Nonstructured Abstract
Leptin is an adipocyte-derived hormone and cytokine that regulates energy balance through a wide range of functions, including several important to cardiovascular health. Increased circulating leptin, a marker of leptin resistance, is common in obesity and independently associated with insulin resistance and cardiovascular disease (CVD) in humans. Mechanisms of leptin resistance include genetic mutation, leptin self regulation, limited tissue access and cellular or circulating molecular regulation. Evidence suggests that central leptin resistance causes obesity and that obesity-induced leptin resistance injures numerous peripheral tissues, including liver, pancreas, platelets, vasculature, and myocardium. This metabolic- and inflammatory-mediated injury may result from either resistance to leptin’s action in selective tissues, or excess leptin action from adiposity associated hyperleptinemia. In this sense, the term “leptin resistance” encompasses a complex pathophysiological phenomenon. The leptin axis has functional interactions with elements of metabolism, such as insulin, and inflammation, including mediators of innate immunity such as interleukin-6. Leptin is even purported to physically interact with C-reactive protein (CRP), resulting in leptin resistance, which is particularly intriguing given CRP’s well-studied relationship to CVD. Given that plasma levels of leptin and inflammatory markers are correlated and also predict cardiovascular risk, it is conceivable that part of this risk may be mediated through leptin-resistance related insulin resistance, chronic inflammation, type II diabetes, hypertension, atherothrombosis and myocardial injury. Leptin resistance and its interactions with metabolic and inflammatory factors, therefore, represent potential novel diagnostic and therapeutic targets in obesity-related cardiovascular disease.
Keywords: obesity, leptin resistance, inflammation, atherosclerosis, cardiovascular disease
Introduction
Since its discovery over a decade ago, leptin has been established as a key regulator of energy balance (1,2). Increased circulating leptin, a marker of leptin resistance, is common in obesity and independently associated with insulin resistance (3) and cardiovascular disease (CVD) (4–7) in humans. These associations may reflect the marked metabolic dysregulation that occurs in leptin resistance due to leptin’s key homeostatic physiological functions. Inflammation compounds these metabolic disturbances since leptin regulates components of innate and adaptive immunity, including T lymphocytes and monocytes (8). Leptin also has structural and functional resemblance to pro-inflammatory cytokines such as interleukin-6 (IL-6) (8) and may modulate C-reactive protein (CRP) (9). Therefore, it is conceivable that the convergence of increased levels of leptin and inflammatory markers (10) in CVD has a functional basis rather than mere association. The intent of this review is to integrate knowledge on leptin, leptin resistance, metabolism and inflammation to provide a cohesive clinical perspective regarding their interactions in obesity-related CVD. To this end, we discuss fundamentals of leptin, the concept and mechanisms of leptin resistance, as well as potential pathways from leptin resistance to CVD.
Fundamentals of Leptin
Ob/ob Mice and the Discovery of Leptin
In 1950, Ingalls et al. (11) described a new mutant strain of obese mice (ob/ob) which are characterized by severe obesity from increased energy intake (hyperphagia) and decreased energy expenditure (reduced metabolic rate, thermogenesis and physical activity). In 1973, Coleman (12) reported weight normalization in ob/ob mice when their circulation was connected to wild-type mice, suggesting ob/ob mice were deficient in a circulating factor involved in energy balance. Employing positional cloning two decades later, Friedman and colleagues (13) at Rockefeller University isolated the ob gene coding for this circulating factor. Shortly thereafter, recombinant ob gene product was administered to ob/ob mice, correcting their obesity (14). The ob gene product was subsequently named leptin, after the Greek, leptos, meaning ‘thin.’ Leptin’s discovery stimulated considerable research into the hormone’s biology, physiometabolic function and impact in human disease.
Leptin: A Pleiotropic Hormone and Cytokine
Leptin is primarily expressed in adipocytes and numerous human and animal studies (15–18) have shown that leptin levels increase with adiposity, presumably to inform the brain regarding the quantity of stored fat. Leptin also has a structural and functional relation to pro-inflammatory cytokines, such as IL-6 (19), reinforcing its classification as an “adipocytokine” (20). These observations, coupled with the wide expression of leptin and/or its receptor in peripheral tissues, including monocytes and lymphocytes, vascular tissue, pancreas, skeletal muscle and myocardium (21), suggest that leptin is pleiotropic in action and a pivotal link in obesity-related disease. Indeed, leptin is involved in several processes relevant to CVD, including insulin signaling, immunity, vascular function and arterial pressure regulation.
Leptin Receptor and Signaling
Leptin signals by engaging an IL-6 type glycoprotein 130 cytokine receptor, encoded by the diabetes (db) gene (22,23). Six leptin receptor isoforms are known (Ob-Ra–f) (24), varying in length, location and functionality. They share identical extracellular ligand-binding domains (second CK-F3) , but differ in their intracellular domains (24). Most is known about Ob-Rb (also known in humans as Ob-RL; L=long), which is highly expressed in the hypothalamus and is the only isoform that activates the janus kinase signal transduction and translation (JAK/STAT) system. Ob-Rb and other isoforms may signal via mitogen activated protein kinases (MAPK), phosphatidylinositol 3-kinase, and nitric oxide pathways. Ob-Ra is widely distributed in peripheral tissues, shows signaling capability, and is thought to transport leptin across the blood-brain barrier (25). Some isoforms may function in leptin clearance (Ob-Rc, Rd) (24) or buffering (Ob-Re; also known as soluble leptin receptor) (26).
Leptin Resistance
Concept
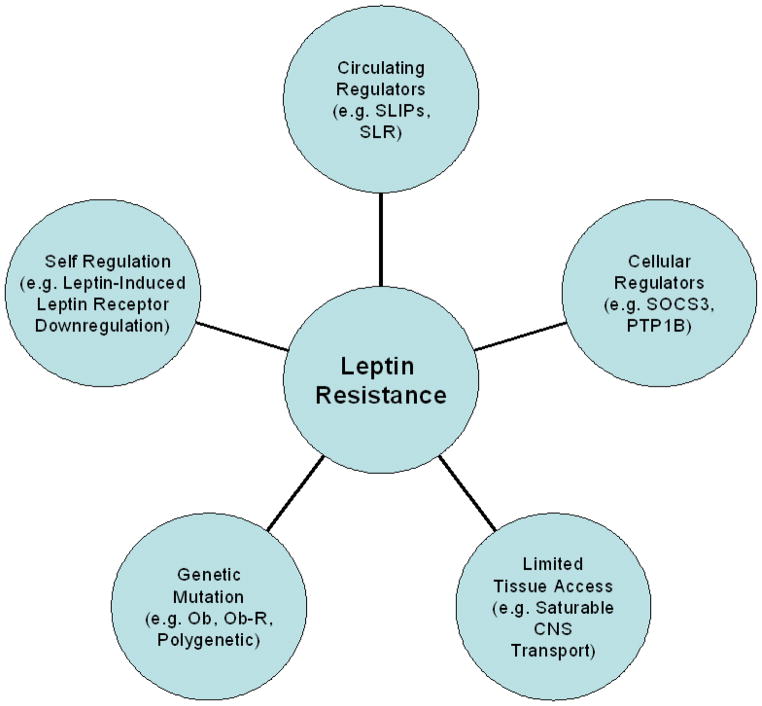
In the context of obesity and disease, leptin resistance began as the idea that the body’s biomolecular milieu decreases overall sensitivity to leptin action such that normal or, classically, elevated levels produce an inadequate response (relative leptin deficiency). This concept (27) is reinforced by the observation that the majority of obese individuals are not leptin deficient, but actually have elevated serum leptin concentrations (28). The leptin resistance theory gained further credence when a randomized, placebo controlled, dose-escalation trial of recombinant leptin was disappointing in producing weight loss in obese adults (with 20–30 times normal physiologic concentrations of leptin necessary to produce significant weight reduction) (29). It is not clear whether high endogenous leptin levels are appropriately high in the setting of increased adiposity and central resistance to leptin action. However, it is clear that these levels, even with the addition of large amounts of exogenous leptin, are not signaling sufficiently to maintain a healthy weight. Thus, in total, these observations imply that the majority of obese patients operate on a resistant, or flat, leptin dose-response curve, at least for weight regulation. This phenomenon of leptin resistance may have several possible underlying mechanisms (Fig. 1).
