Abstract
The cation cis-[Ru(bpy)2(5CNU)2]2+ (bpy = 2,2′-bipyridine, 5CNU = 5-cyanouracil) was synthesized and investigated for use as a potential dual-action photodynamic therapy agent. The complex undergoes efficient photoinduced 5CNU ligand exchange for solvent water molecules, thus simultaneously releasing biologically active 5CNU and generating [Ru(bpy)2(H2O)2]2+. The latter binds covalently to ds-DNA, such that photolysis results in the generation of three potential therapeutic agents from a single molecule.
The chemotherapy agent 5-flurouracil (5FU) has been in use for over 20 years for the treatment of malignancies including colorectal and breast cancers.1 The mode of action of 5FU is multifaceted and involves its incorporation into DNA and RNA, as well as inhibition of thymidylate synthase, an enzyme important in DNA synthesis and repair.1 Other derivitives of uracil also exhibit cellular activity including 5-cyanouracil (5CNU), which has been shown to inhibit pyrimidine catabolism in vivo.2 Complexation of 5CNU to a photoactive transition metal complex represents a manner in which this drug may be caged in an inactive state until delivered to its target, at which time it can be released by irradiation with low energy light.
In addition to the photoinduced release of the caged 5CNU drug, the transition metal portion of the complex itself may be biologically active. It has been shown by us and others that ruthenium and dirhodium complexes hold great potential for photodynamic thereapy (PDT).3-6 In addition, we have also reported photoactive complexes inspired by the mode of action of cisplatin; in these cases, the complex is stable in the dark but undergoes ligand exchange and binds to DNA upon irradiation.7 These complexes include cis-[Ru(bpy)2(NH3)2]2+ (bpy = 2,2′-bipyridine) and cis-[Rh2(O2CCH3)2(CH3CN)6]2+,7 where a 34-fold increase in toxicity towards Hs27 human skin fibroblasts was reported for the latter upon irradiation with visible light.7b It should also be noted that ruthenium complexes are now recognized as promising anticancer drugs, with some being subjected to clinical and pre-clinical trials.8
Nitriles undergo photoinduced ligand exchange more efficiently than other monodentate ligands when bound to Ru(II), includig pyridine and NH3.9,10 As such, complexes with various nitrile ligands are promising PDT candidates. In this vein, we hypothesized that two 5CNU molecules coordinated to ruthenium through their nitrile substituents would efficiently undergo ligand exchange with solvent upon irradiation, thus releasing two equivalents of the drug and the diaqua Ru(II) complex. The present work focuses on the synthesis of the new complex cis-[Ru(bpy)2(5CNU)2]2+ (1) and the investigation of its photophysical properties and potential for its use as a dual-action photochemotherapeutic agent.
The reaction of 50 mg (0.1 mmol) of cis-Ru(bpy)2Cl2 with 2.2 eq AgCF3SO3 in 10 mL methanol at room temperature resulted in the precipation of AgCl and the formation of red cis-Ru(bpy)2(CF3SO3)2. After filtration to remove AgCl, the solution was refluxed with a 5-fold excess of 5CNU for 2 h to generate the yellow complex 1 (Supporting Information). The 1H NMR spectrum of 1 is consistent with two 5CNU molecules and two bpy ligands coordinated to the metal center (Supporting Information), also evidenced in the crystal structure of the product shown in Figure 1a. The Ru–N bond lengths to the 5CNU ligand are 2.034(3) and 2.025(3) Å, while those to the bpy nitrogen atoms positioned trans to 5CNU are 2.044(3) Å. Slightly longer Ru-N bonds were found for the two bpy nitrogen atoms positioned trans to another bpy ligand, 2.061(3) and 2.067(3) Å. These distances are similar to those previously reported for cis-[Ru(bpy)2(CH3CN)2]2+ (2).11
Figure 1.
