB serum (Gemini Bio-Products). One hundred eighty microliter of cell suspension (either MC or WB mixed 1:1 with RPMI) was added to each well of the premade plates containing the specific TLR ligands. For the ICS assays, cells were incubated for 6 hours at 37 °C in 5% CO2. For the TLR3 and TLR9 ligands BFA was added for the last 3 hours only at a final concentration of 10 μg/ml, which provides optimal detection of intracellular cytokine production in response to these ligands (13). After culture, cells were treated with a final concentration of 2mM EDTA for 15 min at 37°C, then spun down and resuspended in 100μl of 1x BD FACS Lysing Solution, sealed and stored frozen at −80°C until staining. For plates containing WB, the entire mixture was added to 1400 μl BD FACS Lysing Solution in deep 96-well plates (VWR) and stored frozen at −80°C until staining. An identical set of plates was incubated in parallel for 18hr without BFA; at 18hr, these plates were spun and 100μl of supernatant was removed and frozen at −80°C for later Luminex analysis.
Staining, Acquisition and Analysis
Preparation of the samples for flow cytometric analysis was performed as described (13). A detailed description of antibody (source, clone and dilution), machine set up, and data acquisition according to the recently accepted MiFlowCyt Standards (14) can be found in the Supplementary Data. Briefly, frozen plates were thawed, spun, and pellets resuspended in 200 μl BD FACS Permeabilizing Solution and incubated at room temperature for 10 min. After one wash in PBS containing 0.5% bovine serum albumin and 0.1% sodium azide (PBSAN), cells were stained in a final volume 100 μl PBSAN for 30–60 minutes at room temperature. After two further washes with PBSAN, cells were resuspended in PBS containing 1% paraformaldehyde and immediately analyzed on an LSRII Flow Cytometer (Becton Dickinson) set up according to published guidelines (15). Compensation beads (CompBeads, Becton Dickinson) were used to standardize voltage settings and used as single stain positive and negative controls as described (16). A total of 200,000 events were acquired for adult and neonatal MC and 1,000,000 for WB. Compensation was set in FlowJo (TreeStar, OR) or Labkey Flow (Labkey, WA), and samples were analyzed compensated.
Luminex-based assessment of cytokines in culture supernatant
Supernatants were thawed at room temperature, then filtered through a 1.2 μm filter plate (Millipore) into a clean 96-well plate to remove any remaining cellular debris using a multiscreen HTS vacuum manifold (Millipore, MA). The Luminex assay was performed using the Upstate/Millipore “Flex Kit” system using the high biotin protocol and overnight incubation at 4°C. Cytokines measured were IL-6, IL-10, IL-12p40, IL-12p70, TNFα, IFN-α2, and IFN-γ. Samples were diluted 1:2 (and if needed to fall within the standard curve, 1:10 or 1:20) in either RPMI with 10% humn AB serum for MC supernatants or a 1:1 mixture of RPMI and human serum diluent (Millipore, MA) for WB supernatants. Beadlytes and biotin were used at ½ of the manufacturer’s standard strength. Assays were read using a BioPlex 200 system (Bio-Rad) and analysis was performed using Excel (Microsoft) and an in-house database.
Human Interleukin-23 (p19/p40) ELISA
Filtered supernatants were diluted 1:4 in diluent contained in the human IL-23 (p19/p40) ELISA kit (eBioscience), and assays performed according to the manufacturer’s specifications. Plates were read at 450 nm with 570 nm subtraction. A sigmoid logistic curve was used to generate the standard curve.
Statistical analysis
Graphs were prepared using Excel (Microsoft). For statistical analysis of the Luminex results, the Prism analysis program (GraphPad) was used to compare the neonates and the adults for a given cytokine determination in response to a specific stimulus using the non-parametric Wilcoxon rank-sum test. For statistical analysis of the flow cytometry results, scatterplots with super-imposed boxplots and the Wilcoxon rank-sum test were used to compare the neonates and the adults. In order to assess the level of statistical significance corrected for multiple comparisons, we calculated q-values to control the rate of false discoveries as described (17) using the ‘q-value’ add-on package for R (18). We also computed the more conservative Bonferroni-corrected p-values for control of overall false positivity rates (19) and found no difference in our results between q-values or Bonferroni-corrected p-values at the 0.05 level. We here only report our results for q-values ≤0.01 as these yield an expected number of false discoveries of less than 0.5 for each of the different analytical categories of primary interest (e.g., comparison of adult and neonatal MC results for all cytokines assayed by Luminex; comparison of the percentage of positive adult and neonatal monocytes, cDCs and pDCs for any or all of the cytokines assayed by flow cytometry in whole blood).
To assess polyfunctionality (i.e., the ability of an individual cell to produce one vs. more than one cytokine in response to a specific stimulus), we included the percent of all cells in a given cytokine-combination category (there are 15 possible cytokine-combination categories for 4 cytokines, in which at least one cytokine is positive (2^4 − 1 = 15)); the percent of all cells in which a given cytokine was positive, and separately the percent of all cells in which any of the cytokines was positive; and the percent of reactive cells (i.e., cells producing at least one cytokine) that were positive for only 1 cytokine (polyfunctional degree (pfd1), and separately the percent of reactive cells expressing 2 (pfd2), 3 (pfd3), or 4 (pfd4) cytokines. Ternary plots (20) were created to examine the distribution of the polyfunctional degree for each subject. The ternary plot consists of an equilateral triangle with one point per subject inside the triangle. Each of the three corners of the triangle represents one of the three possible polyfunctional degrees – pfd1, pfd2, pfd3+. The position of the point within the triangle indicates the distribution of the possible degrees. For example, if a point is in the center of the triangle, the subject has equal percentages of cells which are positive for 1, 2, or 3+ cytokines. If a point is near the pfd1 corner, most of the subject’s reactive cells are positive for only 1 cytokine. Annotated, light grid marks appear inside the triangle to indicate the percentage of each type of pfd represented by a point in any section of the triangle. For each group, the mean and standard deviation of polyfunctional degree was computed as well.
Results
Single-cell analysis of cytokine production by neonatal vs. adult innate immune cells
We previously described a flow cytometric platform for the analysis of TLR responses at the single-cell level (13). Our polychromatic single-cell high-throughput flow-cytometry based approach allowed us to determine the cellular composition of the innate immune system in neonatal and adult MC and WB in quantitative detail (Supplementary Figure 1). We found that the pDC content of adult and neonatal WB and MC samples were similar, whereas neonatal WB preparations contained a higher percentage of monocytes, and adult WB and MC samples contained more cDCs compared to the respective neonatal samples (Supplementary Figure 2). As a result, neonatal MC and WB samples contained a higher ratio of pDCs:cDCs compared to adult samples (~1:3 vs. ~1:6, respectively), as was previously reported (4, 11).
Using this flow cytometric approach, we assessed the production of TNF-α, IL-6, IL-12/23p40, and IFN-α2 at the single-cell level in response to low, medium and high concentrations of each of the TLR ligands. Supplementary Figure 3 shows, for example, the TLR7/8 ligand dose-response profile for TNF-α, IL-6, and IL-12/23p40 production by adult and neonatal monocytes (monocytes did not produce detectable IFN-α2 in response to any of the stimuli employed; data not shown). We found that differences between neonatal and adult samples were most pronounced at the highest TLR ligand concentration tested, and, for this reason, present below only the flow cytometric data obtained with the high concentrations of each TLR ligand.
The two-dimensional dot plots for monocytes stimulated with an optimal concentration of the TLR7/8 ligand 3M-003 in Figure 1, show that individual cells may have no detectable cytokine production or may produce one or more of these cytokines. The cumulative percentages of cells producing one or any of the possible combinations of these cytokines are depicted in color-coded stacked bar graphs in Figures 2, 3 and 4, where the total height of the bar corresponds with the total response (i.e., the percentage of cells producing any cytokine), and each colored segment corresponds with the percentage of cells producing a specific cytokine or combination of cytokines. The total response, percentage of cells producing each cytokine, and the amount of each cytokine produced (as indicated by the mean fluorescence intensity, MFI) are detailed in Table 1. In addition, to provide a more intuitive means to visualize the degree with which a given cell population responded to TLR stimulation by producing either any 1, any 2 or any 3 cytokines, ternary plots — which depict the degree of polyfunctionality of cells from each subject — are shown below the stacked bar graphs in Figures 2–4.