Figure 1. Mechanisms of leptin resistance.
CNS = central nervous system; Ob = leptin gene; Ob-R = leptin receptor gene; PTP1B = protein tyrosine phosphatase 1B; SLR = soluble leptin receptor; SLIPs = serum leptin-interacting proteins; SOCS3 = suppressor-of cytokine-signaling-3.
Mechanisms
Genetic Mutation
Leptin resistance can be inherited, albeit not commonly. According to the laws of feedback signaling, an ob gene mutation producing leptin that is secreted, but ineffective at signaling, could lead to hyperleptinemia and leptin resistance. Similar results could be obtained through leptin receptor mutation. In fact, diabetic (db/db) mice and Zucker fatty (fa/fa) rats have dysfunctional leptin receptors, causing marked hyperleptinemia and leptin resistance (30). While cases are seen in humans, such mutations are uncommon in the typical obese population (31). Thus, Mendelian inheritance patterns in leptin or its receptor are not major players in leptin resistance in the general population. However, polygenetic inheritance patterns in other gene products exerting influence on the leptin axis may still contribute significantly to generational transmission of genetic predisposition to leptin resistance.
Self Regulation
Like other biological signaling pathways, leptin appears to regulate its own receptor and signaling and receptor downregulation may promote pathological leptin resistance (32). Reduced hypothalamic leptin receptor expression and leptin signaling are seen in rodent models of age-related (33) and diet-induced (34) obesity. The reduction appears to be a direct byproduct of increased central leptin. This is supported by rodent models in which chronically elevated leptin, due to transgene overexpression, decreased hypothalamic leptin receptor expression and protein levels, impairing leptin signaling (35). Consistent with this, increasing central leptin desensitizes its physiological responses over time (36) rendering lean rodents more prone to diet-induced obesity (37). Hence, obesity promotes hyperleptinemia, which in turn self promotes leptin resistance and further obesity, making leptin resistance both a consequence and cause of obesity.
Limited Tissue Access
Resistance to leptin’s action may occur via limited tissue access, such as at the blood-brain barrier. While debate over leptin’s exact port and route of entry into the central nervous system continues, the current thought is that leptin entry is via Ob-Ra as a saturable, unidirectional system, located in the vascular endothelium and choroid plexus epithelium of the blood-brain barrier (38,39). Saturation in this transport mechanism could lead to leptin resistance. Perhaps this is why obese hyperleptinemic mice (40) and humans (41) have a decreased cerebrospinal fluid to serum leptin ratio. Indeed, leptin administration into the cerebrospinal fluid of hyperleptinemic obese mice resulted in short-term weight loss (40). However, in the long-term, limiting leptin’s brain access could be protective in the sense that it could prevent high central leptin levels and thus receptor downregulation and impaired signaling. The blood-brain-barrier appears to be a site for leptin regulation and resistance, though such theories demand further testing.
Molecular
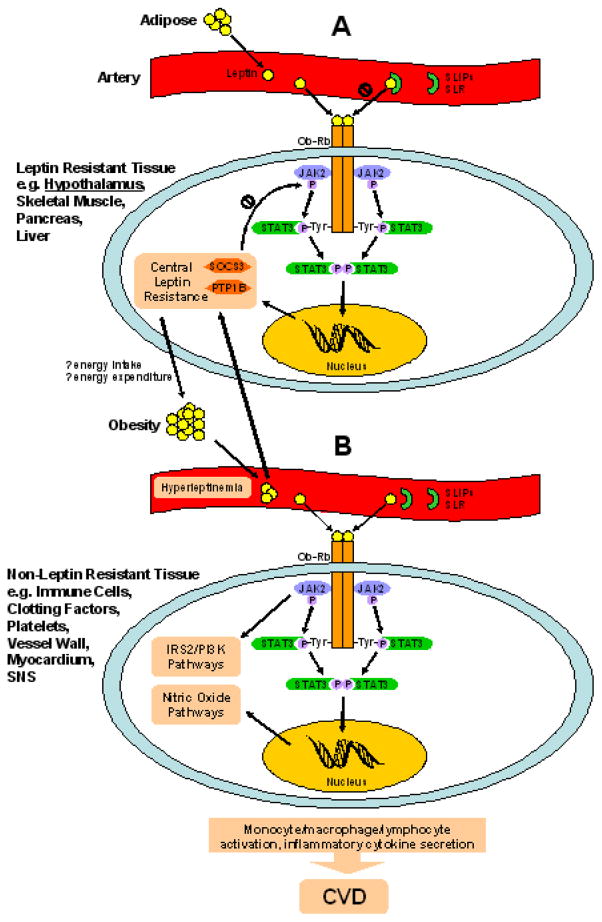
Cellular and circulating molecules can inhibit leptin to cause resistance (Fig. 2).
Figure 2. Theoretical cellular and molecular mechanisms of leptin pathophysiology.