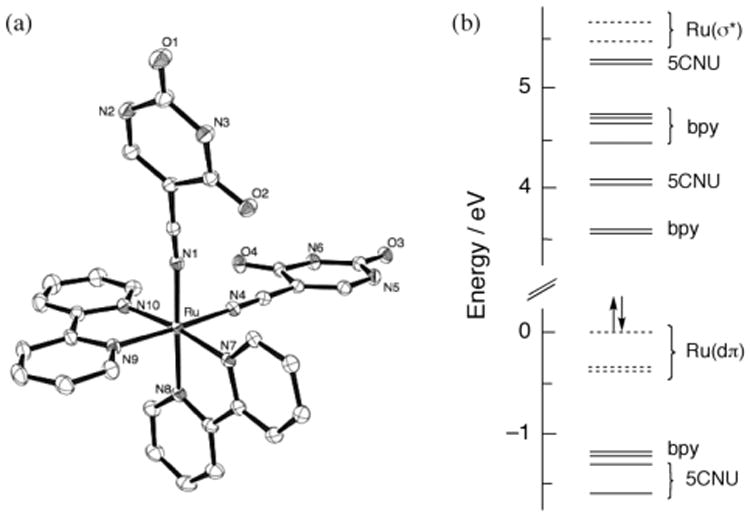
(a) Thermal ellipsoid plot (drawn with 30% probability ellipsoids; hydrogen atoms removed for the sake of clarity) and (b) calculated molecular orbital diagram of 1.
The RuIII/II couple of 1 is observed at +1.45 V vs SCE in CH3CN (0.1 M Bu4NPF6), at a similar potential to that measured for 2 at +1.44 vs SCE,12,13 and related Ru(II) complexes.14,15 Two high energy transitions are observed in the electronic absorption spectrum of the chloride salt of 1 in water at 243 nm (ε = 31 000 M−1 cm−1) and 284 nm (ε = 48 300 M−1 cm−1) assigned as ligand-centered (LC) ππ* transitions of bpy, with maxima at 240 nm (ε = 17 400 M−1 cm−1) and 283 nm (ε = 52 500 M−1 cm−1) in 2 in CH3CN. The shoulder at 315 nm (ε = 21 300 M−1 cm−1) in 1 is not present in the absorption spectrum of 2 or in free 5CNU (λmax = 275 nm, ε = 11 700 M−1 cm−1 in water), and can therefore be assigned to a metal-to-ligand charge transfer (MLCT) transition from the Ru(II) center to the 5CNU ligand. The absorption maximum at 410 nm (ε = 7 800 M−1 cm−1) in 1 (H2O) is consistent with the Ru→bpy MLCT transition observed at 425 nm (ε = 8 590 M−1 cm−1) in 2 in CH3CN.16
Density functional theory (DFT) calculations were perfromed on 1 in CH3CN. The minimized structure from the calculations is consistent with the crystal structure, as is evident from the Ru–N bond lenghts to the 5CNU ligands calculated to be 2.038 Å and shorter than the other Ru–N bonds in the complex (Table S2, Supporting Information). Moreover, the calculated orbital energies support the assignments of the electronic absorption transitions (details in Supporting Informatin). The molecular orbital (MO) diagram calculated for 1 is shown in Figure 1b. The electron density of the three highest occupied molecular orbitals (HOMOs) is localized on the metal center; these MOs are labeled Ru(dπ) in Figure 1b. The LUMO (lowest unoccupied molecular orbital) and LUMO+1 are bpy π* orbitals, while the electron densities of the LUMO+2 and LUMO+3 are localized on the 5CNU ligands. Accordingly, inspection of Figure 1b reveals that Ru→bpy, followed by Ru→5CNU, are expected to be the lowest energy transitions in 1. It should also be noted that the Ru(σ*) orbitals lie at signficantly greater energies, ∼5.5 eV above the HOMO, and comprise the LUMO+10 and LUMO+11 (Figure 1).
Irradiation of the chloride salt of 1 in water with visible light (λirr < 395 nm) results in spectral changes, with a decrease in the MLCT absorption at 410 nm, as shown in Figure 2. A species with a maximum at ∼450 nm forms within 2 min of irradiation (Figure 2, inset, bold line), attributed to the mono-aqua intermediate cis-[Ru(bpy)2(5CNU)(H2O)]2+ (3), similar to that previously reported for the photochemistry of 2.10 This peak deceases in intensity with continued photolysis with a concomitant increase of the product peak at 490 nm (Figure 2). The peak at 490 nm (ε = 9 300 M−1 cm−1) is known to correspond to the di-aqua complex cis-[Ru(bpy)2(H2O)2]2+ (4),17 consistent with the photoinduced exchange of each 5CNU ligand with a solvent water molelcule. The quantum yield for the disappearance of 1 to generate the mono-aqua intermediate 3 was measured to be 0.16 ± 4 (λrr = 400 nm, see Supporting Information for details), a slighly lower value than that reported for 2 (Φ = 0.21, λirr = 400 nm).10 It should be noted that no spectral changes are observed when the sample is kept in the dark under similar experimental conditions.
Figure 2.