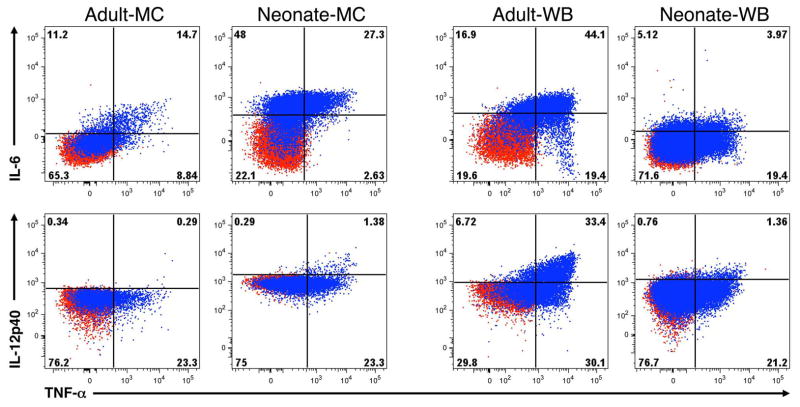
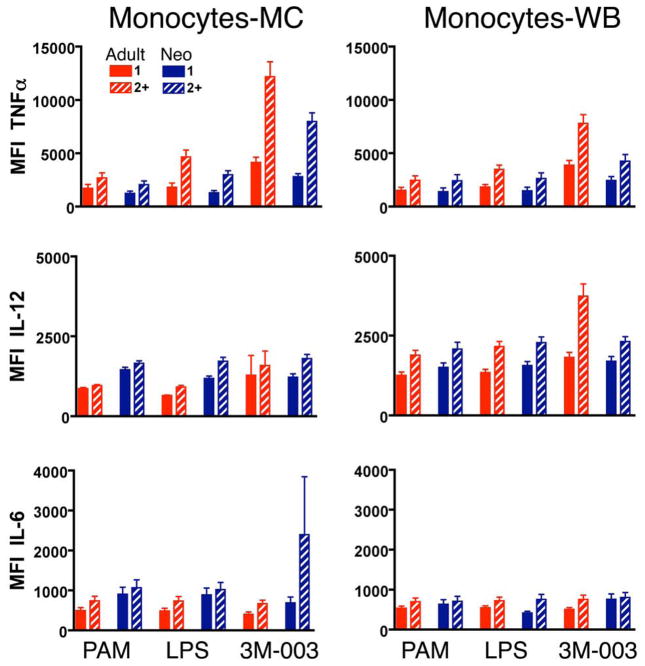
Figure 1. Example of intracellular cytokine production by adult or neonatal monocytes in MC and WB samples.
Shown are results for MC (left) and WB (right) from adult or neonatal monocytes. Samples were either unstimulated (red) or stimulated with TLR4 ligand LPS (100 ng/ml, blue) for 6 h in the presence of Brefeldin A, then expression of IL-6, IL-12/23p40, and TNF-α were determined by multiparameter flow cytometry. Note that we set quadrants for the analysis of a given cytokine expression so that unstimulated samples were <1% positive in ICC for every cytokine for both neonate and adult in both MC and WB.
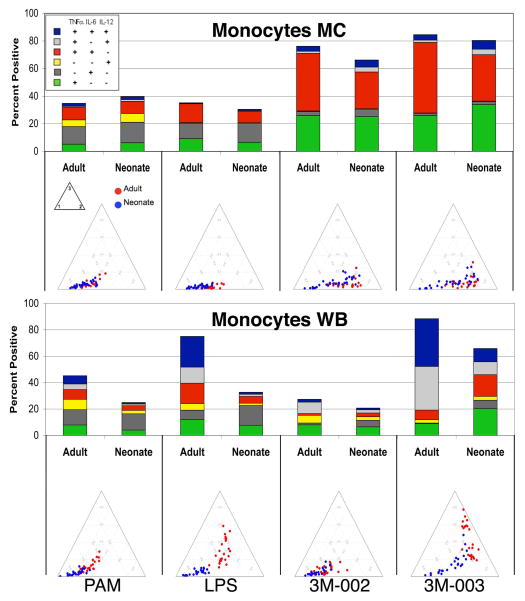
Figure 2. Neonatal monocytes in WB are less responsive and less polyfunctional to TLR stimulation than adult WB monocytes, while there is little difference between monocytes in MC.
MC or WB samples from 25 adults and 25 neonates were stimulated with the indicated TLR ligands (only results for the maximal concentration for each ligand are shown) for 6 hours, and expression of intracellular TNF-α, IL-6, and IL12/23p40 by monocytes was determined by multiparameter flow cytometry. A) Results (mean ± SD) are the percentage and the mean fluorescence intensity (MFI) of monocytes producing an individual cytokine or the total percentage of cells producing any cytokine, with the top and bottom panels showing monocytes in MC and WB, respectively. The probability that differences are significant after correction for multiple comparisons are shown as q-values (see statistical methods for description); blue and red are used to indicate values for which neonates and adults, respectively, were significantly different. B) Top- Stacked bar graphs in which the overall height of the bar indicates the total percentage of monocytes producing any cytokine and the height of each color (coded as shown in the insert) indicates the percentage of monocytes expressing an individual cytokine or cytokine combination. Bottom - ternary plots, in which each circle depicts the degree of polyfunctionality of cells from one adult (red circles) or neonate (blue circles). Circles closer to the left lower corner of the triangles are monocytes that expressed any one of the cytokines tested, circles closer to the right lower corner are monocytes that expressed any 2, and those closer to the top corner expressed all 3 (see methods for a more detailed description of ternary plots).
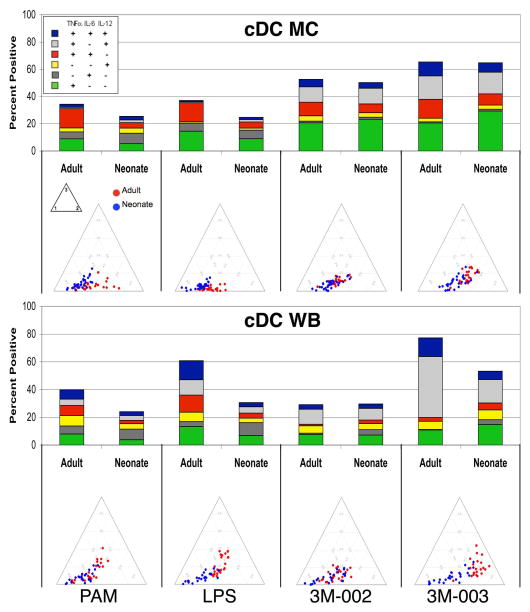
Figure 3. Neonatal cDCs are less responsive and less polyfunctional to TLR stimulation. The difference is more pronounced in WB as compared to MC.
MC or WB samples from 25 adults and 25 neonates were stimulated with the indicated TLR ligands (only results for the maximal concentration for each ligand are shown) for 6 hours, and expression of intracellular TNF-α, IL-6, and IL12/23p40 by cDCs was determined by multiparameter flow cytometry. A) Results (mean ± SD) are the percentage and the mean fluorescence intensity (MFI) of cDCs producing an individual cytokine or the total percentage of cells producing any cytokine, with the top and bottom panels showing monocytes in MC and WB, respectively. B) Top - stacked bar graphs in which the overall height of the bar indicates the total percentage of cDCs producing any cytokine and the height for each color (coded as shown in the insert) indicates the percentage of cDC expressing an individual cytokine or cytokine combination. Bottom - ternary plots, in which each circle depicts the degree of polyfunctionality of cells from one adult (red circles) or neonate (blue circles). See Figure 2 legend for further details.
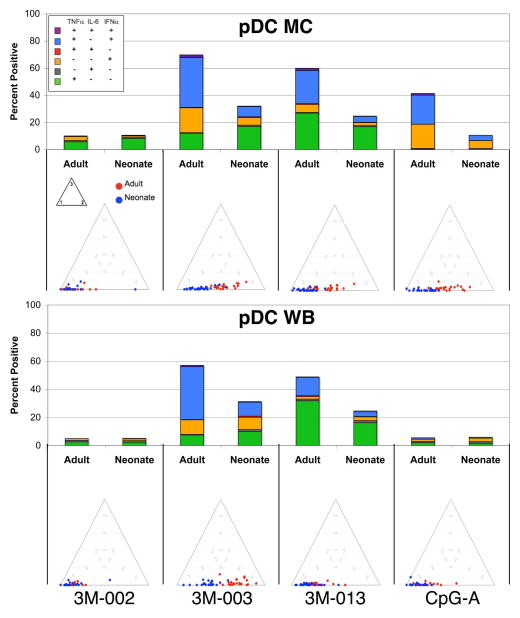
Figure 4. Neonatal pDCs are less responsive and less polyfunctional to TLR7/8 and TLR7 stimulation for both MC & WB.