(A) In leptin resistant tissue (e.g., hypothalamic cell illustrated), serum leptin-interacting proteins (SLIPs) and soluble leptin receptor (SLR) may bind circulating adipose-secreted leptin and inhibit its action. Free leptin engages the long form of its receptor (Ob-Rb), which homodimerizes. Intracellularly, activated janus kinase 2 (JAK2) phosphorylates a specific tyrosine docking site (Tyr1138) on Ob-Rb. Signal transduction and translation protein 3 (STAT3) recognizes and binds to activated Tyr1138 via its src homology 2 (SH2) domain. The Ob-Rb/JAK2 complex activates STAT3, which homodimerizes, then translocates to the nucleus to modulate gene transcription. STAT3 upregulates expression of suppressor-of cytokine-signaling-3 (SOCS3) and protein tyrosine phosphatase 1B (PTP1B), which block JAK2 phosphorylation. It is thought that central leptin resistance promotes obesity, driving greater hyperleptinemia. (B) In non-leptin resistant tissue (e.g., immune cell illustrated) exposed to hyperleptinemia, Ob-Rb may signal excessively through multiple signaling pathways, including JAK/STAT, insulin receptor substrate-2/phosphatidylinositol 3-kinase (IRS-2/PI3K), and nitric oxide that may ultimately promote cardiovascular disease (CVD) through tissue specific mechanisms.
Cellular
Intracellular suppressor-of cytokine-signaling-3 (SOCS3) is induced by and inhibits leptin JAK/STAT signaling (27,42), a negative feedback mechanism shared with other tyrosine kinase receptors including cytokine and insulin receptors. More recently, protein tyrosine phosphatase 1B was found to similarly regulate leptin signaling in cells (43). Related molecules, such as additional SOCS family members (44), cytokine-inducible SH2 protein (45), JAK binding protein (46), and STAT-induced STAT inhibitor-1 (47) appear to regulate intracellular cytokine signaling and are candidate components of the intracellular leptin negative feedback loop.
Circulating
Extracellular circulating factors may bind leptin, altering its bio-availability and -activity. In 2006, Allan Zhao and coworkers (9) isolated five serum leptin-interacting proteins (SLIPs) in human blood utilizing leptin-affinity chromatography, mass spectrometry and immunochemical analysis. The group identified SLIP-1 as CRP (9) and SLIP-2 as APO-J (also known as clusterin) (48), while SLIPs 3–5 are yet to be further characterized. The concept of circulating leptin binding proteins is not new. In fact, Ob-Re, the soluble leptin receptor, is known to bind leptin in the circulation (49), reducing its free concentration and activity (50). Recently described SLIPs were distinct from and present in significantly higher concentrations than soluble leptin receptor.
The Zhao report frames a role for human CRP in the induction of leptin resistance via direct physical interaction (9). In vitro investigation demonstrated the ability of human CRP to directly inhibit leptin binding to its receptor and related cell signaling in HEK293 cells or hypothalamic neurons. This is in contrast to IL-6, which also appears to bind to CRP, but without a change in cell signaling (51). In ob/ob mice, increased human CRP, from continuous infusion or transgene expression, attenuated the physiological actions of exogenous leptin on food intake, body weight, blood glucose and lipid metabolism (9). Moreover, physiologic leptin concentrations boosted CRP expression in vitro in human primary hepatocytes. Correspondingly, studies in healthy humans show a strongly positive independent association between leptin and CRP blood concentrations (52,53). Clinical investigations of whether leptin administration in vivo enhances CRP concentration were positive in non-obese individuals (54,55), but negative in obese subjects (56), perhaps attributable to leptin resistance. Therefore, preliminary evidence points to an adipo-hepato axis whereby leptin enhances CRP expression and CRP, in turn, may antagonize leptin action. These findings are provocative for a functional CRP effect, as well as for an interface of metabolism and inflammation in the pathogenesis of cardiometabolic disease.
However, the role of CRP in the induction of leptin resistance and as a mediator in CVD is not yet established. Limiting acceptance is the absence of an explicit mechanism by which CRP causes leptin resistance. For instance, regarding leptin’s central action, it is not known whether CRP inhibits leptin inside or outside the central nervous system. Furthermore, Zhao and colleague’s proposed leptin-CRP interaction prompted a robust correspondence and data against such an interaction (57–59). A reply from the Zhao group offered explanations for the apparent discrepancies (48). This debate emphasizes the need for validation of leptin-CRP interaction in humans, and careful experimental technique in this pursuit, particularly as species specificity limits the utility of rodent models (60).
Pathways from Leptin Resistance to Cardiovascular Disease
Innate Immunity, Inflammation and Atherosclerosis
Leptin may be directly atherogenic (61). Ob/ob mice are resistant to atherosclerosis (62) and leptin receptors have been detected in human atherosclerotic lesions (63). Considering atherosclerosis is increasingly viewed as an inflammatory disease, driven by lipoproteins, metabolic signals, hemodynamic stress and the integrated activity of immune cells and inflammatory cytokines, it is intriguing that leptin and inflammatory pathways demonstrate reciprocal modulation and shared association with cardiovascular risk. Leptin regulates human immune functions (64) while activation of innate immunity induces leptin in rodents (65) and humans (66–68). T cell hyporesponsiveness is a core manifestation of leptin deficiency in mice (69) and humans (64). In fact, several immune cells implicated in atherosclerosis, including T lymphocytes, monocytes and macrophages bear the leptin receptor and are generally activated by leptin (8). Leptin stimulates central T cell production and a peripheral shift in favor of T helper (Th) 1 adaptive immune responses (pro-inflammatory) as opposed to Th2 responses (anti-inflammatory). Further augmenting the inflammatory milieu that fosters atherosclerosis, leptin promotes intimal monocyte recruitment (70), elicits macrophage foam cell formation (71) and induces secretion of pro-inflammatory (8), atherogenic cytokines. In this setting, leptin itself can be considered to be a pro-inflammatory cytokine.
Notably, multiple downstream inflammatory biomarkers, including CRP, are independent predictors of CVD (66). Despite correlations with CRP, several macrophage and T-cell cytokines, including IL-18, IL-6 and tumor necrosis factor-α levels have also been associated with coronary artery calcification, incident myocardial infarction (MI) and recurrent CVD in most (72–74) but not all (75) prospective epidemiological studies. Recent evidence suggests that leptin induces CRP expression in human coronary artery endothelial cells (76) where CRP itself may promote atherosclerosis (77). Overall, multiple immune and inflammatory pathways, which may be modulated by leptin, hyperleptinemia and leptin resistance, have been implicated in atherosclerosis in humans as well as in experimental models (10).
A limited pool of data is available on the association of leptin with measures of subclinical atherosclerosis in humans. In a study population of 200 type II diabetics without clinical manifestations of CVD, we found an association between plasma leptin levels and coronary artery calcification even after controlling for established risk factors, adiposity and CRP (7). Recently, we also reported a positive relationship between plasma leptin and coronary calcification in 860 non-diabetic healthy adults independent of established CVD risk factors (78). In 126 normal-weight or obese, but otherwise healthy patients, an association of leptin with intima-media thickness of the common carotid artery was attenuated after adjustment for body mass index (BMI) (79). In addition, several small studies failed to establish an association between leptin and intima-media thickness, including in obese or type I diabetic children (80), healthy elderly men (81), and healthy obese women (82). Thus, the relation of leptin to subclinical atherosclerosis in humans requires further study.