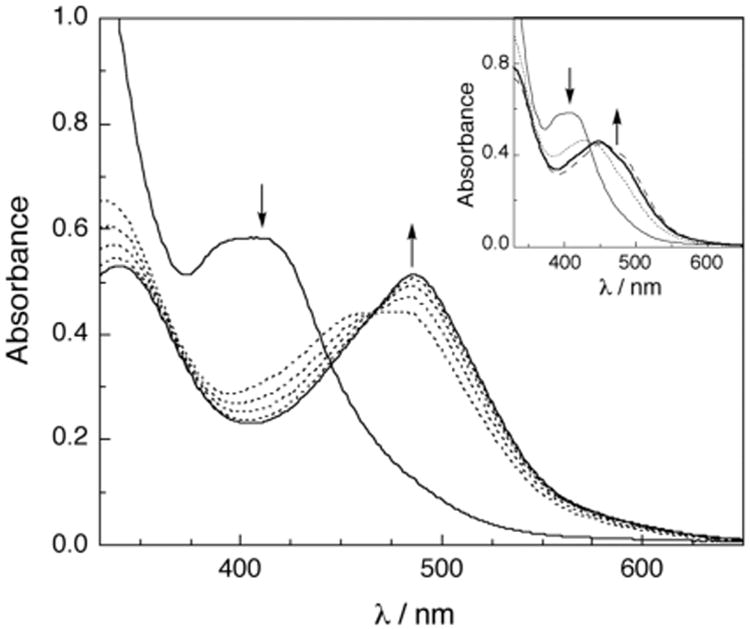
Changes to the electronic absorption spectrum of 77 μM 1 upon irradiation in H2O at tirr = 0, 5, 7.5, 10, 12.5, and 15 min; inset: 0, 1, 2, and 3 min (λirr > 395 nm).
When 1 (80 μM) is photolyzed for 90 s (Supporting Information), the majority of the product formed is the mono-aqua complex, 3, with a maximum at ∼450 nm. If that solution is then stored in the dark, no further reactivity is observed (Figure S2), a fact that is indicative of the requirement of a photon for the conversion of 3 to the final product, 4. This experiment clearly supports the conclusion that the formation of the bis-aqua product 4 from 1 requires the sequential absorption of two photons, one to effect the exchange of each 5CNU ligand.
The photolysis of 1 with visible light was also monitored by 1H NMR spectroscopy in D2O. Prior to irradiation, a peak corresponding to the C–H ring proton of the two bound 5CNU molecules is observed at 8.02 ppm. This peak decreases in intensity upon irradiation with the appearance of two new resonances at 8.00 ppm and 8.18 ppm, assigned to the same 5CNU proton in cis-[Ru(bpy)2(5CNU)(D2O)]2+ (3a) and free 5CNU in D2O, respectively. Continued photolysis results in further decrease of the 5CNU peak corresponding to 3a and greater intensity of the free 5CNU peak, consistent with the formation of the bis-aqua product. Spectral changes are also observed for the bpy protons, but overlapping resonances of the reactant, intermediate and product preclude assignments to be made.
The covalent binding of cisplatin to DNA results in decreased mobility of linearized plasmid in agrose gel electrophoresis. 7,18 Our group has shown that certain complexes are capable of covalently binding to DNA when activated by light.7 Agrose mobility shift gels were conducted in order to test the ability of 1 to bind to DNA when irradiated. Photolysis of 1 (λrr ≥ 395 nm, tirr = 15 min) in the presence of linearized pUC18 plasmid results in a decrease of mobility of the ds-DNA as a function of increased complex concentration (Figure 3a).19 In contrast, when the plasmid is incubated in the dark for 20 min no change in the DNA mobility is observed (Figure 3b).
Figure 3.
