Abstract
Welding is a common industrial process used to join metals and generates complex aerosols of potentially hazardous metal fumes and gases. Most long-time welders experience some type of respiratory disorder during their time of employment. The use of animal models and the ability to control the welding fume exposure in toxicology studies have been helpful in developing a better understanding of how welding fumes affect health. There are no studies that have performed a side-by-side comparison of the pulmonary responses from an animal toxicology welding fume study with the lung responses associated with chronic exposure to welding fume by a career welder. In this study, post-mortem lung tissue was donated from a long-time welder with a well-characterized work background and a history of extensive welding fume exposure. To simulate a long-term welding exposure in an animal model, Sprague-Dawley rats were treated once a week for 28 weeks by intratracheal instillation with 2 mg of a stainless steel, hard-surfacing welding fume. Lung tissues from the welder and the welding fume-treated rats were examined by light and electron microscopy. Pathological analysis of lung tissue collected from the welder demonstrated inflammatory cell influx and significant pulmonary injury. The poor and deteriorating lung condition observed in the welder examined in this study was likely due to exposure to very high levels of potentially toxic metal fumes and gases for a significant number of years due to work in confined spaces. The lung toxicity profile for the rats treated with welding fume was similar. For tissue samples from both the welder and treated rats, welding particle accumulations deposited and persisted in lung structures and were easily visualized using light microscopic techniques. Agglomerates of deposited welding particles mostly were observed within lung cells, particularly alveolar macrophages. Analysis of individual particles within the agglomerates showed that these particles were metal complexes with iron, chromium, and nickel being the most common metals present. In conclusion, long-term exposure to specific welding fume can lead to serious chronic lung disease characterized by significant particle deposition and persistence as demonstrated in both a human case study and rat model. Not only were the lung responses similar in the human and rat lungs, as evidenced by inflammatory cell influx and pulmonary disease, but the composition of individual welding particles and agglomerations in situ was comparable.
Keywords: human, microscopy, pulmonary toxicity, rat, welding fume
INTRODUCTION
Welding is a common industrial process used to join metals. Welding processes generate complex aerosols of potentially hazardous metal fumes that may contain iron (Fe), chromium (Cr), manganese (Mn), and nickel (Ni) and gases, such as carbon monoxide, nitrogen oxide, and ozone. Approximately, 340 000 workers were employed full-time as welders, cutters, solderers, and brazers in the USA in 2010 (Bureau of Labor Statistics, 2012). Millions of more workers worldwide perform duties related to welding operations but are not classified as full-time welders, such as boilermakers, pipefitters, construction workers, shipbuilders, automotive workers, and farmers. Employment of full-time welders in the USA is expected to grow 15% to nearly 400 000 workers by the year 2020 (Bureau of Labor Statistics, 2012).
Most long-time welders or workers exposed to welding fumes on a daily basis experience some type of respiratory disorder during their time of employment. Pulmonary effects have included siderosis, bronchitis, metal fume fever, lung function changes, a susceptibility to upper and lower respiratory infections, and the possible development of lung cancer (reviewed by Sferlazza and Beckett, 1991; Martin et al., 1997; Antonini, 2003). Due to the many different types of industries and welding processes, welders are a heterogeneous workforce. Welders work in a number of settings, such as confined, poorly ventilated spaces (e.g. boilers, underground mines, ship hulls, pipelines, building crawl spaces) or in well-ventilated indoor and outdoor open-air sites. Because of this, the health of welders has been difficult to assess because of differences in worker populations, work area ventilation, welding processes and materials used, and confounding occupational exposures other than to welding aerosols. Thus, questions remain unanswered regarding the causality and possible underlying mechanisms associated with the potential adverse effects of welding fume exposure.
The use of animal models and the ability to control the welding fume exposure in toxicology studies have been helpful in developing a better understanding of how welding fumes affect health. Analysis of the bronchoalveolar lavage fluid (BALF) collected from the lungs of rats that have been exposed to welding fume by either intratracheal instillation or inhalation has shown significant lung cell toxicity, air–blood barrier damage, inflammation, and an inflammatory cytokine response to specific welding fumes (Antonini et al., 1996, 2007, 2009, 2011a). It was observed that stainless steel welding fumes induced more lung toxicity as compared to mild steel fumes, a response most likely attributable to the presence of toxic metals, such as Cr and Ni, in the stainless steel fumes (Taylor et al., 2003).
Most welding fume toxicology studies have been short-term and used high concentrations of welding fume. Animals have been exposed to welding fumes by intratracheal instillation and inhalation methods. Due to expense, complexity, and the need for a specialized staff trained in aerosol physics, inhalation toxicology, and engineering to construct a welding fume generation system, studies of animal inhalation exposure to welding fumes are limited (Yu et al., 2001, 2003; Antonini et al., 2006). Intratracheal instillation is the more widely used procedure to deliver welding particulates into lungs of laboratory animals. For this procedure, welding fume is collected onto filters, suspended in an aqueous medium, and delivered directly into the lungs of animals. Advantages to this method over the more physiologic inhalation exposure route include simplicity, relatively low cost, and importantly, the delivery of a well-defined dose of particles (Brain et al., 1976; Henderson et al., 1995; Driscoll et al., 2000). However, a large bolus of welding particulates is delivered to the lungs by intratracheal instillation that is likely to have a much higher particle concentration than may be inhaled in the workplace over time. In addition, a number of potentially toxic gases (e.g. ozone, nitrogen oxide, nitrogen dioxide) are generated during welding processes that are not present when administering welding fumes by intratracheal instillation. Thus, there is a need to correlate the lung response results from the animal welding fume toxicology studies using intratracheal instillation treatments with the findings from actual welder case studies.
As discussed, much research has been performed using animal models to assess the lung effects of welding fume. A question that needs to be answered is whether or not rat lungs respond and process inhaled welding particles in the same manner as human lungs. Currently, there are no studies that have performed a side-by-side comparison of the pulmonary responses from an animal toxicology welding fume study with the lung responses associated with chronic exposure to welding fume by a career welder. In this study, post-mortem lung tissue was recovered from a long-time welder with a well-characterized work background and a history of extensive welding fume exposure over 35 years. To simulate a long-term welding exposure in an animal model, male Sprague-Dawley rats were treated once a week for 28 weeks by intratracheal instillation with 2 mg of a stainless steel, hard-surfacing welding fume. Lung tissues from the welder and the welding fume-treated rats were fixed, processed, and examined by electron microscopy. Histopathology, lung distribution, and elemental mapping of deposited welding particles and agglomerates were assessed and compared.