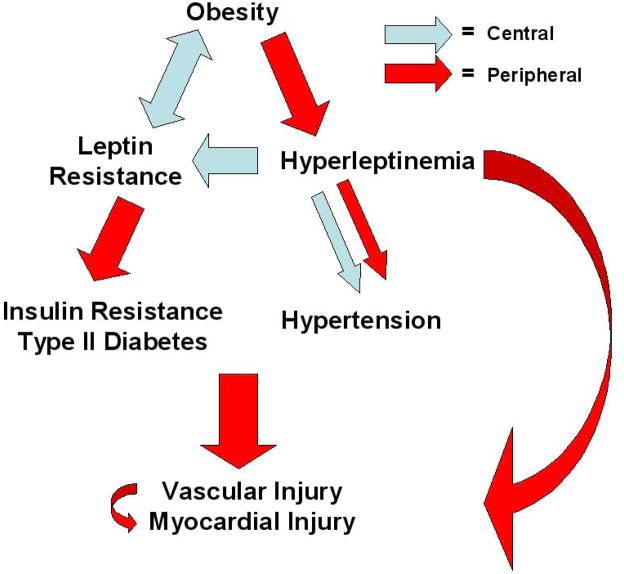
Several clinical studies have correlated leptin levels with cardiovascular events. The first of these reports, from Soderberg et al., saw a positive association of plasma leptin with first MI, independent of traditional risk factors, in a small population based case-control study (4). In the largest of these studies to date, a case-control analysis of over 1000 hypercholesterolemic patients, Wallace et al. (5) found that plasma leptin positively predicted acute cardiovascular events (MI, need for revascularization, death), after adjusting for BMI, plasma glucose, lipids and CRP. Other investigations have connected hyperleptinemia with stroke (83,84) and major adverse cardiac events (6) including restenosis after percutaneous coronary intervention (85). In contrast, conflicting data comes from two small analyses in which plasma leptin levels were unrelated to (86) or negatively predicted (87) CVD. All told, the majority of data favors positive cardiovascular risk prediction for both inflammatory markers and leptin. Thus, it is important to further dissect the diverse mechanisms that may underlie this convergence in CVD (Fig. 3).
Figure 3. Overview of leptin resistance and hyperleptinemia in obesity-related cardiovascular disease.
A leptin resistant/hyperleptinemic state is a putative link between obesity and diverse vascular and myocardial injury via direct effects or intermediary disorders. Site of effect (central versus peripheral) is depicted.
Insulin Resistance and Diabetes
Diabetes and insulin resistance are major cardiovascular risk factors. It is not surprising that leptin interacts with other hormonal regulators of energy metabolism, such as insulin. Interestingly, leptin deficient mice (11,14) and humans (60,64,88) have diabetic features, which correct with leptin replacement even before weight loss. One can infer that leptin resistance (a state of relative leptin deficiency) would lead to insulin resistance and diabetes. This idea of leptin as an insulin-sensitizing hormone and leptin deficiency, or resistance, as a potential link between obesity and diabetes has been reviewed recently (89). Indeed, leptin-induced SOCS3 results in resistance to insulin receptor signaling. As a cautionary note, the bulk of literature comes from in vitro and animal studies and there have been some inconsistencies between reports. However, one cannot overlook the accumulating body of work supporting the direct involvement of leptin in glucose homeostasis.
Human data shows that basal plasma leptin and insulin concentrations parallel each other (90,91). Elevated leptin is associated with hyperinsulinemia and insulin resistance, independent of BMI (3). Consistent with a bidirectional adipoinsular axis, insulin and glucose appear to stimulate leptin secretion in adipocytes (92–94). Indeed, insulinoma elevates leptin levels (95). In response, leptin decreases insulin secretion via direct action on leptin receptors in pancreatic B-cells (96), while enhancing skeletal muscle glucose uptake and oxidation (97,98), and suppressing hepatic glucose production (99). Furthermore, leptin might reduce lipotoxicity (triglyceride accumulation in nonadipose tissue which contributes to insulin resistance) and consequently improve hepatic, muscular and whole body insulin sensitivity (100). Although leptin therapy was disappointing in trials of common obesity (perhaps due to leptin resistance), it has shown success in other patient populations. For instance, leptin therapy improved diabetic measures in children (60,64) and adults (101) with familial leptin deficiency, and in lipoatrophic diabetes (102). Taken together, these data imply that leptin resistance may induce insulin resistance and diabetes. In view of recent evidence that inflammatory cell infiltration promotes adiposity, insulin resistance and type II diabetes (103) and that elevated IL-6 and CRP predict incident type II diabetes (104–106), it is intriguing to consider a leptin-CRP interaction as a possible mechanism linking these disorders.
Hypertension
Hypertension is a major cardiovascular risk factor linked to hyperleptinemia and leptin resistance. Cross-sectional investigations in human subjects show increasing leptin concentrations with increasing blood pressure in subjects in both the normotensive (107) and hypertensive range (108–110). Moreover, hypertensive, overweight women have higher leptin levels than their normotensive counterparts (111). Possible causal pathways for hyperleptinemia in hypertension, based mainly on animal studies, were described in detail in recent reviews (112–115). Briefly, chronic leptin-mediated central sympathetic activation, originating in the hypothalamus, results in a systemic pressor effect that is believed to play a chief role in obesity-related hypertension. In the kidney, sympathoactivation, along with decreased natriuresis leading to volume retention, may contribute to increased blood pressure. Conversely, there is suggestion, but lack of consensus, that leptin decreases arterial tone in the vascular wall through direct actions on the endothelium and smooth muscle although uncertainty surrounds the mechanism of action, acute versus chronic effects and the relevance to human physiology. Regardless, chronic infusion or transgenic overexpression of leptin induces hypertension pointing to a sympathetic pressor effect as the dominant hemodynamic action of leptin, at least over the longer-term.
The paradoxical notion of a leptin-mediated pressor effect persisting in the setting of an otherwise leptin-resistant state might be reconciled by the concept of selective leptin resistance (116) (Table 1). It posits that certain actions of leptin (e.g., sympathoexcitatory actions) persist despite resistance to others. This concept is supported by data from murine models of genetic (117) and diet-induced obesity (118). Yet, how leptin resistance blocks some actions of leptin, but not others, is not clearly understood, especially outside of rodent models. A leading hypothesis is that post-receptor leptin signaling encounters greater feedback (e.g., higher SOCS-3 concentration) in resistant tissues, compared to non-resistant tissues (119). Other possibilities include differential leptin tissue access and circulating leptin inhibitors. Such hypotheses call for further testing.
Table 1.