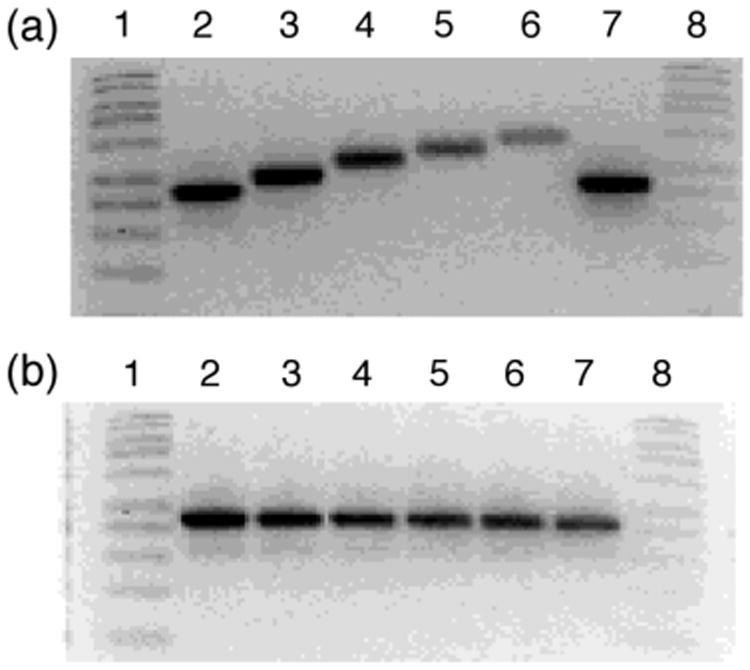
Imaged ethidium bromide stained agarose gel of 50 μM linearized pUC18 plasmid (10 mM phophate buffer, pH = 8.3) in the presence of various concentrations of 1. Lanes 1 and 8, 1kb DNA molecular weight standard; lanes 2 and 7, linearized plasmid alone; lanes 3 – 6, 25 μM, 75 μM, 150 μM, and 250 μM complex (a) irradiated with λirr > 395 nm (tirr = 15 min) and (b) incubated in dark for 15 min at 298 K.
The work presented here demonstrates that, upon photolysis of 1 in aqueous media, complex 4 is formed which subsequently covelently binds to DNA. Moreover, two equivalents of the biologically active 5CNU molecule are released in the process, rendering this type of complex a potential dual-action photochemotherapy agent.
Supplementary Material
Acknowledgments
C.T. and K.R.D. thank the National Science Foundation (CHE 0911354) for partial funding of this work and C.T. thanks the Ohio Supercomputer Center for their generous support. R.N.G. thanks the National Institutes of Health for a Ruth L. Kirschstein National Research Service Award/MARC Fellowship (GM 833552).
Footnotes
Supporting Information. Synthesis details, complete crystal structure and cyrstalligraphic data, and dark activity. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Longley DB, Harkin DP, Johnston PG. Nature Reviews Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 2.(a) Gentry GA, Morse PA, Dorsett MT. Cancer Research. 1971;31:909–912. [PubMed] [Google Scholar]; (b) Porter DJT, Chestnut WG, Merrill BM, Spector T. J Biol Chem. 1992;267:5236–5242. [PubMed] [Google Scholar]
- 3.(a) Angeles-Boza AM, Bradley PM, Fu PKL, Wicke SE, Bacsa J, Dunbar KR, Turro C. Inorg Chem. 2004;43:8510–8519. doi: 10.1021/ic049091h. [DOI] [PubMed] [Google Scholar]; (b) Bradley PM, Angeles-Boza AM, Dunbar KR, Turro C. Inorg Chem. 2004;43:2450–2452. doi: 10.1021/ic035424j. [DOI] [PubMed] [Google Scholar]; (c) Angeles-Boza AM, Bradley PM, Fu PKL, Shatruk M, Hilfiger MG, Dunbar KR, Turro C. Inorg Chem. 2005;44:7262–7264. doi: 10.1021/ic0500715. [DOI] [PubMed] [Google Scholar]; (d) Aguirre JD, Angeles-Boza AM, Chouai A, Pellois JP, Turro C, Dunbar KR. J Am Chem Soc. 2009;131:11353–11360. doi: 10.1021/ja9021717. [DOI] [PubMed] [Google Scholar]; (e) Joyce LE, Aguirre JD, Angeles-Boza AM, Chouai A, Fu PKL, Dunbar KR, Turro C. Inorg Chem. 2010;49:5371–5376. doi: 10.1021/ic100588d. [DOI] [PubMed] [Google Scholar]
- 4.(a) Liu Y, Hammitt R, Lutterman DA, Joyce LE, Thummel RP, Turro C. Inorg Chem. 2009;48:375–385. doi: 10.1021/ic801636u. [DOI] [PubMed] [Google Scholar]; (b) Zhao R, Hammitt R, Thummel RP, Liu Y, Turro C, Snapka RM. Dalton Trans. 2009;48:10926–10931. doi: 10.1039/b913959a. [DOI] [PubMed] [Google Scholar]; (c) Sun Y, Joyce LE, Dickson NM, Turro C. Chem Comm. 2010;46:2426–2428. doi: 10.1039/b925574e. [DOI] [PubMed] [Google Scholar]; (d) Sun Y, Joyce LE, Dickson NM, Turro C. Chem Comm. 2010;46:6759–6761. doi: 10.1039/c0cc02571b. [DOI] [PubMed] [Google Scholar]