METHODS
Case report
Our laboratory received donated lung tissue from the right upper lung lobe post-mortem from a full-time career welder who had >35 years of occupational exposure to a variety of different welding fumes. The individual had welded for the Air National Guard for 30 years, as well as welded in his home shop during his work years and for at least 15 years after his retirement. At work, about half of his time was spent welding 4130 chrome-molybdenum (Mo) steel that contained predominately Fe, Cr, Mo, Ni, and Mn. He welded tail pipes of various military fighter aircraft composed of inconel (Ni, Mo, Cr, and Fe) and hastelloy (Ni, Cr, cobalt, tungsten, and Mn) alloys. For many years, he completed crack repairs on F-106 tailpipes by climbing into and welding in the enclosed space without ventilation or breathing apparatus. Other common extended fume exposures included stainless steel, aluminum (Al), magnesium, and titanium (Ti). At home, he welded primarily 4130 chrome-Mo steel, carbon steel, stainless steel, cast Fe, and Al. At annual welding certification tests, he would weld using beryllium. In his early years, he performed electric arc welding using 7018, 6010, and 6011 electrodes, oxy-acetylene with flux-coated brazing rod, and silver soldering with flux and acid wash. Most of the welding in his later years was done using tungsten inert gas welding with thoriated tungsten electrodes.
In the year 2000, the welder, who was 63 years old at the time, was diagnosed with chronic obstructive pulmonary disease (COPD) and occupational asthma due to welding fume exposure. From 2000 until his death in 2010, a continual increase in dyspnea on exertion was reported that occurred very gradually over the first 6 years before rapidly declining over the last 2 years of his life. In association with his dyspnea on exertion, which was his most severe symptom, he reported no wheezing. He had a non-productive cough for many years that produced little phlegm. No history of hay fever or post-nasal drip was reported. In 2007, he underwent an extensive cardiac evaluation that revealed no obstructive coronary disease, as well as normal ventricular function. In addition to exercise, he listed triggers for the shortness of breath as air pollution, cigarette smoke, car exhaust, specifically diesel, and weather changes. He was an ex-smoker of 2 packs per day, from age 20 to 46, which is equivalent to a 52 pack-year smoking history. Chest computed tomography scans that were performed in 2004 and 2008 indicated a progression of confluent emphysema and diagnosis of obliterative bronchiolitis, marked diffuse bronchial wall thickening, residual scarring along the left major fissure and lingula, and a small, right lung nodule. In addition in 2008, pulmonary function testing using spirometry demonstrated that total lung capacity was 9.63 l (160% predicted), functional residual capacity-pleth was 7.02 l (193% predicted), residual volume was 6.13 l (128% predicted), forced vital capacity (FVC) 2.64 l (65% predicted), forced expiratory volume in 1 s (FEV1) was 0.92 l (31% predicted), and a FEV1/FVC ratio of 37%. Post-bronchodilator values showed an FVC of 3.03 l (74% predicted) and an FEV1 of 1.06 l (36% predicted), which reflected a 15% change after bronchodilator administration. After this 2008 examination, he was diagnosed with COPD and chronic airway obstruction, chronic obstructive asthma, bronchiectasis, and emphysema after examination.
Animal exposure
Welding fume
Bulk samples of welding fumes were provided by Lincoln Electric Company (Cleveland, OH, USA). The fumes were generated in a cubical open front fume chamber (volume = 1 m3) by a skilled welder using a manual or semi-automatic technique appropriate to the electrode and collected on 0.2-μm nuclepore filters (Nuclepore, Pleasanton, CA, USA). The fumes were generated by a shielded manual metal arc welding process using a flux-covered stainless steel hard-surfacing electrode (Wearshield 15 CrMn; Lincoln Electric). The particle size of the collected fume sample was determined using scanning electron microscopy (SEM) and found to be in the respirable size range with a count mean diameter of <1 μm. A portion of the collected fume sample was digested, and the metals were analyzed by inductively coupled plasma-atomic emission spectroscopy, according to the National Institute for Occupational Safety and Health (NIOSH) method 7300 modified for microwave digestion (NIOSH, 1994). The metals that were measured included: Ag, Al, As, Ba, beryllium, Ca, Cd, cobalt, Cr, Cu, Fe, K, La, Li, magnesium, Mn, Mo, Na, Ni, P, Pb, Sb, Se, Sr, Te, Ti, Tl, V, Zn, and Zr. Metal content of blank filters were analyzed for control purposes. The collected welding fume was composed of Fe (19.3%), Mn (50.9%), Cr (8.46%), and Ni (0.09%), as well as flux materials like K (12.1%) and Na (6.73%). A more detailed characterization of this particular welding fume is presented in Antonini et al. (2010).
Animal treatment
Male Sprague-Dawley [Hla: (SD) CVF] rats from Hilltop Lab Animals (Scottdale, PA, USA), weighing 250–300 g and free of viral pathogens, parasites, mycoplasmas, Helicobacter, and cilia-associated respiratory bacillus, were used for all exposures. The rats were acclimated for at least 6 days after arrival and were housed in ventilated polycarbonate cages on Alpha-Dri cellulose chips and hardwood Beta-chips as bedding, and provided high-efficiency particulate-filtered air, irradiated Teklad 2918 diet, and tap water ad libitum. The animal facilities are specific pathogen-free, environmentally controlled, and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All animal procedures used during the study were reviewed and approved by the NIOSH Animal Care and Use Committee.
The welding fume sample was prepared in sterile saline and sonicated for 1 min in a Sonifier 450 Cell Disruptor (Branson Ultrasonics, Danbury, CT, USA) to disperse the particulates. Rats were lightly anesthetized by an intraperitoneal injection of 0.6 ml of a 1% solution of sodium methohexital (Brevital; Eli Lilly, Indianapolis, IN, USA). Rats were intratracheally instilled with 2 mg per animal of the welding fume in 300 μl of sterile saline once a week for 28 weeks. Most intratracheal instillation studies utilize a single, large bolus of instillate to treat laboratory animals. However, multiple instillations at smaller doses may be more representative of an inhalation exposure than a large single instillation (Reasor and Antonini, 2000). Control animals were intratracheally instilled with 300 μl of sterile saline. Animals were euthanized 1 week after the 28 weekly treatments to assess lung responses.