Theoretical model of selective leptin resistance and hyperleptinemia in cardiovascular disease pathophysiology.
| Resistant | Non-Resistant | |
|---|---|---|
| Tissue | Hypothalamus (metabolic centers), skeletal muscle, pancreas, liver | Immune cells, clotting factors, platelets, vessel wall, myocardium, sympathetic nervous system |
| Leptin concentration | High | High |
| Pathophysiology | Insensitivity to leptin (relative leptin deficiency) causes disease | Retained sensitivity to leptin in the setting of hyperleptinemia causes disease |
| Disease manifestations | Obesity, insulin resistance, diabetes | Hypertension, atherothrombosis, myocardial disease |
| Response to exogenous leptin | Disease improvement | Disease worsening |
| Potential response to targeted treatment of leptin resistance/hyperleptinemia | Disease improvement | Disease improvement |
Thrombosis
Leptin may be prothrombotic. Ob/ob mice show impaired thrombus formation in response to vascular injury and this is reversed by leptin replacement (120,121), suggesting leptin signaling promotes arterial thrombosis. One target of such signaling may be platelets. Ob-Rb is found on platelets (122) and leptin enhances platelet aggregation in the presence, but not in the absence, of this receptor (121). While high concentrations of leptin corresponding to levels in obese individuals increase platelet aggregation, lower concentrations do not (122). This suggests leptin’s prothrombotic effect might be limited to obese hyperleptinemic individuals, thus acting as a unique link between obesity and cardiovascular events. Coagulation-fibrinolysis balance represents a second possible system for leptin mediated thrombosis. Leptin levels have been positively correlated with plasminogen activator inhibitor-1 (123–125), fibrinogen (126), von Willebrand factor (126), and factor VIIa (127), and negatively correlated with tissue plasminogen activator (125,128), tissue factor pathway inhibitor (129), and protein C (129). These findings from various human cohorts imply that increased leptin concentration, either as a marker or through direct effect, favors coagulation over fibrinolysis. The concept of leptin as prothrombotic is of clinical importance because it could directly implicate leptin resistance in acute CVD beyond a relationship with atherosclerosis. It is noteworthy, in fact, that leptin was an independent predictor of acute cardiovascular events in patients with angiographically confirmed coronary disease (6). Overall, whether via platelets or clotting factors, high leptin may confer risk for thrombosis.
Myocardial Injury
Leptin signaling may alter cardiomyocyte structure and function, as thoroughly reviewed recently by Yang and Barouch (130). Evidence implicates the leptin axis in decreased cardiomyocyte contractility and through nitric oxide, B-adrenergic intermediates, reactive oxygen species, ceramide, and pro-inflammatory factors. Contractility may also be impaired by cardiac lipotoxicity secondary to decreased fatty acid oxidation in late stage leptin resistance in contrast to the increased fatty acid oxidation that characterizes early leptin resistance and protects from steatosis. This example draws an important distinction between early and late stage leptin resistance, which may turn out to be conceptually similar to the progression seen in insulin resistance towards type II diabetes. Besides anti-steatosis, additional benefits of short-term hyperleptinemia may include compensatory cardiac hypertrophy and protection from ischemia/reperfusion injury. Yet chronically, leptin-related cardiomyocyte hypertrophy, proliferation, apoptosis, and extracellular matrix reorganization may all contribute to maladaptive cardiac remodeling in obesity. Hypertrophic and proliferative effects may account for the observation that, independent of blood pressure, fasting plasma leptin levels are positively associated with left ventricular hypertrophy in hypertensive patients (131).
Leptin signaling pathways in the myocyte are complicated by different isoform signaling capabilities, but as detailed (130), significant progress has been made in elucidating pathological disturbances in cardiac disease, including in the JAK/STAT, MAPK, nitric oxide, and B-adrenergic pathways. In myocardium, leptin deficiency and hyperleptinemia generally appear to produce the same result, perhaps because hyperleptinemia reflects a state of leptin resistance, and thus functional leptin deficiency. However, whether leptin resistance occurs in the myocardium itself is not yet firmly established. Faced with these issues, we can expect continued challenge in attempting to isolate the physiological actions of leptin from pathological ones in leptin deficiency, resistance, or excess.
Leptin Resistance and the Obesity Epidemic in Evolutionary Terms
Leptin resistance and the surfacing of the obesity epidemic are logical when viewed in the context of evolution. Rapid environmental changes introduced by industrialization, namely increased food availability and decreased physical exertion, were in sync with the rise in obesity. This environmental change presumably had such an impact because it exerted its influence on a human biological system with a propensity for obesity. Such propensity likely stems from an evolutionary survival advantage, in the face of periodic famine, injury or infection, for those best equipped to store energy at times of nutritional abundance. Inflammation-related induction of CRP or other leptin inhibitors with attenuation of central leptin signaling would promote adiposity, the most efficient energy depot in humans. Evolutionarily preprogrammed to store energy, it is not surprising that entry of humans into the energy dense modern society has resulted in the emergence of obesity as a major public health issue. It appears our biological system regulating energy balance has taken on a maladaptive role, which may lead to a host of negative cardiometabolic consequences.
Conclusions and Outstanding Questions
Following a doubling in obesity over the last quarter century, approximately one-third of American adults were classified as obese and two-thirds overweight (132). These figures highlight the need to better understand the etiology of obesity and find effective treatments. Leptin resistance may be an interface of metabolic dysregulation with inflammation in the pathogenesis of obesity, its co-morbidities, and ultimately CVD. Such an interface provides one possible pathophysiological explanation for the convergence of leptin and inflammatory biomarkers in prediction of CVD in humans.
Several questions need to be addressed. For example, harmful leptin effects arising in an otherwise leptin resistance state raise the question of selective leptin resistance. If this exists, how does it occur? Are cellular and circulating inhibitors of leptin signaling major players in leptin resistance and capable of tissue selective effects in humans? Such questions underscore that much is yet to be defined, but should help focus basic and translational research. The potential clinical implications of leptin resistance are provocative. First, leptin resistance may represent an integrated marker for the inextricably linked disease states of obesity, metabolic syndrome, insulin resistance, type II diabetes, hypertension, atherothrombosis and myocardial disease (133). More study is needed to determine whether leptin resistance markers would be valuable in future approaches to cardiovascular risk stratification in clinical practice. Second, knowledge of the mechanisms of leptin resistance, particularly tissue selectivity, cellular mechanisms and circulating modulators (e.g., SLIPs) may direct us toward therapeutic interventions aimed at overcoming leptin resistance. For example, molecules that alter the bioavailability of circulating leptin (e.g., interfere with leptin interactions with SLIPs) or modify post-receptor signaling (e.g., block SOCS3 actions) might represent new targets for therapeutic development. These therapeutic possibilities add promise in combating the obesity epidemic and its devastating consequences. This background provides impetus for further investigation of leptin resistance.