MC or WB samples from 25 adults and 25 neonates were stimulated with the indicated TLR ligands (only results for the maximal concentration for each ligand are shown) for 6 hours, and expression of intracellular TNF-α, IL-6, and IFN-α by pDCs was determined by multiparameter flow cytometry. A) Results (mean ± SD) are the percentage and the mean fluorescence intensity (MFI) of pDCs producing an individual cytokine or the total percentage of cells producing any cytokine, with the top and bottom panels showing pDC in MC and WB, respectively. B) Top - stacked bar graphs in which the overall height of the bar indicates the total percentage of pDCs producing any cytokine and the height for each color (coded as shown in the insert) indicates the percentage of pDC expressing an individual cytokine or cytokine combination. Bottom - ternary plots, in which each circle depicts the degree of polyfunctionality of cells from one adult (red circles) or neonate (blue circles). See Figure 2 legend for further details.
Table I.
Flow cytometric comparison of neonatal and adult responses to TLR stimulation.a
| Monocytes - MC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | PAM | LPS | 3M-002 | 3M-003 | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IL-12/23p40 | % positive | 10.1 ± 1.3 | 12.6 ± 0.9 | n.s. | 1.5 ± 0.1 | 2.2 ± 1.2 | n.s. | 6.1 ± 0.6 | 9.7 ± 1.1 | n.s. | 6.6 ± 0.8 | 10.6 ± 1.1 | n.s. |
| MFI | 910 ± 37 | 1577 ± 84 | <0.0001 | 745 ± 41 | 1544 ± 134 | <0.0001 | 1064 ± 63 | 1869 ± 139 | <0.0001 | 1023 ± 56 | 1968 ± 156 | <0.0001 | |
| IL-6 | % positive | 27.0 ± 2.4 | 26.4 ± 3.5 | n.s. | 25.7 ± 1.6 | 22.1 ± 3.3 | n.s. | 49.7 ± 3.3 | 37.2 ± 5.1 | n.s. | 59.5 ± 2.8 | 41.5 ± 4.7 | n.s. |
| MFI | 537 ± 73 | 792 ± 164 | n.s. | 545 ± 76 | 790 ± 163 | n.s. | 652 ± 83 | 827 ± 163 | n.s. | 722 ± 91 | 882 ± 170 | n.s. | |
| TNF-α | % positive | 18.3 ± 2.4 | 18.2 ± 2.1 | n.s. | 23.7 ± 2.4 | 16.2 ± 1.8 | n.s. | 74.6 ± 3.9 | 62.0 ± 4.4 | n.s. | 86.4 ± 1.8 | 78.9 ± 2.3 | n.s. |
| MFI | 2214 ± 486 | 1285 ± 239 | 0.006 | 2315 ± 508 | 1313 ± 221 | 0.006 | 5783 ± 736 | 2835 ± 295 | <0.0001 | 7509 ± 1009 | 3881 ± 363 | <0.0001 | |
| TOTAL % positive | 35.4 ± 2.2 | 38.3 ± 2.7 | n.s. | 34.9 ± 1.8 | 29.4 ± 3.2 | n.s. | 75.6 ± 3.0 | 66.3 ± 4.3 | n.s. | 84.3 ± 1.8 | 79.6 ± 2.1 | n.s. | |
| Monocytes - WB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | PAM | LPS | 3M-002 | 3M-003 | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IL-12/23p40 | % positive | 21.6 ± 2.2 | 6.0 ± 0.7 | <0.0001 | 44.9 ± 2.7 | 5.9 ± 0.9 | <0.0001 | 17.4 ± 3.1 | 7.1 ± 1.1 | 0.0073 | 76.3 ± 2.2 | 24.7 ± 3.4 | <0.0001 |
| MFI | 1525 ± 145 | 1799 ± 153 | n.s. | 2203 ± 184 | 1897 ± 162 | n.s. | 1557 ± 136 | 1889 ± 170 | n.s. | 4603 ± 507 | 2303 ± 178 | <0.0009 | |
| IL-6 | % positive | 28.2 ± 4.0 | 18.1 ± 3.3 | n.s. | 51.3 ± 4.1 | 22.8 ± 3.8 | <0.0001 | 5.1 ± 0.7 | 9.8 ± 1.8 | n.s. | 45.6 ± 4.7 | 34.8 ± 4.5 | n.s. |
| MFI | 548 ± 61 | 683 ± 124 | n.s. | 647 ± 68 | 685 ± 118 | n.s. | 475 ± 45 | 697 ± 121 | n.s. | 675 ± 74 | 806 ± 129 | n.s. | |
| TNF-α | % positive | 25.3 ± 2.1 | 10.2 ± 1.4 | <0.0001 | 65.6 ± 2.5 | 16.3 ± 2.4 | <0.0001 | 20.3 ± 3.2 | 13.2 ± 1.8 | n.s. | 88.5 ± 1.4 | 58.1 ± 4.6 | <0.0001 |
| MFI | 1896 ± 395 | 1865 ± 549 | n.s. | 3056 ± 423 | 1760 ± 471 | <0.0009 | 1996 ± 354 | 2089 ± 436 | n.s. | 7622 ± 885 | 3355 ± 559 | <0.0009 | |
| TOTAL % positive | 44.7 ± 3.0 | 24.9 ± 3.0 | <0.0001 | 76.8 ± 1.5 | 32.7 ± 3.5 | <0.0001 | 27.2 ± 3.1 | 20.3 ± 2.4 | n.s. | 88.5 ± 1.2 | 65.8 ± 4.1 | <0.0001 | |
| cDC - MC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | PAM | LPS | 3M-002 | 3M-003 | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IL-12/23p40 | % positive | 7.2 ± 0.7 | 9.7 ± 1.0 | n.s | 3.3 ± 0.4 | 5.2 ± 0.6 | n.s. | 21.9 ± 2.9 | 19.2 ± 2.1 | n.s. | 32.0 ± 3.5 | 26.6 ± 2.0 | n.s. |
| MFI | 814 ± 38 | 1682 ± 140 | <0.0001 | 882 ± 48 | 1794 ± 135 | <0.0001 | 2114 ± 169 | 2808 ± 191 | 0.003 | 3599 ± 291 | 3929 ± 240 | n.s. | |
| IL-6 | % positive | 21.6 ± 2.4 | 14.8 ± 1.8 | n.s. | 21.2 ± 1.5 | 12.1 ± 1.3 | <0.0003 | 17.8 ± 1.6 | 12.6 ± 2.2 | n.s. | 26.6 ± 1.6 | 17.4 ± 2.4 | 0.0036 |
| MFI | 598 ± 98 | 555 ± 102 | n.s. | 562 ± 90 | 511 ± 88 | n.s. | 557 ± 85 | 505 ± 101 | n.s. | 614 ± 92 | 519 ± 100 | n.s. | |
| TNF-α | % positive | 26.1 ± 3.3 | 13.9 ± 1.5 | n.s. | 30.7 ± 2.8 | 16.7 ± 2.1 | 0.0025 | 48.7 ± 3.9 | 46.4 ± 4.4 | n.s. | 63.8 ± 3.1 | 60.3 ± 4.1 | n.s. |
| MFI | 4487 ± 827 | 2975 ± 578 | 0.0091 | 4826 ± 942 | 3150 ± 639 | 0.0048 | 7275 ± 950 | 4784 ± 668 | 0.0005 | 10026 ± 1069 | 5734 ± 683 | <0.0001 | |
| TOTAL % positive | 34.1 ± 3.1 | 24.6 ± 2.0 | n.s | 37.2 ± 2.5 | 23.6 ± 2.3 | 0.0024 | 52.9 ± 3.6 | 50.1 ± 4.4 | n.s. | 65.8 ± 2.9 | 63.7 ± 3.9 | n.s. | |
| cDC - WB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | PAM | LPS | 3M-002 | 3M-003 | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IL-12/23p40 | % positive | 21.4 ± 2.6 | 11.7 ± 1.7 | 0.0053 | 35.3 ± 2.7 | 12.3 ± 1.8 | <0.0001 | 19.3 ± 2.5 | 16.9 ± 2.3 | n.s. | 67.3 ± 3.7 | 31.1 ± 3.8 | <0.0001 |
| MFI | 1799 ± 180 | 2582 ± 282 | n.s. | 2470 ± 215 | 2893 ± 282 | 2264 ± 212 | 3497 ± 306 | n.s. | 6827 ± 852 | 3985 ± 335 | n.s. | ||
| IL-6 | % positive | 21.7 ± 3.1 | 14.4 ± 2.4 | n.s. | 32.6 ± 2.7 | 17.5 ± 2.4 | 0.0003 | 5.4 ± 0.6 | 10.3 ± 1.6 | n.s. | 17.6 ± 2.4 | 15.7 ± 2.0 | n.s. |
| MFI | 456 ± 73 | 577 ± 112 | n.s. | 509 ± 72 | 591 ± 110 | 357 ± 49 | 621 ± 116 | n.s. | 412 ± 56 | 620 ± 112 | n.s. | ||
| TNF-α | % positive | 26.4 ± 3.1 | 12.7 ± 1.8 | 0.0002 | 52.1 ± 2.9 | 18.0 ± 2.3 | <0.0001 | 22.4 ± 2.6 | 21.4 ± 3.0 | n.s. | 73.8 ± 3.6 | 43.3 ± 4.9 | <0.0001 |
| MFI | 3515 ± 617 | 3685 ± 832 | n.s. | 4425 ± 585 | 3639 ± 727 | 3280 ± 457 | 3853 ± 683 | n.s. | 6151 ± 585 | 4176 ± 633 | n.s. | ||
| TOTAL % positive | 39.3 ± 3.5 | 24.0 ± 2.7 | 0.0031 | 61.1 ± 2.7 | 30.5 ± 3.0 | <0.0001 | 28.0 ± 2.7 | 28.5 ± 3.3 | n.s. | 76.3 ± 3.2 | 53.2 ± 4.7 | <0.0001 | |