To relate the pulmonary (intratracheal instillation) dosing paradigm employed in this study to workplace exposures of welders, we utilized a mathematical calculation (Antonini et al., 2010; Sriram et al., 2010) to determine the daily lung burden of a welder on an 8-h work schedule. Incorporating factors such as fume concentration (5 mg m−3, previous threshold limit value for welding fumes), human minute ventilation volume (20 000 ml min−1 × 10−6 m3 ml−1), exposure duration (8 h day−1 × 60 min h−1), and deposition efficiency (predicted as 15%; ICRP, 1994; Antonini et al., 2006), it was determined that the daily lung burden of a welder is about 7.2 mg. Using surface area of alveolar epithelium (rat = 0.4 m2; human = 102 m2; Stone et al., 1992) as dose metric, the daily lung burden for a similar exposure in the rat amounts to 0.0282 mg. Factoring the cumulative dosing paradigm used in this study (2 mg × 28 instillations = 56 mg) and the estimated daily lung burden for rat (0.0282 mg), the number of welder exposure days was derived for the exposure paradigm as 56 mg/0.0282 mg = 1985.8 days. Thus, the pulmonary exposure regimen used in this study mimicked worker exposures of about 10 years at 200 working days per year.
Evaluation of lung responses
At 1 week after the last of the 28 weekly treatments, rats were deeply anesthetized with an intraperitoneal injection of Sleepaway (>100 mg kg−1 body weight of sodium pentobarbital; Fort Dodge Animal Health, Fort Dodge, IA, USA), and then exsanguinated by severing the abdominal aorta. BAL was performed on right lungs that were lavaged with a 1 ml 100 g−1 body weight aliquot of calcium- and magnesium-free phosphate-buffered saline (PBS), pH 7.4. The first fraction of recovered BALF was centrifuged at 500 g for 10 min, and the resultant cell-free supernatant was analyzed for lung injury by measuring albumin levels colorimetrically at 628 nm based on albumin binding to bromcresol green using an albumin BCG diagnostic kit (Sigma Chemical Co., St Louis, MO, USA). The right lungs were further lavaged with 6-ml aliquots of PBS until 30 ml were collected. These samples also were centrifuged for 10 min at 500 g and the cell-free BALF discarded. The cell pellets from all washes for each rat were combined, washed, and resuspended in 1 ml of PBS. Total cell numbers recovered by BAL were determined using a Coulter Multisizer II and AccuComp software (Coulter Electronics, Hialeah, FL, USA). Cells were differentiated using a Cytospin 3 centrifuge (Shandon Life Sciences International, Cheshire, UK). Cell suspensions (5 × 104 cells) were spun for 5 min at 800 r.p.m. and pelleted onto a slide. Cells (200/rat) were identified after labeling with Leukostat stain (Fisher Scientific, Pittsburgh, PA, USA) as alveolar macrophages (AMs), neutrophils (PMNs), and lymphocytes (LYMPHs). Non-lavaged left lungs from the treated rats were preserved by 10% neutral buffered formalin by airway fixation at total lung capacity. Portions of fixed left lungs were embedded in paraffin and sectioned for light microscopy and histopathological analysis or electron microscopy.
Microscopic analysis
Electron microscopy
For the human tissue sample collected from the welder, a 5-μm lung section was placed on a carbon planchet, deparaffinized, and dried. Individual particles within welding agglomerates that had deposited in lung tissue structures were viewed using a Hitachi S4800 field emission SEM and X-ray system (Bruker, Madison, WI, USA). Elemental profiles of multiple welding particles that had deposited in the lung tissue were determined by energy-dispersive X-ray analysis (SEM–EDS) at 20 kV. For the rat lung samples, tissues were post-fixed with osmium tetroxide and embedded in epoxy resin. Welding particles within lung structures were imaged using a JEOL 1220 transmission electron microscope (TEM; JEOL Inc., Tokyo, Japan) at 80 kV. In addition, a 1.0-μm section, from embedded blocks prepared for TEM, was attached to a carbon planchet via a hot plate. Individual welding particles within rat lung tissue structures were imaged by backscatter imaging on a Hitachi S4800 field emission scanning electron microscope and then analyzed by a Bruker EDS for elemental analysis.
Statistical analysis
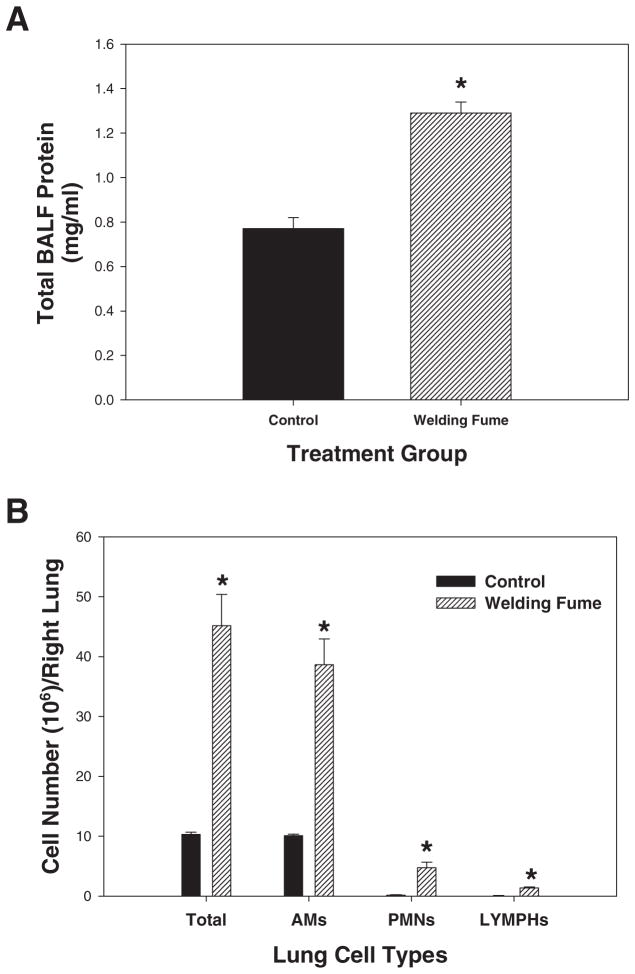
The lung injury and inflammation data in Fig. 6 are presented as means ± standard error of measurement. Analysis of effects in Fig. 6 compared two treatments—the welding fume-treated and control groups; because of this, data were analyzed using a Student’s t-test (JMP 9 Statistical Discovery Software; SAS Inc., Belmont, CA). Differences were considered statistically significant at P < 0.05.