Abbreviations
- BMI
body mass index
- CVD
cardiovascular disease
- CRP
C-reactive protein
- IL
interleukin
- JAK/STAT
janus kinase signal transduction and translation
- MAPK
mitogen activated protein kinases
- MI
myocardial infarction
- SLIPs
serum leptin-interacting proteins
- SOCS3
suppressor-of cytokine-signaling-3
Footnotes
The authors have no conflicts of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 2.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 3.Mantzoros CS, Liolios AD, Tritos NA, et al. Circulating insulin concentrations, smoking, and alcohol intake are important independent predictors of leptin in young healthy men. Obes Res. 1998;6:179–86. doi: 10.1002/j.1550-8528.1998.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 4.Soderberg S, Ahren B, Jansson JH, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409–18. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 5.Wallace AM, McMahon AD, Packard CJ, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–6. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 6.Wolk R, Berger P, Lennon RJ, Brilakis ES, Johnson BD, Somers VK. Plasma leptin and prognosis in patients with established coronary atherosclerosis. J Am Coll Cardiol. 2004;44:1819–24. doi: 10.1016/j.jacc.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Reilly MP, Iqbal N, Schutta M, et al. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–8. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 8.Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13. [PubMed] [Google Scholar]
- 9.Chen K, Li F, Li J, et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat Med. 2006;12:425–32. doi: 10.1038/nm1372. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 11.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41:317–8. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 12.Coleman DL. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973;9:294–8. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 14.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 15.Harigaya A, Nagashima K, Nako Y, Morikawa A. Relationship between concentration of serum leptin and fetal growth. J Clin Endocrinol Metab. 1997;82:3281–4. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- 16.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy A, Gettys TW, Watson P, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 18.Frederich RC, Lollmann B, Hamann A, et al. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest. 1995;96:1658–63. doi: 10.1172/JCI118206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Basinski MB, Beals JM, et al. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–9. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 21.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 22.Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 23.Baumann H, Morella KK, White DW, et al. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 25.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–95. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 26.Lollmann B, Gruninger S, Stricker-Krongrad A, Chiesi M. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and, e in different mouse tissues. Biochem Biophys Res Commun. 1997;238:648–52. doi: 10.1006/bbrc.1997.7205. [DOI] [PubMed] [Google Scholar]
- 27.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz MW, Prigeon RL, Kahn SE, et al. Evidence that plasma leptin and insulin levels are associated with body adiposity via different mechanisms. Diabetes Care. 1997;20:1476–81. doi: 10.2337/diacare.20.9.1476. [DOI] [PubMed] [Google Scholar]
- 29.Heymsfield SB, Greenberg AS, Fujioka K, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–75. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 31.Considine RV, Considine EL, Williams CJ, et al. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995;95:2986–8. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249–56. doi: 10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–7. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 34.Wilsey J, Scarpace PJ. Caloric restriction reverses the deficits in leptin receptor protein and leptin signaling capacity associated with diet-induced obesity: role of leptin in the regulation of hypothalamic long-form leptin receptor expression. J Endocrinol. 2004;181:297–306. doi: 10.1677/joe.0.1810297. [DOI] [PubMed] [Google Scholar]
- 35.Wilsey J, Zolotukhin S, Prima V, Shek EW, Matheny MK, Scarpace PJ. Hypothalamic delivery of doxycycline-inducible leptin gene allows for reversible transgene expression and physiological responses. Gene Ther. 2002;9:1492–9. doi: 10.1038/sj.gt.3301835. [DOI] [PubMed] [Google Scholar]
- 36.Scarpace PJ, Matheny M, Zhang Y, et al. Central leptin gene delivery evokes persistent leptin signal transduction in young and aged-obese rats but physiological responses become attenuated over time in aged-obese rats. Neuropharmacology. 2002;42:548–61. doi: 10.1016/s0028-3908(02)00003-5. [DOI] [PubMed] [Google Scholar]
- 37.Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia. 2005;48:1075–83. doi: 10.1007/s00125-005-1763-x. [DOI] [PubMed] [Google Scholar]
- 38.Bjorbaek C, Elmquist JK, Michl P, et al. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology. 1998;139:3485–91. doi: 10.1210/endo.139.8.6154. [DOI] [PubMed] [Google Scholar]
- 39.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 40.Van Heek M, Compton DS, France CF, et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest. 1997;99:385–90. doi: 10.1172/JCI119171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D., Jr Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2:589–93. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 42.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 43.Cheng A, Uetani N, Simoncic PD, et al. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 44.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–21. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 45.Emilsson V, Arch JR, de Groot RP, Lister CA, Cawthorne MA. Leptin treatment increases suppressors of cytokine signaling in central and peripheral tissues. FEBS Lett. 1999;455:170–4. doi: 10.1016/s0014-5793(99)00874-1. [DOI] [PubMed] [Google Scholar]
- 46.Endo TA, Masuhara M, Yokouchi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–4. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 47.Naka T, Narazaki M, Hirata M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–9. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 48.Zhao A. Is leptin an important physiological regulator of CRP? Nat Med. 2007;13:19–21. doi: 10.1038/nm0107-18. (letter; author reply) [DOI] [PubMed] [Google Scholar]
- 49.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–8. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 50.Zastrow O, Seidel B, Kiess W, et al. The soluble leptin receptor is crucial for leptin action: evidence from clinical and experimental data. Int J Obes Relat Metab Disord. 2003;27:1472–8. doi: 10.1038/sj.ijo.0802432. [DOI] [PubMed] [Google Scholar]
- 51.Pignatti P, Ciapponi L, Galle P, et al. High circulating levels of biologically inactive IL-6/SIL-6 receptor complexes in systemic juvenile idiopathic arthritis: evidence for serum factors interfering with the binding to gp130. Clin Exp Immunol. 2003;131:355–63. doi: 10.1046/j.1365-2249.2003.02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kazumi T, Kawaguchi A, Hirano T, Yoshino G. C-reactive protein in young, apparently healthy men: associations with serum leptin, QTc interval, and high-density lipoprotein-cholesterol. Metabolism. 2003;52:1113–6. doi: 10.1016/s0026-0495(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 53.Shamsuzzaman AS, Winnicki M, Wolk R, et al. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–5. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 54.Mackintosh RM, Hirsch J. The effects of leptin administration in non-obese human subjects. Obes Res. 2001;9:462–9. doi: 10.1038/oby.2001.60. [DOI] [PubMed] [Google Scholar]