| pDC - MC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | 3M-002 | 3M-003 | 3M-013 | CpG-A | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IFN-α | % positive | 3.6 ± 0.9 | 1.3 ± 0.3 | n.s. | 58.1 ± 2.2 | 13.5 ± 1.8 | <0.0003 | 32.3 ± 2.9 | 7.0 ± 1.0 | <0.0003 | 40.9 ± 3.6 | 9.8 ± 1.3 | <0.0003 |
| MFI | 2314 ± 1926 | 562 ± 92 | 0.0072 | 1784 ± 237 | 833 ± 46 | <0.0001 | 1509 ± 225 | 1089 ± 166 | 0.005 | 2978 ± 239 | 1661 ± 148 | <0.0001 | |
| IL-6 | % positive | 1.6 ± 0.3 | 1.5 ± 0.2 | n.s. | 3.3 ± 0.4 | 2.4 ± 0.3 | n.s. | 3.3 ± 0.3 | 1.4 ± 0.2 | <0.0003 | 2.3 ± 0.5 | 1.1 ± 0.2 | n.s. |
| MFI | 1537 ± 1277 | 219 ± 31 | n.s. | 330 ± 54 | 235 ± 23 | n.s. | 448 ± 170 | 226 ± 25 | n.s. | 233 ± 33 | 390 ± 127 | n.s. | |
| TNF-α | % positive | 6.7 ± 1.3 | 9.2 ± 1.7 | n.s. | 52.3 ± 2.7 | 26.2 ± 2.1 | <0.0003 | 54.8 ± 3.1 | 22.2 ± 2.1 | <0.0003 | 27.5 ± 2.8 | 9.2 ± 1.2 | <0.0003 |
| MFI | 1786 ± 381 | 1077 ± 171 | n.s. | 2379 ± 454 | 1188 ± 197 | 0.0002 | 2622 ± 498 | 1058 ± 206 | <0.0001 | 2326 ± 505 | 1008 ± 182 | <0.0001 | |
| TOTAL % positive | 10.2 ± 1.4 | 10.7 ± 1.6 | n.s. | 70.1 ± 1.7 | 32.2 ± 2.0 | <0.0003 | 60.1 ± 2.8 | 24.7 ± 2.2 | <0.0003 | 45.8 ± 3.7 | 15.5 ± 1.6 | <0.0003 | |
| pDC - WB | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Parameter measured | 3M-002 | 3M-003 | 3M-013 | CpG-A | ||||||||
| Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | Adult | Neonate | q-value | ||
| IFN-α | % positive | 1.4 ± 0.2 | 1.9 ± 0.3 | n.s. | 50 ± 2.9 | 19 ± 1.3 | <0.0001 | 15 ± 2.0 | 6.7 ± 0.6 | <0.0001 | 2.6 ± 0.6 | 3.6 ± 0.6 | n.s. |
| MFI | 2448 ± 1959 | 439 ± 144 | n.s. | 2156 ± 301 | 835 ± 73 | <0.0009 | 1359 ± 228 | 645 ± 69 | n.s. | 1372 ± 42 | 3057 ± 2057 | n.s. | |
| IL-6 | % positive | 1.4 ± 0.3 | 1.5 ± 0.3 | n.s. | 2.8 ± 0.6 | 3.0 ± 0.5 | n.s. | 2.6 ± 0.4 | 2.2 ± 0.4 | n.s. | 1.1 ± 0.2 | 1.3 ± 0.2 | n.s. |
| MFI | 223 ± 28 | 248 ± 55 | n.s. | 226 ± 24 | 195 ± 18 | n.s. | 210 ± 24 | 218 ± 27 | n.s. | 283 ± 75 | 793 ± 595 | n.s. | |
| TNF-α | % positive | 3.1 ± 0.5 | 3.0 ± 0.6 | n.s. | 51.1 ± 3.1 | 22.5 ± 2.1 | <0.0001 | 47.2 ± 2.9 | 20.7 ± 2.3 | <0.0001 | 3.3 ± 0.5 | 2.5 ± 0.5 | n.s. |
| MFI | 835 ± 136 | 977 ± 117 | n.s. | 1687 ± 259 | 1043 ± 121 | n.s. | 1603 ± 267 | 916 ± 121 | n.s. | 1012 ± 246 | 855 ± 133 | n.s. | |
| TOTAL % positive | 5.1 ± 0.5 | 5.2 ± 0.6 | n.s. | 57.2 ± 2.7 | 31.4 ± 1.9 | <0.0001 | 48.9 ± 2.7 | 24.7 ± 2.1 | <0.0001 | 5.5 ± 0.7 | 6.1 ± 0.9 | n.s. | |
Shown are the mean ± SD of percentage and the mean fluorescence intensity (MFI) of cells producing an individual cytokine, as well as the total percentage of cells producing any cytokine. The probability that differences are significant after correction for multiple comparisons is shown as q-values (see statistical methods for description).
n.s. = non-significant.
Context-dependent differences in cytokine production by neonatal vs. adult monocytes
We found no obvious difference between neonatal and adult MC samples in the total percentage of monocytes responding to any of the TLR ligands, nor in their degree of polyfunctionality (Figure 2; Table 1). However, neonatal monocytes in MC samples produced significantly more IL-12/23p40 per cell (i.e., the MFI was greater) than adult monocytes for each of the TLR ligands tested (Table 1). The fraction of neonatal monocytes in MC producing IL-6 and the MFI for IL-6 were consistently greater compared to adult monocytes, though these differences were not significant after correction for multiple comparisons. On the other hand, adult monocytes in MC produced significantly more TNF-α per cell in response to all TLR ligands tested.
Results for monocytes in WB were substantially different. The total percentage of cytokine-producing cells, the percentages producing IL-12/23p40 and TNF-α, and polyfunctionality were all lower for neonatal than adult monocytes in WB after TLR2, TLR4, and TLR7/8 stimulation (Figure 2). These differences resulted primarily from a substantial increase in the percentages of cytokine-producing adult monocytes and their polyfunctionality in response to these ligands in WB compared to MC. There were two exceptions to this general trend: the percentage of IL-6- producing neonatal and adult monocytes in WB was equivalent for all TLR ligands but LPS; responses to the TLR8 ligand 3M-002 were inhibited in WB compared to MC and were similar for adult and neonatal monocytes. The TLR9 ligand CpG-A did not induce a measurable response in either neonatal or adult monocytes (data not shown).
cDCs also exhibit context-dependent differences in cytokine production
The findings with cDCs to a large extent paralleled those obtained with monocytes. We detected no obvious difference between neonatal and adult MC samples in the total percentage of responding cDCs, in percentages producing individual cytokines and cytokine combinations, nor in their degree of polyfunctionality (Figure 3; Table 1) for each of the stimuli save LPS, to which a significantly larger fraction of adult than neonatal cDCs responded. The amounts (i.e., the MFI) of IL-12/23p40 produced by individual neonatal cDCs in MC were higher as compared to adults, whereas the TNF-α MFIs were lower. By contrast in WB samples, as observed for monocytes, the total percentage of responding cDCs, percentage producing IL-12/23p40 and TNF-α, as well as the degree of polyfunctionality were all significantly lower for neonatal cDCs with the same two exceptions: the percentage of IL-6-producing neonatal and adult monocytes in WB was equivalent for all TLR ligands but LPS and responses to the TLR8 ligand 3M-002 were inhibited in WB compared to MC and were similar for adult and neonatal monocytes. And as for monocytes, the TLR9 ligand CpG-A did not induce a measurable response in either neonatal or adult cDC (data not shown).