Fig. 6.
(A) Total protein and (B) lung cell differential measured in BALF collected from rat lungs after 28 weeks of weekly treatments of 2 mg of welding fume. Control animals were treated with sterile saline. Values are means ± standard error (n = 4); *, significantly different from saline control group (P < 0.05).
RESULTS
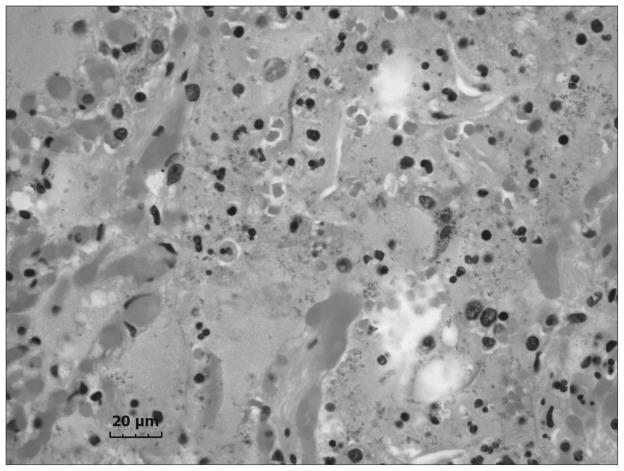
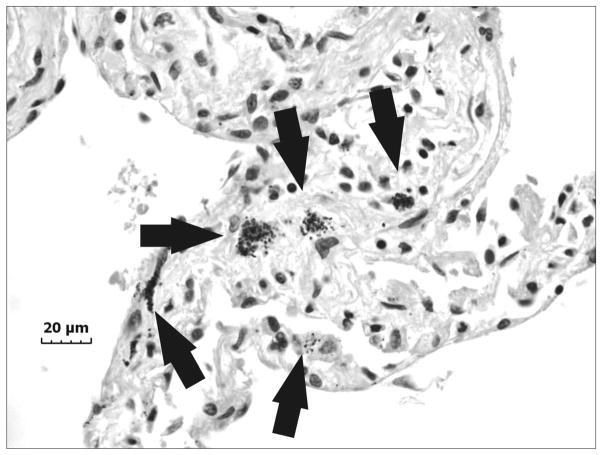
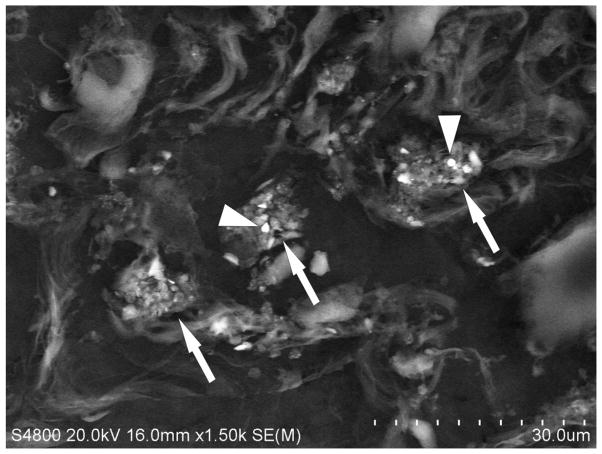
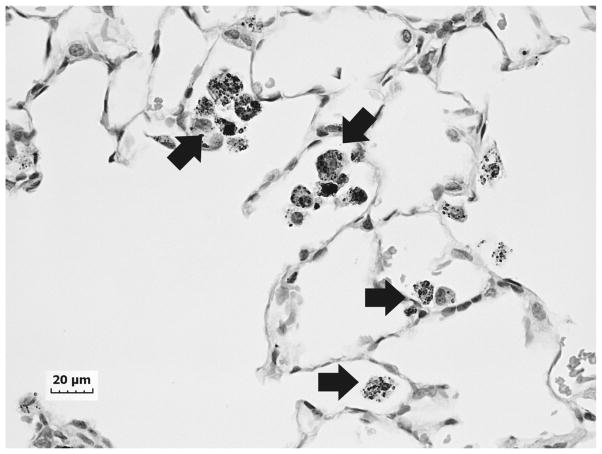
An accumulation of fluid, a grainy material consisting of protein and fat in the alveoli of the lungs, and inflammatory cell influx were generally present throughout the sample collected from the right upper lung lobe of the exposed welder’s lungs (Fig. 1). On closer examination, numerous welding particle agglomerates were observed to have accumulated and persisted in AMs throughout the human lungs as shown by the particles in the light micrograph in Fig. 2 (arrows). SEM analysis of deposited particle aggregations (Fig. 3, arrows) in the lung tissue samples depicted particles of different morphologies that are often associated with welding fume, including large more spherical, opaque particles (Fig. 3, arrowheads).
Fig. 1.
Representative light micrograph of diseased lung tissue collected at autopsy from a welder who was exposed to high concentrations of welding fume throughout his career. Micron bar equals 20 μm.
Fig. 2.
Representative light micrograph of lung tissue collected at autopsy from a welder who was exposed to high concentrations of welding fume throughout his career depicting areas of particle accumulation (arrows). Micron bar equals 20 μm.
Fig. 3.
Representative scanning electron micrograph of lung tissue collected at autopsy from a welder who was exposed to high concentrations of welding fume throughout his career depicting areas of welding fume accumulation (arrows). Note the presence of large more spherical, opaque particles (arrowheads). Micron bar equals 30 μm.
In comparison to the findings from the human lungs, granulomatous areas were observed throughout the rat lungs after 28 weeks of welding fume exposure (Fig. 4). Similar to the observations in the human lung sample, multiple deposits of welding particle agglomerates were observed throughout the rat lungs (Fig. 4, arrows). The deposited particles displayed a golden color that is consistent with welding particles rich in Fe. TEM analysis showed many of the deposited particles to be aggregates of much smaller ultrafine or nanometer-sized particles with some much larger, more spherical particles present as well (Fig. 5). To determine if chronic welding fume exposure increased protein and cellular accumulation in the air spaces of rats, bronchoalveolar lavage was performed on the lungs at 7 days after the 28-week treatment regimen. Statistically significant elevations in total protein, total cells, AMs, PMNs, and LYMPHs were recovered from the lungs of rats treated with welding fume for 28 weeks compared to saline controls (Fig. 6).
Fig. 4.