- 55.Hukshorn CJ, Lindeman JH, Toet KH, et al. Leptin and the proinflammatory state associated with human obesity. J Clin Endocrinol Metab. 2004;89:1773–8. doi: 10.1210/jc.2003-030803. [DOI] [PubMed] [Google Scholar]
- 56.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–9. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 57.Farooqi IS, O'Rahilly S. Is leptin an important physiological regulator of CRP? Nat Med. 2007;13:16–7. doi: 10.1038/nm0107-16. (letter) [DOI] [PubMed] [Google Scholar]
- 58.Hutchinson WL, Coll AP, Gallimore JR, Tennent GA, Pepys MB. Is leptin an important physiological regulator of CRP? Nat Med. 2007;13:17–8. doi: 10.1038/nm0107-17. (letter) [DOI] [PubMed] [Google Scholar]
- 59.Gertler A, Niv-Spector L, Reicher S. Is leptin an important physiological regulator of CRP? Nat Med. 2007;13:18–9. doi: 10.1038/nm0107-18. (letter) [DOI] [PubMed] [Google Scholar]
- 60.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–84. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 61.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Yen TT, Allan JA, Pearson DV, Schinitsky MR. Dissociation of obesity, hypercholesterolemia and diabetes from atherosclerosis in ob/ob mice. Experientia. 1977;33:995–6. doi: 10.1007/BF01945927. [DOI] [PubMed] [Google Scholar]
- 63.Kang SM, Kwon HM, Hong BK, et al. Expression of leptin receptor (Ob-R) in human atherosclerotic lesions: potential role in intimal neovascularization. Yonsei Med J. 2000;41:68–75. doi: 10.3349/ymj.2000.41.1.68. [DOI] [PubMed] [Google Scholar]
- 64.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest. 2002;110:1093–103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grunfeld C, Zhao C, Fuller J, et al. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–7. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 67.Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Wardlaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. J Clin Endocrinol Metab. 2003;88:1285–91. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- 68.Bornstein SR, Licinio J, Tauchnitz R, et al. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J Clin Endocrinol Metab. 1998;83:280–3. doi: 10.1210/jcem.83.1.4610. [DOI] [PubMed] [Google Scholar]
- 69.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 70.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzman M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 71.O'Rourke L, Gronning LM, Yeaman SJ, Shepherd PR. Glucose-dependent regulation of cholesterol ester metabolism in macrophages by insulin and leptin. J Biol Chem. 2002;277:42557–62. doi: 10.1074/jbc.M202151200. [DOI] [PubMed] [Google Scholar]
- 72.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 73.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000;101:2149–53. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- 74.Reilly MP, Rohatgi A, McMahon K, et al. Plasma cytokines, metabolic syndrome, and atherosclerosis in humans. J Investig Med. 2007;55:26–35. doi: 10.2310/6650.2007.06013. [DOI] [PubMed] [Google Scholar]
- 75.Pai JK, Pischon T, Ma J, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 76.Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:e302–7. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- 77.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 78.Qasim A, Mehta N, Tadesse MG, et al. Adipokines, Insulin Resistance and Coronary Artery Calcification. J Am Coll Cardiol. 2008 doi: 10.1016/j.jacc.2008.04.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciccone M, Vettor R, Pannacciulli N, et al. Plasma leptin is independently associated with the intima-media thickness of the common carotid artery. Int J Obes Relat Metab Disord. 2001;25:805–10. doi: 10.1038/sj.ijo.0801623. [DOI] [PubMed] [Google Scholar]
- 80.Mangge H, Schauenstein K, Stroedter L, Griesl A, Maerz W, Borkenstein M. Low grade inflammation in juvenile obesity and type 1 diabetes associated with early signs of atherosclerosis. Exp Clin Endocrinol Diabetes. 2004;112:378–82. doi: 10.1055/s-2004-821023. [DOI] [PubMed] [Google Scholar]
- 81.van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003;157:25–31. doi: 10.1093/aje/kwf160. [DOI] [PubMed] [Google Scholar]
- 82.Oflaz H, Ozbey N, Mantar F, et al. Determination of endothelial function and early atherosclerotic changes in healthy obese women. Diabetes Nutr Metab. 2003;16:176–81. [PubMed] [Google Scholar]
- 83.Soderberg S, Ahren B, Stegmayr B, et al. Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke. 1999;30:328–37. doi: 10.1161/01.str.30.2.328. [DOI] [PubMed] [Google Scholar]
- 84.Soderberg S, Stegmayr B, Stenlund H, et al. Leptin, but not adiponectin, predicts stroke in males. J Intern Med. 2004;256:128–36. doi: 10.1111/j.1365-2796.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 85.Piatti P, Di Mario C, Monti LD, et al. Association of insulin resistance, hyperleptinemia, and impaired nitric oxide release with in-stent restenosis in patients undergoing coronary stenting. Circulation. 2003;108:2074–81. doi: 10.1161/01.CIR.0000095272.67948.17. [DOI] [PubMed] [Google Scholar]
- 86.Couillard C, Lamarche B, Mauriege P, et al. Leptinemia is not a risk factor for ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Diabetes Care. 1998;21:782–6. doi: 10.2337/diacare.21.5.782. [DOI] [PubMed] [Google Scholar]
- 87.Piemonti L, Calori G, Mercalli A, et al. Fasting plasma leptin, tumor necrosis factor-alpha receptor 2, and monocyte chemoattracting protein 1 concentration in a population of glucose-tolerant and glucose-intolerant women: impact on cardiovascular mortality. Diabetes Care. 2003;26:2883–9. doi: 10.2337/diacare.26.10.2883. [DOI] [PubMed] [Google Scholar]
- 88.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84:3686–95. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 89.Yildiz BO, Haznedaroglu IC. Rethinking leptin and insulin action: therapeutic opportunities for diabetes. Int J Biochem Cell Biol. 2006;38:820–30. doi: 10.1016/j.biocel.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 90.Ahren B, Larsson H, Wilhelmsson C, Nasman B, Olsson T. Regulation of circulating leptin in humans. Endocrine. 1997;7:1–8. doi: 10.1007/BF02778056. [DOI] [PubMed] [Google Scholar]
- 91.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–13. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 92.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest. 1997;100:1107–13. doi: 10.1172/JCI119621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koopmans SJ, Frolich M, Gribnau EH, Westendorp RG, DeFronzo RA. Effect of hyperinsulinemia on plasma leptin concentrations and food intake in rats. Am J Physiol. 1998;274:E998–E1001. doi: 10.1152/ajpendo.1998.274.6.E998. [DOI] [PubMed] [Google Scholar]
- 94.Sonnenberg GE, Krakower GR, Hoffmann RG, Maas DL, Hennes MM, Kissebah AH. Plasma leptin concentrations during extended fasting and graded glucose infusions: relationships with changes in glucose, insulin, and FFA. J Clin Endocrinol Metab. 2001;86:4895–900. doi: 10.1210/jcem.86.10.7951. [DOI] [PubMed] [Google Scholar]