Neonatal pDCs are strikingly less responsive and less polyfunctional than adult pDCs
In contrast to the relative similarity of the responses of neonatal and adult monocytes and cDCs in MC samples, neonatal pDCs were consistently less responsive in MC as well as in WB. A substantial fraction of adult pDCs produced IFN-α, TNF-α or both of these cytokines, but these cells produced little or no IL-6 (Figure 4; Table 1) and IL-12/23p40 (data not shown), respectively, i.e. were at most bifunctional and not polyfunctional. By contrast, the total percentages of neonatal cytokine producing pDCs, the percentages producing IFN-α, TNF-α or both of these cytokines, the amounts of these cytokines produced per cell, and their polyfunctionality were all lower compared to adult pDCs in response to the TLR7 (3M-013), TLR7/8 (3M-003) and TLR9 (CpG-A) ligands. Responses to the TLR9 ligand were inhibited in WB compared to MC, and, as expected, there was little response by pDCs to the TLR8 ligand (3M-002).
Polyfunctionality may be a regulated function for IL-6 but not for other cytokines
Polyfunctionality by innate immune cells could reflect a qualitative difference in the response by specific cells to a TLR ligand or a quantitative difference in which cells that are more responsive are more likely to produce multiple cytokines. We reasoned that if polyfunctionality reported quantitative rather than qualitative differences, then polyfunctionality should show a positive correlation with the amount of cytokine(s) produced per cell (i.e., the MFI). When this relationship was evaluated for cells expressing one vs. two or more cytokines, we found that polyfunctional monocytes (Figure 5) and cDCs (Supplementary Figure 4) produced more TNF-α and IL-12/23p40 under most conditions tested. Similarly, polyfunctional pDCs produced more IFN-α and somewhat more TNF-α (Supplementary Figure 4). These relationships were apparent with adult and neonatal cells, although the magnitude of the differences in MFI between monofunctional and polyfunctional cells was generally stronger in the adult. Thus, for these cytokines, polyfunctionality and MFI appeared to function as parallel quantitative indices of cellular responsiveness. By contrast, there was little correlation between polyfunctionality and the MFI of IL-6 for any of the ligands or cell types in either adult or neonatal samples, except the neonatal IL-6 monocyte MC response to 3M-003. This finding suggests that IL-6 may be regulated in a qualitatively different manner than the other cytokines tested. Preliminary results suggest that the degree of polyfunctionality is influenced by soluble factors as well as by cell-cell interactions (Supplementary Figure 5).
Figure 5. The degree of polyfunctionality may be a specifically regulated function for some but not all cytokines.
MC or WB samples from 25 adults and 25 neonates were stimulated with the indicated TLR ligands (only results for the maximal concentration for each ligand are shown) for 6 hours, and expression of intracellular TNF-α, IL-6, and IL12/23p40 by monocytes was determined by multiparameter flow cytometry. The y-axis depicts the MFI (mean fluorescent intensity) for the indicated cytokine for monocytes that produced only the indicated cytokine (solid bar, monofunctional cells) or monocytes that produced the indicated cytokine plus any of the others (hatched bar, polyfunctional cells) with results for adults shown in red and neonates in blue.
Neonatal innate immune cells produced lower levels of Th1-supporting but higher levels of Th17-supporting and anti-inflammatory cytokines compared to adult cells
As an additional approach by which to address responses of neonatal and adult innate immune cells to TLR stimulation, we used multiplexed bead array-based detection of cytokines in 18 hr culture supernatants. This approach quantifies the total amount of cytokine secreted by all cells present in the culture, i.e., it provides a more global view of differences between neonatal and adult cells. It also allows the measurement of cytokines, such as the heterodimeric cytokines IL-12p70 and IL-23 that share a common p40 subunit, for which sufficiently sensitive and specific antibodies usable in flow cytometry were not available. To assess possible concentration-dependent differences between adult and neonatal cells, we used 3 concentrations of each TLR ligand known to yield low, medium and high responses based on our previous studies (13).
For cytokines assayed both by flow cytometry at 6 hr (Figure 2–4) and in culture supernatants at 18 hr (Figure 6–8), the results were congruent and consistent with the exception that unstimulated neonatal samples secreted low levels of certain cytokines (IL-6, IL-1b, IL-12p40, and IL-10) into the culture supernatant over the 18hr incubation. This higher level of cytokine production detected in culture supernatants of unstimulated neonatal samples by Luminex is possibly biologically relevant (21–23), and was therefore not subtracted from the stimulated samples but shown side by side. Of the cytokines detectable at higher levels in neonatal than adult unstimulated samples after 18hr culture, only IL-6 also appeared to be present at a higher MFI in unstimulated neonatal samples in the 6hr flow cytometric analysis (but not statistically significant). As our overall analysis however focused on the comparison of the neonatal to the adult response after TLR-specific stimulation, we positioned the unstimulated flow cytometric samples as a biological negative control; this has been identified as the most appropriate approach for flow cytometric analysis of stimulation experiments (24).
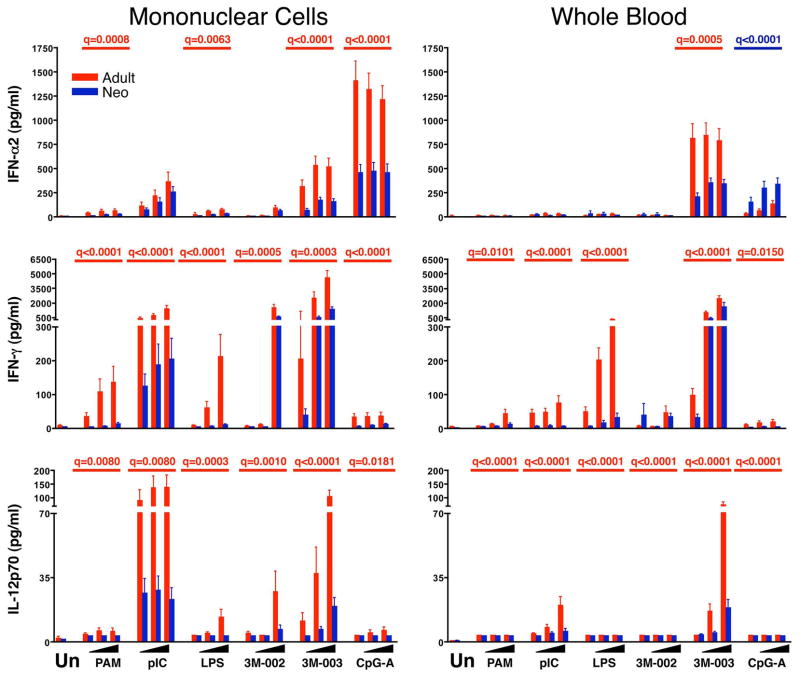
Figure 6. Diminished production of type 1 cytokines by neonatal cells.
Results are the mean ± SE concentrations of IFN-α2 (top), IFN-γ (middle), and IL-12p70 (bottom) in 18 hr culture supernatant for MC (left panels) or WB (right panels), comparing adult (red bars and q values) to neonatal (blue bars and q values) samples (n = 25 each). Cultures were stimulated with nothing (unstimulated, Un) or with increasing concentrations from left to right (indicated by the black triangles) of PAM3CSK4 (TLR2/6), pI:C (TLR3), LPS (TLR4), 3M-002 (TLR8), 3M-003 (TLR7/8), or CpGA (TLR9). The probability that differences are significant after correction for multiple comparisons is shown as q-values (see statistical methods for description) but only if there was a significant difference between neonatal and adult samples.