Representative light micrograph of lung tissue collected from a rat that had been exposed to welding fume (2 mg per week) for 28 weeks. Multiple deposits of welding particles were observed throughout the lungs (arrows). Micron bar equals 20 μm.
Fig. 5.
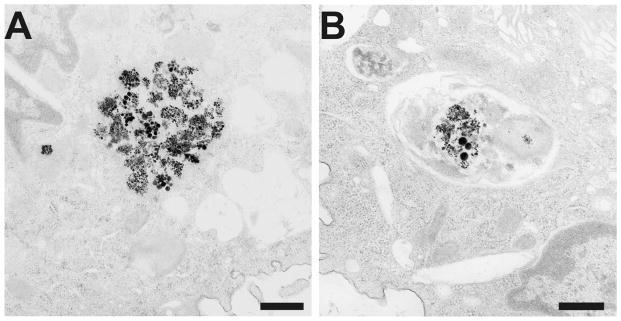
Representative transmission electron micrographs of aggregates of welding particles that had deposited in phagolysosomes of AMs in rat lungs after 28 weeks of weekly treatments of 2 mg of welding fume (A and B). Micron bar equals 500 nm.
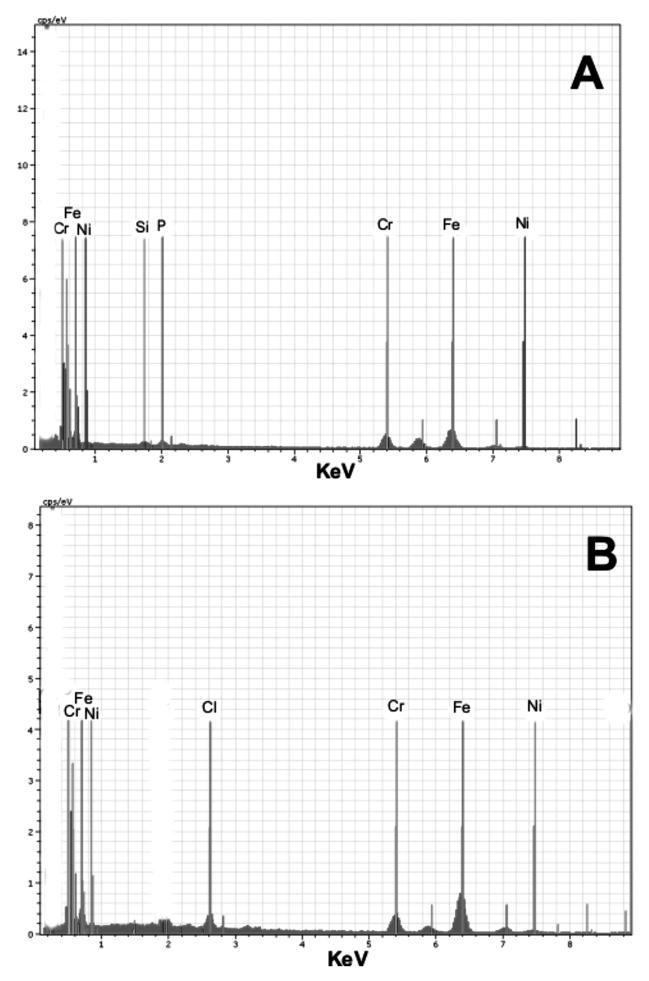
Elemental analysis of individual welding particles with agglomerations that had accumulated in the lung samples collected from the welder was performed by SEM–EDS (Fig. 7). Nearly every particle that was examined was determined to be metallic and was composed of different metals commonly found in welding fumes. SEM–EDS elemental analysis indicated that the metals were complexed together but distributed heterogeneously among the different individual particles that were scanned. Particles were composed of mostly Fe, Cr, and Ni with some presence of silicates in the human lung sample (Fig. 7A). SEM–EDS analysis of the lung samples collected from the rats exposed to welding fume demonstrated a similar elemental profile as to what was observed for the human lung samples (Fig. 7B). Cr, Fe, and Ni were the predominant metals still present in the rat lungs after 28 weeks of treatment.
Fig. 7.
Elemental spectra of one random metallic particle agglomerate (high backscattered electron image contrast) in one random area of deposition from (A) human and (B) rat lungs exposed to welding fume as determined by SEM–EDS analysis of random welding particles in preserved lung tissue samples. (The labeled thin vertical lines are guidelines to the positions of the spectral peaks for the elements and should not be confused with the spectra themselves.)
DISCUSSION
When generated, welding fumes generally appear as chain-like agglomerates composed of nanometer-sized primary particles (Zimmer and Biswas, 2001; Antonini et al., 2006, 2011b; Sowards et al., 2010). In the lung tissue samples collected from a welder with extensive exposure to welding fume and rats repeatedly exposed to welding fume for 28 weeks in this study, welding particle agglomerates deposited and persisted in lung structures and were easily visualized using light and electron microscopy. Agglomerates of deposited welding particles mostly were observed within lung cells, particularly AMs. Previous case studies have indicated deposited welding particles in lungs to have diameters that mostly ranged from 0.2 to 1.0 μm in size with some particles as small as 10 nm (Hull and Abraham, 2002). Larger more, spherical opaque particles that differ in appearance from the smaller primary particles also have been observed in tissue samples and are characteristic of a welding fume exposure (Stern et al., 1983). Similar observations were made in this study in regards to size and morphology of individual particles that had deposited in the lung tissue samples of the welder.
The persistence of the welding particles in AMs likely has toxicological implications. Welding fume exposure has been observed to have immunosuppressive and immunotoxic effects in welders and exposed laboratory animals (reviewed by Zeidler-Erdely et al., 2012). The presence of specific metals (e.g. Cr) from welding particles in AMs has been shown to reduce immune defenses in rats by altering AM immune cytokine cell signaling and the ability of AMs to respond to bacterial challenge (Antonini et al., 2004; Antonini and Roberts, 2007). Importantly, it was observed that individual welding particles and smaller particle agglomerations, especially in the rat lung tissue samples, had deposited in lung interstitial areas. Due to the presence of pulmonary capillaries within the interstitium, the nanometersized welding particles and/or their associated solubilized metals would have direct access to the bloodstream and possibly translocate to and have potentially toxic effects in other organ systems (e.g. brain, heart).