- 95.D'Adamo M, Buongiorno A, Maroccia E, et al. Increased OB gene expression leads to elevated plasma leptin concentrations in patients with chronic primary hyperinsulinemia. Diabetes. 1998;47:1625–9. doi: 10.2337/diabetes.47.10.1625. [DOI] [PubMed] [Google Scholar]
- 96.Seufert J, Kieffer TJ, Leech CA, et al. Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus. J Clin Endocrinol Metab. 1999;84:670–6. doi: 10.1210/jcem.84.2.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–7. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 98.Wang JL, Chinookoswong N, Scully S, Qi M, Shi ZQ. Differential effects of leptin in regulation of tissue glucose utilization in vivo. Endocrinology. 1999;140:2117–24. doi: 10.1210/endo.140.5.6681. [DOI] [PubMed] [Google Scholar]
- 99.Rossetti L, Massillon D, Barzilai N, et al. Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J Biol Chem. 1997;272:27758–63. doi: 10.1074/jbc.272.44.27758. [DOI] [PubMed] [Google Scholar]
- 100.Lee Y, Wang MY, Kakuma T, et al. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276:5629–35. doi: 10.1074/jbc.M008553200. [DOI] [PubMed] [Google Scholar]
- 101.Licinio J, Caglayan S, Ozata M, et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci U S A. 2004;101:4531–6. doi: 10.1073/pnas.0308767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–8. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 103.Ferrante AW., Jr Obesity-induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–14. doi: 10.1111/j.1365-2796.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 104.Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med. 2007;167:1676–85. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- 105.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 106.Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–21. doi: 10.2337/diacare.25.11.2016. [DOI] [PubMed] [Google Scholar]
- 107.Schorr U, Blaschke K, Turan S, Distler A, Sharma AM. Relationship between angiotensinogen, leptin and blood pressure levels in young normotensive men. J Hypertens. 1998;16:1475–80. doi: 10.1097/00004872-199816100-00011. [DOI] [PubMed] [Google Scholar]
- 108.Hirose H, Saito I, Tsujioka M, Mori M, Kawabe H, Saruta T. The obese gene product, leptin: possible role in obesity-related hypertension in adolescents. J Hypertens. 1998;16:2007–12. doi: 10.1097/00004872-199816121-00023. [DOI] [PubMed] [Google Scholar]
- 109.Agata J, Masuda A, Takada M, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997;10:1171–4. doi: 10.1016/s0895-7061(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 110.Barba G, Russo O, Siani A, et al. Plasma leptin and blood pressure in men: graded association independent of body mass and fat pattern. Obes Res. 2003;11:160–6. doi: 10.1038/oby.2003.25. [DOI] [PubMed] [Google Scholar]
- 111.Itoh K, Imai K, Masuda T, et al. Relationship between changes in serum leptin levels and blood pressure after weight loss. Hypertens Res. 2002;25:881–6. doi: 10.1291/hypres.25.881. [DOI] [PubMed] [Google Scholar]
- 112.Haynes WG. Role of leptin in obesity-related hypertension. Exp Physiol. 2005;90:683–8. doi: 10.1113/expphysiol.2005.031237. [DOI] [PubMed] [Google Scholar]
- 113.Correia ML, Haynes WG. Obesity-related hypertension: is there a role for selective leptin resistance? Curr Hypertens Rep. 2004;6:230–5. doi: 10.1007/s11906-004-0074-9. [DOI] [PubMed] [Google Scholar]
- 114.Beltowski J. Role of leptin in blood pressure regulation and arterial hypertension. J Hypertens. 2006;24:789–801. doi: 10.1097/01.hjh.0000222743.06584.66. [DOI] [PubMed] [Google Scholar]
- 115.Mathew B, Patel SB, Reams GP, Freeman RH, Spear RM, Villarreal D. Obesity-hypertension: emerging concepts in pathophysiology and treatment. Am J Med Sci. 2007;334:23–30. doi: 10.1097/MAJ.0b013e3180959e4e. [DOI] [PubMed] [Google Scholar]
- 116.Mark AL, Correia ML, Rahmouni K, Haynes WG. Selective leptin resistance: a new concept in leptin physiology with cardiovascular implications. J Hypertens. 2002;20:1245–50. doi: 10.1097/00004872-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–42. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- 118.Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–8. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- 119.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–9. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 120.Bodary PF, Westrick RJ, Wickenheiser KJ, Shen Y, Eitzman DT. Effect of leptin on arterial thrombosis following vascular injury in mice. JAMA. 2002;287:1706–9. doi: 10.1001/jama.287.13.1706. [DOI] [PubMed] [Google Scholar]
- 121.Konstantinides S, Schafer K, Koschnick S, Loskutoff DJ. Leptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesity. J Clin Invest. 2001;108:1533–40. doi: 10.1172/JCI13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakata M, Yada T, Soejima N, Maruyama I. Leptin promotes aggregation of human platelets via the long form of its receptor. Diabetes. 1999;48:426–9. doi: 10.2337/diabetes.48.2.426. [DOI] [PubMed] [Google Scholar]
- 123.Thogersen AM, Soderberg S, Jansson JH, et al. Interactions between fibrinolysis, lipoproteins and leptin related to a first myocardial infarction. Eur J Cardiovasc Prev Rehabil. 2004;11:33–40. doi: 10.1097/01.hjr.0000116824.84388.a2. [DOI] [PubMed] [Google Scholar]
- 124.De Mitrio V, De Pergola G, Vettor R, et al. Plasma plasminogen activator inhibitor-I is associated with plasma leptin irrespective of body mass index, body fat mass, and plasma insulin and metabolic parameters in premenopausal women. Metabolism. 1999;48:960–4. doi: 10.1016/s0026-0495(99)90190-7. [DOI] [PubMed] [Google Scholar]
- 125.Soderberg S, Olsson T, Eliasson M, Johnson O, Ahren B. Plasma leptin levels are associated with abnormal fibrinolysis in men and postmenopausal women. J Intern Med. 1999;245:533–43. doi: 10.1046/j.1365-2796.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 126.Chu NF, Spiegelman D, Hotamisligil GS, Rifai N, Stampfer M, Rimm EB. Plasma insulin, leptin, and soluble TNF receptors levels in relation to obesity-related atherogenic and thrombogenic cardiovascular disease risk factors among men. Atherosclerosis. 2001;157:495–503. doi: 10.1016/s0021-9150(00)00755-3. [DOI] [PubMed] [Google Scholar]
- 127.Guagnano MT, Romano M, Falco A, et al. Leptin increase is associated with markers of the hemostatic system in obese healthy women. J Thromb Haemost. 2003;1:2330–4. doi: 10.1046/j.1538-7836.2003.00445.x. [DOI] [PubMed] [Google Scholar]
- 128.Skurk T, van Harmelen V, Lee YM, Wirth A, Hauner H. Relationship between IL-6, leptin and adiponectin and variables of fibrinolysis in overweight and obese hypertensive patients. Horm Metab Res. 2002;34:659–63. doi: 10.1055/s-2002-38253. [DOI] [PubMed] [Google Scholar]
- 129.Malyszko J, Wolczynski S, Malyszko J, Mysliwiec M. Leptin correlates with some hemostatic parameters in CAPD patients. Nephron. 2002;92:721–4. doi: 10.1159/000064074. [DOI] [PubMed] [Google Scholar]
- 130.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–59. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 131.Paolisso G, Tagliamonte MR, Galderisi M, et al. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–52. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 132.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 133.Ukkola O, Kesaniemi YA. Leptin and high-sensitivity C-reactive protein and their interaction in the metabolic syndrome in middle-aged subjects. Metabolism. 2007;56:1221–7. doi: 10.1016/j.metabol.2007.04.019. [DOI] [PubMed] [Google Scholar]