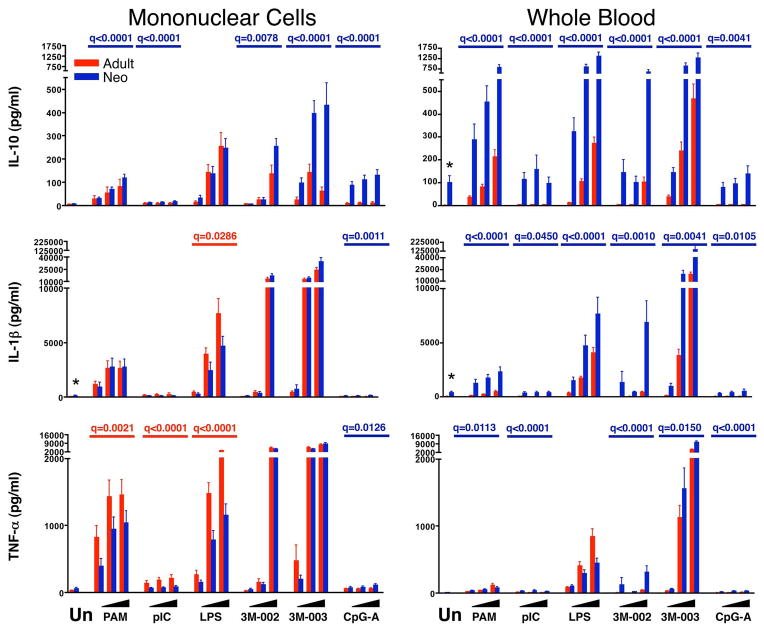
Figure 8. Neonatal WB makes more IL-10 as compared to adult.
Results are the mean ± SE concentrations of TNF-α (top), IL-1β (middle), and IL-10 (bottom) in 18 hr culture supernatant for MC (left panels) or WB (right panels), comparing adult (red bars and q values) to neonatal (blue bars and q values) samples (n = 25 each). Cultures were stimulated with nothing (unstimulated, Un) or with increasing concentrations from left to right (indicated by the black triangles) of PAM3CSK4 (TLR2/6), pI:C (TLR3), LPS (TLR4), 3M-002 (TLR8), 3M-003 (TLR7/8), or CpGA (TLR9). The probability that differences are significant after correction for multiple comparisons is shown as q-values (see statistical methods for description) but only if there was a significant difference between neonatal and adult samples.
We found that monocytes clearly were the dominant source of the cytokines produced after stimulation, with the exception of IFN-α, which was produced primarily by pDCs. Adult MC and WB secreted more TNF-α, paralleling greater TNF-α MFIs for adult vs. neonatal monocytes, particularly for the TLR2/6 and TLR4 ligands (Figure 8). Neonatal MC and, in particular, WB secreted as much or more IL-6 than adult samples, paralleling the consistent trend for greater IL-6 MFIs by neonatal vs. adult monocytes (and cDCs) (Figure 7). Adult MC and WB produced more IFN-α in response to the TLR7/8 ligand 3M-003, paralleling greater IFN-α production by adult pDCs; this was also the case for IFN-α production in response to CpG-A for MC, though not for WB, in which IFN-α production by adult but not by neonatal MC was suppressed compared to adult MC samples. IFN-α production in response to pI:C was suppressed equally in neonatal and adult WB as compared to the respective MC samples (Figure 6). The only cytokine where supernatant assays did not parallel flow cytometric MFIs was IL-12/23p40, where the significantly greater MFIs of neonatal monocytes and cDCs observed by flow cytometry at 6 hr were not consistently reflected by a higher abundance of IL-12/23p40 in 18hr culture supernatants (Figure 7).
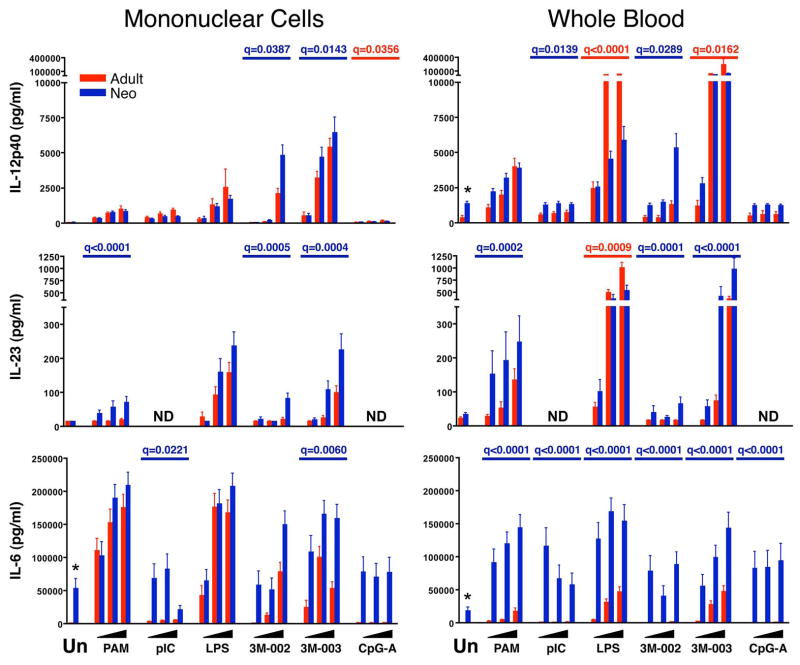
Figure 7. Relatively greater production of Th17-inducing cytokines by neonatal compared to adult cells.
Results are the mean ± SE concentrations of IL-12p40 (top), IL-23 (middle), and IL-6 (bottom) in 18 hr culture supernatant for MC (left panels) or WB (right panels), comparing adult (red bars and q values) to neonatal (blue bars and q values) samples (n = 25 each). Cultures were stimulated with nothing (unstimulated, Un) or with increasing concentrations from left to right (indicated by the black triangles) of PAM3CSK4 (TLR2/6), pI:C (TLR3), LPS (TLR4), 3M-002 (TLR8), 3M-003 (TLR7/8), or CpGA (TLR9). The probability that differences are significant after correction for multiple comparisons is shown as q-values (see statistical methods for description) but only if there was a significant difference between neonatal and adult samples. ND = Not determined.
In addition to these findings, the supernatant assays also revealed a consistent qualitative difference between adult and neonatal cells in the production of cytokines that promote Th1 vs. Th17 type CD4 T cell responses. Thus, neonatal MC and WB cells produced significantly less IFN-α, IL-12p70 and IFN-γ, which promote Th1 responses, than adult samples in nearly all contexts (Figure 6). By contrast, neonatal MC and particularly WB produced more IL-23, IL-6, and IL-1β than adult samples; these cytokines are known to promote Th17 responses (Figures 7 and 8). The only exceptions were the somewhat greater production of IL-23 by adult than neonatal WB in response to LPS and of IFN-α by neonatal compared to adult WB in response to CpG-A. We also found that neonatal as compared to adult samples consistently produced more of the immunoregulatory and anti-inflammatory cytokine IL-10 (Figure 8).
Discussion
The underlying mechanisms that lead to the higher susceptibility of neonates to infection, as well as their often suboptimal immune response to immunization have not been fully delineated. Peculiarities in the neonatal innate immune response to pathogen associated molecular patterns (PAMPs) have been postulated to be at least partly responsible, and differences between the neonatal and adult response to stimulation via TLRs have been identified previously. These previous studies, however, did not provide a comprehensive overview of the complex reactions involved, as they focused on only few stimuli, target cells, or functional readouts. We here provide the most comprehensive comparison yet of the innate immune response to TLR stimulation by monocytes, cDCs and pDCs from neonates and adults in MC and in WB, through analysis of both of the global response of these samples (cytokines in the culture supernatant) and the responses of individual cells (intracellular cytokine expression). We found that innate immune cells from neonates had a consistently and significantly reduced capacity compared to adults to produce IL-12p70, IFN-α and IFN-γ, a more modest and less consistently reduced capacity to produce TNF-α, a similar or even greater capacity to produce IL-6, IL-23 and IL-1β, and a strikingly greater capacity to produce IL-10. On the single-cell level, our study provides novel insights demonstrating that neonatal innate immune cells in general were less polyfunctional, in that they less often produced 2 or more of the tested cytokines simultaneously compared to adult cells. Most of the differences we detected between neonate and adult were more pronounced when assays were performed in WB as compared to MC, indicating the presence of regulatory factors contained in adult and neonatal WB. Together our data support the notion that the neonatal innate immune system is not in general less able than their adult counterpart to respond to TLR stimulation, but rather has a qualitatively different response.