As was observed with the persistence of the welding agglomerates in the lungs in this study, both animal and worker studies have indicated that welding particle deposits, depending on the type of fume, can persist in the lungs for a significant period of time. It was determined that the elimination half-time (t1/2) for stainless steel particles in the lungs was 47 days compared to only 18 days for mild steel particles after a single, bolus, intratracheal instillation (Antonini et al., 1996). Similarly, stainless steel welding fumes (t1/2 of 38.5 days) and mild steel fumes (t1/2 of 24.8 days) were observed to persist in the lungs of rats after a short-term inhalation exposure of only 3 days (Antonini et al., 2011a). In humans, the retention half-time of mostly insoluble particles in the alveolar region has been estimated to be up to 700 days (Oberdorster, 2004).
The fate of the deposited welding particles and metals can be quite complicated. Differences in solubility and valence states of the different metals present in the welding fumes may influence the bioavailability and lung clearance of the metals after deposition. Lam et al. (1979) demonstrated that the metal constituents of welding fume were cleared in different phases. In the initial phase, intact welding particles were cleared from the body within days up to a week by mucociliary and lung macrophage clearance. The clearance rates of each element of the fume were similar during this initial phase, indicating that the eliminated particles were transported unchanged, without solubilization or separation of constituents. At the later phase, specific elements were cleared at much different rates with biological half-lives of several weeks, indicative of tissue solubilization of the deposited welding particles. Elemental spectra of deposited welding particles in this study demonstrated a similar metal profile when comparing the rat and welder lungs. Analysis showed that the metals were complexed together but distributed heterogeneously and that Fe, Cr, and Ni were the most common metals present. The presence of Cr and Ni in the welder’s lungs was indicative of exposure to stainless steel, inconel, and hastelloy welding fumes that was confirmed by work history.
The career welder examined in this study had been diagnosed with COPD and occupational asthma as diagnosed by spirometry with worsening dyspnea on exertion in his early 60s due to welding fume exposure. His history of cigarette smoking (52 pack-year smoking history) as a contributor to his poor respiratory health also should not be ignored. Pathological analysis of lung tissue collected post-mortem from the welder clearly demonstrated inflammatory cell influx and evidence of significant pulmonary injury. The poor condition of the welder’s lungs was not surprising due to his documented breathing problems in the last years of his life. In addition, he reported a non-productive cough that produced little phlegm that persisted for many years. Chronic bronchitis, a condition characterized by a chronic, productive cough, is a common respiratory complaint of welders (Sferlazza and Beckett, 1991; Martin et al., 1997; Antonini, 2003). However, the prevalence of cigarette smoking among welders makes it difficult to determine if welding fume exposure is a causative factor for the development of chronic bronchitis.
Differing observations have been made when examining the effect of welding fume inhalation on pulmonary function of exposed workers. This may be due to differences in ventilation of the exposure area, duration of exposure, the types of welding processes and materials used, or exposure to confounding factors, such as tobacco smoke. In a literature review, Sferlazza and Beckett (1991) concluded that exposure to welding fume alone had little effect on pulmonary function. However, specific groups of welders who are exposed to high concentrations of welding fume may be more susceptible to the development of lung function decrements. It has been documented that shipyard welders, who are oftentimes exposed to higher fume conditions because of work in confined, poorly ventilated areas, like the welder described in this case study, had greater reductions in pulmonary function than welders who worked in well-ventilated areas (Oxhoj et al., 1979; Akbar- Khanzadeh 1980, 1993; Mur et al., 1985). In addition, stainless steel welders have been shown to be more susceptible to lung function changes as compared to welders exposed to non-stainless steel fumes (Sobaszek et al., 2000; Hannu et al., 2005; Brand et al., 2010). Moreover, in regards to occupational asthma, El-Zein et al. (2003) observed that the incidence of airway hyper-responsiveness increased 11.9% in apprentice welders from the time of baseline lung function tests taken at the start of the apprenticeship to when follow-up tests were taken 15 months later at the end of their training. Also, Banga et al. (2011) reported that welding fumes were among the top five workplace exposures associated with the development of occupational asthma among Michigan workers, with a large majority of subjects suffering chronic respiratory symptoms.
The histological findings of the lungs observed in the rats treated with welding fume were similar to what was seen with the welder case study. There was significant inflammatory cell (AMs, PMNs, and LYMPHs) influx into the lungs and an increase in the presence of protein in airspaces after 28 weekly intratracheal instillations with a welding fume that contained many of the same metals that were inhaled by the welder on a chronic basis. Based on the findings from animal studies, the types of metals and fumes, specifically stainless steel, that the welder was exposed to have been shown to be highly biologically reactive (Antonini et al., 1998; Leonard et al., 2010) and the most pneumotoxic (Antonini et al., 1996, 2011a; Taylor et al., 2003). Interestingly, despite the high weekly dose of welding fume, no evidence of pulmonary fibrosis was observed in the lungs of the treated rats. Interstitial fibrosis has been observed to be appear in rats as early as 60 days and become prominent by 90 days after exposure to relatively high levels (105–118 mg m−3) of stainless steel welding fume for 2 h a day (Yu et al., 2001). In addition, the same group of investigators indicated that the rats exposed for 60–90 days to high concentrations of stainless steel welding fumes did not recover from the lung fibrosis and X-ray abnormalities, lung function impairment, and pulmonary inflammation that had developed (Yu et al., 2003; Sung et al., 2004; Yang et al., 2009). The difference in the fibrotic responses observed in these studies with this study may be due to the fact that animals in this study were exposed to welding fume by intratracheal instillation and thus not exposed to any of the potential toxic and irritating gases, such as nitrogen dioxide, that are also generated during the welding processes (Guidotti, 1978; Stern et al., 1983).
Pulmonary fibrosis has been reported in welders. Welders are commonly exposed to a number of different metals such as Fe Cr, Ti, and Al that are associated with pneumoconiosis and lung disease (Abraham et al., 1994, 1997). In a search of 3600 indexed pathology cases, Stern et al. (1983) discovered pulmonary fibrosis in 29 welders. Using a rat macrophage in vitro bioassay, they observed a manual metal arc-stainless steel welding fume to have distinct fibrogenic potential, although only a small percentage of the welders that demonstrated pulmonary fibrosis in the study were exposed to that particular fume. Thus, they proposed that exposure to nitrogen dioxide gas during welding may be the causative agent associated with the fibrogenic responses in the welders. In agreement, Verhoff and Muller (2000) could not correlate deposition of specific metals common in welding fume, in particular Fe, with fibrotic processes in the lung samples of 43 welders. Buerke et al. (2002) examined 15 welders with interstitial pulmonary fibrosis and concluded that a causal relationship exists between pulmonary fibrosis and welding when exposures are for long periods of time at very high concentrations. More recent reports have observed cases of pulmonary fibrosis in welders that is associated with siderosis, a condition characterized by the accumulation of significant amounts of Fe in the lungs (McCormick et al., 2008; Ji et al., 2012). Exposure to aluminum welding fume, as was the case with the welder in this study, also has been associated with severe pneumoconiosis and pulmonary fibrosis (Vallyathan et al., 1982; Hull and Abraham, 2002). Because of the presence of a small right lung nodule and the observation of scarring in the left major fissure and lingula as determined by computed tomography scan before the welder’s death, it is likely that the welder in this case study suffered from some degree of pulmonary fibrosis. This would not be surprising based on the welder’s history of chronic exposure to high levels of metal particles and gases generated during welding.
Acknowledgments
The investigators of the study would like to acknowledge the family of the deceased welder who arranged for the donation of the lung tissue and Dr Brenton A. Meda of Great Falls, MT for the harvesting, processing, and fixation of the donated lung samples.
Footnotes
Disclaimer—The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Abraham JL, Hunt A, Burnett BR. Steel (Fe-Cr) particles as a marker of welding fume exposure, frequency of occurrence and prevalence in a series of over 400 human lungs. Ann Occup Hyg. 1994;38(Suppl 1):551–7. [Google Scholar]
- Abraham JL, Hunt A, Burnett BR. Lung pathology and mineralogy associated with high pulmonary burden of metal particles: Fe, Ti, Al, and Cr in a pneumoconiosis database. Ann Occup Hyg. 1997;41(Suppl 1):522–30. [Google Scholar]
- Akbar-Khanzadeh F. Long-term effects of welding fumes upon respiratory symptoms and pulmonary function. J Occup Med. 1980;22:337–41. doi: 10.1097/00043764-198005000-00007. [DOI] [PubMed] [Google Scholar]
- Akbar-Khanzadeh F. Short-term respiratory function changes in relation to workshift welding fume exposures. Int Arch Occup Environ Health. 1993;64:393–7. doi: 10.1007/BF00517944. [DOI] [PubMed] [Google Scholar]
- Antonini JM. Health effects of welding. Crit Rev Toxicol. 2003;33:61–103. doi: 10.1080/713611032. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Afshari AA, Stone S, et al. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J Occup Environ Hyg. 2006;3:194–203. doi: 10.1080/15459620600584352. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Clarke RW, Krishna Murthy GG, et al. Freshly generated stainless steel welding fume induces greater lung inflammation in rats as compared to aged fume. Toxicol Lett. 1998;98:77–86. doi: 10.1016/s0378-4274(98)00103-9. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Keane M, Chen BT, et al. Alterations in welding process voltage affect the generation of ultrafine particles, fume composition, and pulmonary toxicity. Nanotoxicology. 2011b;5:700–10. doi: 10.3109/17435390.2010.550695. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Krishna Murthy GG, Rogers RA, et al. Pneumotoxicity and pulmonary clearance of different welding fumes after intratracheal instillation in the rat. Toxicol Appl Pharmacol. 1996;140:188–99. doi: 10.1006/taap.1996.0212. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR. Chromium in stainless steel welding fume suppresses lung defense responses against bacterial infection in rats. J Immunotoxicol. 2007;4:117–27. doi: 10.1080/15476910701336953. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Chapman RS, et al. Pulmonary toxicity and extrapulmonary tissue distribution of metals after repeated exposure to different welding fumes. Inhal Toxicol. 2010;22:805–16. doi: 10.3109/08958371003621641. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. Short-term inhalation exposure to mild steel welding fume had no effect on lung inflammation and injury but did alter defense responses to bacteria in rats. Inhal Toxicol. 2009;21:182–92. doi: 10.1080/08958370802360661. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. Persistence of deposited metals in the lungs after stainless steel and mild steel welding fume inhalation in rats. Arch Toxicol. 2011a;85:487–98. doi: 10.1007/s00204-010-0601-1. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Stone S, Roberts JR, et al. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol Appl Pharmacol. 2007;223:234–45. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Taylor MD, Millecchia L, et al. Suppression in lung defense responses after bacterial infection in rats pretreated with different welding fumes. Toxicol Appl Pharmacol. 2004;200:206–18. doi: 10.1016/j.taap.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Banga A, Reilly MJ, Rosenman KD. A study of characteristics of Michigan workers with work-related asthma exposed to welding. J Occup Environ Med. 2011;53:415–9. doi: 10.1097/JOM.0b013e31820fd0c3. [DOI] [PubMed] [Google Scholar]
- Brain JD, Knudson DE, Sorokin SP, et al. Pulmonary distribution of particles given by intratracheal instillation or by aerosol inhalation. Environ Res. 1976;11:13–33. doi: 10.1016/0013-9351(76)90107-9. [DOI] [PubMed] [Google Scholar]
- Brand P, Gube M, Gerards K, et al. Internal exposure, effect monitoring, and lung function in welders after acute short-term exposure to welding fumes from different welding processes. J Occup Environ Med. 2010;52:887–92. doi: 10.1097/JOM.0b013e3181f09077. [DOI] [PubMed] [Google Scholar]