The most consistent finding in the published literature analyzing the innate immune response in the human newborn is reduced production of IL-12p70, type I IFNs, and IFN-γ (reviewed in (2, 4)). We also detected a profoundly reduced secretion by neonatal cells of IL-12p70 in response to TLR stimulation. Unfortunately, stable and sufficiently sensitive antibodies to IL-12p70 or p35 are not available, precluding our attempt to demonstrate the responsible cell type(s) on the single cell level. The lower IL-12p70 production in neonatal samples likely also was responsible for the strikingly reduced IFN-γ production we observed in neonatal MC and WB culture supernatants. While DCs or monocytes themselves are not known to produce IFN-γ, NK cells and memory CD4 and CD8 T cells present in MC or WB cultures do produce IFN-γ in response to IL-12p70 and type I IFNs produced by DCs and monocytes (2). We also confirmed the previously reported reduction in IFN-α production in neonatal MC cultures in response to CpGA (11). However, we were unable to confirm the previously described reduction in IFN-α production in response to pI:C stimulation in either MC or WB cultures (10). It is possible that this discrepancy reflects differences in assay specificity, because the Luminex assay we employed detects IFN-α2B only, while the ELISA used in the other study reportedly detects IFN-α2A, -2B, and –2C. Our single-cell flow cytometric analysis, performed with an antibody that detects IFN-α2A and 2B, and to a lesser degree IFN-α C, D, G, H, I, K, and omega, failed to detect IFN-α in cDC in response to pI:C (data not shown); this is likely due to the overall low level of IFN-α production in cDC as compared to pDC (25). Our flow cytometric analysis however confirmed the globally much reduced IFN-α production by neonatal as compared to adult pDCs to all stimuli tested in MC or WB. Given the many subtypes of type 1 IFN and their cell-type specific expression patter (25), a much expanded and more detailed analysis of the of changes in type 1 IFN expression early in life is clearly necessary.
Reduced IL-12p70 by neonatal innate immune cells appears to result from a selective decrease in production of IL-12p35 but not IL-12/23p40 (10, 26–28). Our supernatant results are consistent with these findings, which we extended by demonstrating that neonatal monocytes and cDCs in MC and WB consistently produced as much or more IL-12/23p40 on a per cell basis as their adult counterparts. Thus, even though in some contexts a lower percentage of neonatal cells made IL-12/23p40, the production of greater amounts on a per cell basis was reflected by comparable amounts in MC and WB culture supernatants. Because IL-12p40 can also combine with p19 to form the heterodimer IL-23 (28–30), we were not surprised to find a significantly elevated concentration of IL-23 in the supernatant of neonatal cultures stimulated with TLR2, TLR8, and TLR7/8 ligands in both MC and WB samples. Thus, the innate response early in life appears to be directed away from IL-12p70 towards IL-23 dominance. We confirmed previous reports of higher IL-6 production by neonatal as compared to adult WB samples (31, 32), but were surprised to find that this difference was not observed in parallel MC cultures. This argues for an IL-6-suppressive factor in adult WB, rather than an inherently higher neonatal capacity to produce IL-6. A similar trend was found for IL-1β, with greater production by neonatal WB but not MC samples. Importantly, IL-1β, IL-6 and IL-23 together are key differentiation signals for Th17 lineage T cells, suggesting that at birth the innate immune response to TLR stimulation favors Th17 over Th1 CD4 T cell development (29, 30).
The relatively greater production of the pro-inflammatory cytokines IL-1β and IL-6 by neonatal innate immune cells may be offset by their generally reduced production of TNF-α and greater production of the anti-inflammatory and immunomodulatory cytokine IL-10. Interestingly, the greater production of IL-10 by neonatal vs. adult cells appeared to parallel that of IL-6 and IL-23, and may in fact reflect secondary co-induction of IL-10 by IL-6 and/or IL-23 (31, 33).
Polychromatic (i.e., > 5 colors) flow cytometry offers the ability to identify how many different cytokines a single cell makes, i.e. its polyfunctionality (34). This parameter has been investigated intensely in T cells, but prior to our studies, polyfunctionality in the innate immune system had to our knowledge never been specifically addressed. Our data indicate that WB strongly supported the capacity of adult but not neonatal monocytes or cDCs to make more than 2 cytokines, and that the degree of polyfunctionality was TLR ligand-dependent. The factors contained in WB that led to such ligand-specific, fine-tuned regulation of polyfunctionality are currently not known. Both soluble factors contained in plasma as well as cell-cell interactions appear to be responsible (see Supplementary Figure 5). Contrary to monocytes and cDCs, neonatal as compared to adult pDCs displayed a globally reduced degree of polyfunctionality. We also found that an increase in polyfunctionality for any of the cell types paralleled an increase in MFI for TNF-α, IL-12/23p40 and IFN-α but not for IL-6, suggesting that regulatory mechanisms governing IL-6 are potentially distinct from those governing these other cytokines. The functional relevance of our findings regarding innate immune cell polyfunctionality is currently unclear, but for T cells, polyfunctionality rather than production of any one specific cytokine correlates best with protection from infection (35–38).
By comparing responses of neonatal and adult MC, which were cultured in medium containing pooled 10% human AB serum, to responses in neonatal or adult WB, which was cultured in medium containing autologous plasma, our studies revealed both extrinsic and cell intrinsic factors responsible for the differences between adult and neonatal innate immune function. For example, the reduced production of Th1-promoting cytokines, including IL-12p70, IFN-α, and IFN-γ, and relatively greater production of Th17-promoting cytokines, including IL-23, IL-6 and IL-1β in the neonate, were already evident in MC, indicating a cell intrinsic bias; however, these trends were somewhat amplified in WB, indicating additional contributions of cell extrinsic factors. Similarly, the greater production of IL-10 by neonatal samples was evident in MC but amplified in WB. However, neonatal pDCs were less responsive overall than their adult counterparts both in MC and WB samples. Our findings confirm previous reports indicating that both extrinsic soluble factors as well as extrinsic cell-cell interactions can influence the response to TLR stimulation (31, 39). Most consistent with our findings are previous reports of significantly higher serum concentrations for adenosine in newborn as compared to adult samples; these differences have been shown to directly relate to lower TNF-α and IL-12 but enhanced IL-6, IL-10 and IL-23 production (3, 40). For the TLR4 specific response soluble extrinsic factors leading to different response profiles between neonatal and adult samples may also relate to LPS-binding protein and soluble CD14, both of which are higher in adult than in neonatal samples (2, 3). Together, these results indicate that cell intrinsic and extrinsic differences can differentially affect the responses of neonatal vs. adult monocytes and cDCs, but factors responsible for differences in responses to TLR ligands by neonatal compared to adult pDCs appear to be predominantly cell intrinsic.
We found that neonatal pDC responses were globally impaired, suggesting a proximal defect in TLR signaling, although this may be compounded further by impaired IRF-7 nuclear translocation previously reported by others (41). In contrast to pDCs, cell intrinsic response differences between adult and neonatal monocytes and cDCs were largely qualitative, suggesting that specific regulatory mechanisms further downstream are involved. Consistent with this notion, diminished neonatal IL-12p35 production appears to result from a defect in IRF-3 binding to and remodeling of the IL-12p35 promoter, while more proximal aspects of signaling resulting in IRF-3 translocation from the cytoplasm to the nucleus appear to be intact (42). We currently are in the process of determining if differences in these and other molecular processes account for some of the novel differences between neonate and adult samples identified in our study, such as the degree of polyfunctionality.
In summary, our data show that the neonatal innate immune response does not simply suffer from quantitative deficiencies as compared to the adult, but rather is characterized by a response pattern that is qualitatively different. The foremost example of this is the reduced ability of neonatal vs. adult innate immune cells to produce Th1-supporting cytokines and a shift towards an enhanced capacity to support Th17-type immune responses. Th17 cells secrete cytokines that induce the production of antimicrobial peptides by epithelial cells and mobilize phagocytes to promote resistance against extracellular bacterial and fungal pathogens, particularly at body surfaces, while Th1 cells are predominantly involved in systemic defence against intracellular pathogens (43, 44). Thus our findings suggest that the neonatal innate immune response is more focused on promoting defence against extracellular pathogens, which may pose the most immediate threat to the neonate upon entering the extrauterine environment, than defense against intracellular pathogens. It will be essential to decipher the cell intrinsic and extrinsic factors underlying these differences in great detail in order to gain the understanding necessary to guide optimal interventions (e.g. vaccines) aimed at protecting this most vulnerable population.
Supplementary Material
Footnotes
This work was supported in part by: 1) National Institute of Allergy and Infectious Diseases, NIH; Grant number: N01 AI50023; 2) AllerGen NCE; Grant numbers: 07-A1A, 07-B2B; 3) the Michael Smith Foundation for Health Research. TRK is supported in part by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, and by a CIHR Training Grant in Canadian Child Health Clinician Scientist Program, in partnership with SickKids Foundation, Child & Family Research Institute (BC), Women & Children’s Health Research Institute (Alberta), Manitoba Institute of Child Health.
References
- 1.Klein JO, Baker CJ, Remington JS, Wilson CB. Current Concepts of Infections of the Fetus and Newborn Infant. In: Klein JO, Remington JS, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Elsevier Saunders; Philiadelphia: 2006. pp. 1–24. [Google Scholar]
- 2.Lewis DB, Wilson CB. Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Remington Jack JOKS, Wilson Christopher B, Baker Carol J., editors. Infectious Diseases of the Fetus and Newborn Infant. 6. Elsevier Saunders; Philiadelphia: 2006. pp. 25–138. [Google Scholar]
- 3.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 4.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 5.Joyner JL, Augustine NH, Taylor KA, La Pine TR, Hill HR. Effects of group B streptococci on cord and adult mononuclear cell interleukin-12 and interferon-gamma mRNA accumulation and protein secretion. J Infect Dis. 2000;182:974–977. doi: 10.1086/315796. [DOI] [PubMed] [Google Scholar]
- 6.La Pine TR, Joyner JL, Augustine NH, Kwak SD, Hill HR. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B Streptococci. Pediatr Res. 2003;54:276–281. doi: 10.1203/01.PDR.0000072515.10652.87. [DOI] [PubMed] [Google Scholar]
- 7.Upham JW, Lee PT, Holt BJ, Heaton T, Prescott SL, Sharp MJ, Sly PD, Holt PG. Development of interleukin-12-producing capacity throughout childhood. Infect Immun. 2002;70:6583–6588. doi: 10.1128/IAI.70.12.6583-6588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SM, Suen Y, Chang L, Bruner V, Qian J, Indes J, Knoppel E, van de Ven C, Cairo MS. Decreased interleukin-12 (IL-12) from activated cord versus adult peripheral blood mononuclear cells and upregulation of interferon-gamma, natural killer, and lymphokine-activated killer activity by IL-12 in cord blood mononuclear cells. Blood. 1996;88:945–954. [PubMed] [Google Scholar]
- 9.Drohan L, Harding JJ, Holm B, Cordoba-Tongson E, Dekker CL, Holmes T, Maecker H, Mellins ED. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum Immunol. 2004;65:1356–1369. doi: 10.1016/j.humimm.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 10.De Wit D, Tonon S, Olislagers V, Goriely S, Boutriaux M, Goldman M, Willems F. Impaired responses to toll-like receptor 4 and toll-like receptor 3 ligands in human cord blood. J Autoimmun. 2003;21:277–281. doi: 10.1016/j.jaut.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, Willems F. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood. 2004;103:1030–1032. doi: 10.1182/blood-2003-04-1216. [DOI] [PubMed] [Google Scholar]
- 12.Seghaye MC, Heyl W, Grabitz RG, Schumacher K, von Bernuth G, Rath W, Duchateau J. The production of pro- and anti-inflammatory cytokines in neonates assessed by stimulated whole cord blood culture and by plasma levels at birth. Biol Neonate. 1998;73:220–227. doi: 10.1159/000013980. [DOI] [PubMed] [Google Scholar]
- 13.Jansen K, Blimkie D, Furlong J, Hajjar A, Rein-Weston A, Crabtree J, Reikie B, Wilson C, Kollmann T. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods. 2008;336:183–192. doi: 10.1016/j.jim.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JA, Spidlen J, Boyce K, Cai J, Crosbie N, Dalphin M, Furlong J, Gasparetto M, Goldberg M, Goralczyk EM, Hyun B, Jansen K, Kollmann T, Kong M, Leif R, McWeeney S, Moloshok TD, Moore W, Nolan G, Nolan J, Nikolich-Zugich J, Parrish D, Purcell B, Qian Y, Selvaraj B, Smith C, Tchuvatkina O, Wertheimer A, Wilkinson P, Wilson C, Wood J, Zigon R, Scheuermann RH, Brinkman RR. MIFlowCyt: the minimum information about a Flow Cytometry Experiment. Cytometry A. 2008;73:926–930. doi: 10.1002/cyto.a.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay P, Roederer M. Quality assurance for polychromatic flow cytometry. Nat Protoc. 2006;1:1522–1530. doi: 10.1038/nprot.2006.250. [DOI] [PubMed] [Google Scholar]
- 16.Maecker HT, Rinfret A, D’Souza P, Darden J, Roig E, Landry C, Hayes P, Birungi J, Anzala O, Garcia M, Harari A, Frank I, Baydo R, Baker M, Holbrook J, Ottinger J, Lamoreaux L, Epling CL, Sinclair E, Suni MA, Punt K, Calarota S, El-Bahi S, Alter G, Maila H, Kuta E, Cox J, Gray C, Altfeld M, Nougarede N, Boyer J, Tussey L, Tobery T, Bredt B, Roederer M, Koup R, Maino VC, Weinhold K, Pantaleo G, Gilmour J, Horton H, Sekaly RP. Standardization of cytokine flow cytometry assays. BMC Immunol. 2005;6:13. doi: 10.1186/1471-2172-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storey JD, Taylor JE, Siegmund D. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach. J R Statist Soc B. 2004;66:187–205. [Google Scholar]
- 18.Team, R. D. C. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2007. [Google Scholar]
- 19.Miller RG. Simultaneous Statistical Inference. Springer Verlag; New York: 1981. [Google Scholar]
- 20.Aitchison J. The Statistical Analysis of Compositional Data. 1986. [Google Scholar]
- 21.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchini G, Berggren V, Djilali-Merzoug R, Hansson LO. The birth process initiates an acute phase reaction in the fetus-newborn infant. Acta Paediatr. 2000;89:1082–1086. doi: 10.1080/713794557. [DOI] [PubMed] [Google Scholar]
- 23.Schultz C, Rott C, Temming P, Schlenke P, Moller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–322. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Maecker HT, Trotter J. Flow cytometry controls, instrument setup, and the determination of positivity. Cytometry A. 2006;69:1037–1042. doi: 10.1002/cyto.a.20333. [DOI] [PubMed] [Google Scholar]
- 25.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 26.Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 27.Goriely S, Van Lint C, Dadkhah R, Libin M, De Wit D, Demonte D, Willems F, Goldman M. A defect in nucleosome remodeling prevents IL-12(p35) gene transcription in neonatal dendritic cells. J Exp Med. 2004;199:1011–1016. doi: 10.1084/jem.20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. 2006;36:21–26. doi: 10.1002/eji.200535467. [DOI] [PubMed] [Google Scholar]
- 29.Goriely S, Goldman M. Interleukin-12 family members and the balance between rejection and tolerance. Curr Opin Organ Transplant. 2008;13:4–9. doi: 10.1097/MOT.0b013e3282f406c4. [DOI] [PubMed] [Google Scholar]
- 30.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol. 2008;8:81–86. doi: 10.1038/nri2225. [DOI] [PubMed] [Google Scholar]
- 31.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 32.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 34.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 35.Nebbia G, Mattes FM, Smith C, Hainsworth E, Kopycinski J, Burroughs A, Griffiths PD, Klenerman P, Emery VC. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant. 2008;8:2590–2599. doi: 10.1111/j.1600-6143.2008.02425.x. [DOI] [PubMed] [Google Scholar]
- 36.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun Y, Santra S, Schmitz JE, Roederer M, Letvin NL. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J Virol. 2008;82:8812–8819. doi: 10.1128/JVI.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciuffreda D, Comte D, Cavassini M, Giostra E, Buhler L, Perruchoud M, Heim MH, Battegay M, Genne D, Mulhaupt B, Malinverni R, Oneta C, Bernasconi E, Monnat M, Cerny A, Chuard C, Borovicka J, Mentha G, Pascual M, Gonvers JJ, Pantaleo G, Dutoit V. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 39.Schakel K, von Kietzell M, Hansel A, Ebling A, Schulze L, Haase M, Semmler C, Sarfati M, Barclay AN, Randolph GJ, Meurer M, Rieber EP. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–777. doi: 10.1016/j.immuni.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Danis B, George TC, Goriely S, Dutta B, Renneson J, Gatto L, Fitzgerald-Bocarsly P, Marchant A, Goldman M, Willems F, De Wit D. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38:507–517. doi: 10.1002/eji.200737760. [DOI] [PubMed] [Google Scholar]
- 42.Aksoy E, Albarani V, Nguyen M, Laes JF, Ruelle JL, De Wit D, Willems F, Goldman M, Goriely S. Interferon regulatory factor 3-dependent responses to lipopolysaccharide are selectively blunted in cord blood cells. Blood. 2007;109:2887–2893. doi: 10.1182/blood-2006-06-027862. [DOI] [PubMed] [Google Scholar]
- 43.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson CB, Kollmann TR. Induction of antigen-specific immunity in human neonates and infants. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:183–195. doi: 10.1159/000113493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.