- Buerke U, Schneider J, Rösler J, et al. Interstitial pulmonary fibrosis after severe exposure to welding fumes. Am J Ind Med. 2002;41:259–68. doi: 10.1002/ajim.10055. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. Occupational outlook handbook: welders, cutter, solders, and brazers. U.S. Department of Labor; 2012. [Accessed 05 August 2012]. Available at http://www.bls.gov/ooh. [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, et al. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- El-Zein M, Malo JL, Infante-Rivard C, et al. Incidence of probable occupational asthma and changes in airway calibre and responsiveness in apprentice welders. Eur Respir J. 2003;22:513–8. doi: 10.1183/09031936.03.00000903. [DOI] [PubMed] [Google Scholar]
- Guidotti TL. The higher oxides of nitrogen: inhalation toxicology. Environ Res. 1978;15:443–72. doi: 10.1016/0013-9351(78)90125-1. [DOI] [PubMed] [Google Scholar]
- Hannu T, Piipari R, Kasurinen H, et al. Occupational asthma due to manual metal-arc welding of special stainless steels. Eur Respir J. 2005;26:736–9. doi: 10.1183/09031936.05.00130504. [DOI] [PubMed] [Google Scholar]
- Henderson RF, Driscoll KE, Harkema JR, et al. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Fundam Appl Toxicol. 1995;24:183–97. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- Hull MJ, Abraham JL. Aluminum welding fume-induced pneumoconiosis. Hum Pathol. 2002;33:819–25. doi: 10.1053/hupa.2002.125382. [DOI] [PubMed] [Google Scholar]
- ICRP. Human respiratory tract model for radiological protection: a report of a task group of the international commission on radiological protection. Annals ICRP. 1994;24:267–72. [PubMed] [Google Scholar]
- Ji C, Chen G, Cai HR, et al. An unusual case of Welder’s siderosis with local massive fibrosis: a case report. Chin Med J. 2012;125:552–4. [PubMed] [Google Scholar]
- Lam HF, Hewitt PJ, Hicks R. A study of pulmonary deposition, and the elimination of some constituent metals from welding fume in laboratory animals. Ann Occup Hyg. 1979;21:363–73. doi: 10.1093/annhyg/21.4.363. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Chen BT, Stone SG, et al. Comparison of stainless and mild steel welding fumes in generation of reactive oxygen species. Part Fibre Toxicol. 2010;7:32. doi: 10.1186/1743-8977-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ, Guidotti TL, Langard S. Respiratory hazards of welding. Clin Pulm Med. 1997;4:194–204. [Google Scholar]
- McCormick LM, Goddard M, Mahadeva R. Pulmonary fibrosis secondary to siderosis causing symptomatic respiratory disease: a case report. J Med Case Rep. 2008;2:257. doi: 10.1186/1752-1947-2-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur JM, Teculescu D, Pham QT, et al. Lung function and clinical findings in a cross-sectional study of arc welders. An epidemiological study. Int Arch Occup Environ Health. 1985;57:1–17. doi: 10.1007/BF00383541. [DOI] [PubMed] [Google Scholar]
- NIOSH; NIOSH, editor. NIOSH manual of analytical methods. 4. Washington, DC: U.S. Department of Health and Human Services; 1994. Elements (ICP): method 7300; pp. 1–10. Issue 2, Publication No. 98–119. [Google Scholar]
- Oberdorster G. Kinetics of inhaled ultrafine particles in the organism. In: Heinrich U, editor. Effects of air contaminants on the respiratory tract - interpretations from molecular to meta analysis. Hannover, Fraunhofer: IRB Verlag; 2004. pp. 122–43. [Google Scholar]
- Oxhoj H, Bake B, Wedel H, et al. Effects of electric arc welding on ventilatory lung function. Arch Environ Health. 1979;34:211–7. doi: 10.1080/00039896.1979.10667400. [DOI] [PubMed] [Google Scholar]
- Reasor MJ, Antonini JM. Pulmonary responses to single versus multiple intratracheal instillations of silica in rats. J Toxicol Environ Health A. 2000;62:9–21. doi: 10.1080/00984100050201631. [DOI] [PubMed] [Google Scholar]
- Sferlazza SJ, Beckett WS. The respiratory health of welders. Am Rev Respir Dis. 1991;143:1134–48. doi: 10.1164/ajrccm/143.5_Pt_1.1134. [DOI] [PubMed] [Google Scholar]
- Sobaszek A, Boulenguez C, Frimat P, et al. Acute respiratory effects of exposure to stainless steel and mild steel welding fumes. J Occup Environ Med. 2000;42:923–31. doi: 10.1097/00043764-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Sowards JW, Ramierz AJ, Dickinson DW, et al. Characterization of welding fume from SMAW electrodes - part II. Welding J. 2010;89:82s–90s. [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, et al. Mitochondrial dysfunction and loss of Parkinson’s disease-linked proteins contribute to neurotoxicity of manganese-containing welding fumes. FASEB J. 2010;24:4989–5002. doi: 10.1096/fj.10-163964. [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Gehr P, et al. Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol. 1992;6:235–43. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- Stern RM, Pigott GH, Abraham JL. Fibrogenic potential of welding fumes. J Appl Toxicol. 1983;3:18–30. doi: 10.1002/jat.2550030106. [DOI] [PubMed] [Google Scholar]
- Sung JH, Choi BG, Maeng SH, et al. Recovery from welding-fume-exposure-induced lung fibrosis and pulmonary function changes in sprague dawley rats. Toxicol Sci. 2004;82:608–13. doi: 10.1093/toxsci/kfh289. [DOI] [PubMed] [Google Scholar]
- Taylor MD, Roberts JR, Leonard SS, et al. Effects of welding fumes of differing composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol Sci. 2003;75:181–91. doi: 10.1093/toxsci/kfg173. [DOI] [PubMed] [Google Scholar]
- Vallyathan V, Bergeron WN, Robichaux PA, et al. Pulmonary fibrosis in an aluminum arc welder. Chest. 1982;81:372–4. doi: 10.1378/chest.81.3.372. [DOI] [PubMed] [Google Scholar]
- Verhoff MA, Müller KM. Sideroelastosis of pulmonary vessels after welder dust exposure. Pathologe. 2000;21:229–33. doi: 10.1007/s002920050392. [DOI] [PubMed] [Google Scholar]
- Yang MJ, Yang YS, Sung JH, et al. Recurrent exposure to welding fumes induces insufficient recovery from inflammation. Inhal Toxicol. 2009;21:337–46. doi: 10.1080/08958370802448979. [DOI] [PubMed] [Google Scholar]
- Yu IJ, Song KS, Chang HK, et al. Lung fibrosis in Sprague-Dawley rats, induced by exposure to manual metal arc-stainless steel welding fumes. Toxicol Sci. 2001;63:99–106. doi: 10.1093/toxsci/63.1.99. [DOI] [PubMed] [Google Scholar]
- Yu IJ, Song KS, Chang HK, et al. Recovery from manual metal arc-stainless steel welding-fume exposure induced lung fibrosis in Sprague-Dawley rats. Toxicol Lett. 2003;143:247–59. doi: 10.1016/s0378-4274(03)00154-1. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Erdely A, Antonini JM. Immunotoxicology of arc welding fume: worker and experimental animal studies. J Immunotoxicol. 2012;9:411–25. doi: 10.3109/1547691X.2011.652783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer AT, Biswas P. Characterization of the aerosols resulting from arc welding processes. J Aerosol Sci. 2001;32:993–1008. [Google Scholar]