- 5.(a) Jain A, Wang J, Mashack ER, Winkel BSJ, Brewer K. J Inorg Chem. 2009;48:9077–9084. doi: 10.1021/ic900190a. [DOI] [PubMed] [Google Scholar]; (b) Prussin AJ, II, Zhao S, Jain A, Winkel BSJ, Brewer KJ. J Inorg Bio-chem. 2009;103:427–431. doi: 10.1016/j.jinorgbio.2008.12.008. [DOI] [PubMed] [Google Scholar]; (c) Higgins SLH, White TA, Winkel BSJ, Brewer K. J Inorg Chem. 2011;50:463–470. doi: 10.1021/ic100958r. [DOI] [PubMed] [Google Scholar]
- 6.(a) Farrer NJ, Salassa L, Sadler P. J Dalton Trans. 2009;48:10690–10701. doi: 10.1039/b917753a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Salassa L, Ruiu T, Garino C, Pizarro AM, Bardelli F, Gianolio D, Westendorf A, Bednarski PJ, Lamberti C, Gobetto R, Sadler PJ. Organometallics. 2010;29:6703–6710. [Google Scholar]; (c) Farrer NJ, Woods JA, Salassa L, Zhao Y, Robinson KS, Clarkson G, Mackay FS, Sadler PJ. Angew Chemie, Int Ed. 2010;49:8905–8908. doi: 10.1002/anie.201003399. [DOI] [PubMed] [Google Scholar]; (d) Farrer NJ, Woods JA, Munk VP, Mackay FS, Sadler P. J Chem Res Tox. 2010;23:413–421. doi: 10.1021/tx900372p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ronconi L, Sadler P. J Dalton Trans. 2011;40:262–268. doi: 10.1039/c0dt00546k. [DOI] [PubMed] [Google Scholar]; (e) Liu HK, Sadler P. J Acc Chem Res. 2011;44:348–359. doi: 10.1021/ar100140e. [DOI] [PubMed] [Google Scholar]
- 7.(a) Singh TN, Turro C. Inorg Chem. 2004;43:7260–7262. doi: 10.1021/ic049075k. [DOI] [PubMed] [Google Scholar]; (b) Lutterman DA, Fu PKL, Turro C. J Am Chem Soc. 2006;128:738–739. doi: 10.1021/ja057620q. [DOI] [PubMed] [Google Scholar]
- 8.(a) Antonarakis ES, Emadi A. Cancer Chemother Pharmacol. 2010;66:1–9. doi: 10.1007/s00280-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ang WH, Dyson PJ. Eur J Inorg Chem. 2006:4003–4018. [Google Scholar]
- 9.(a) Pinnick DV, Durham B. Inorg Chem. 1984;23:1440–1445. [Google Scholar]; (b) Cruz AJ, Kirgan R, Siam K, Heiland P, Rillema DP. Inorg Chim Acta. 2010;363:2496–2505. [Google Scholar]
- 10.Liu Y, Turner DB, Singh TN, Angeles-Boza AM, Chouai A, Dunbar KR, Turro C. J Am Chem Soc. 2009;131:26–27. doi: 10.1021/ja806860w. [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay SK, Mitra K, Biswas S, Naskar S, Mishra D, Adhikary B. Trans Metal Chem. 2004;29:1–6. [Google Scholar]
- 12.Steel PJ, Lahousse F, Lerner D, Marzin C. Inorg Chem. 1983;22:1488–1493. [Google Scholar]
- 13.Walsh JL, Durham B. Inorg Chem. 1982;21:329–332. [Google Scholar]
- 14.Caspar JV, Meyer T. J Inorg Chem. 1983;22:2444–2453. [Google Scholar]
- 15.Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, Von Zelewsky A. Coord Chem Rev. 1988;84:85–277. [Google Scholar]
- 16.Brown GM, Callahan RW, Meyer TJ. Inorg Chem. 1975;8:1915–1921. [Google Scholar]
- 17.Durham B, Wilson SR, Hodgson DJ, Meyer TJ. J Am Chem Soc. 1980;102:600–607. [Google Scholar]
- 18.Fang Z, Swavey S, Holder A, Winkel B, Brewer KJ. Inorg Chem Comm. 2002;5:1078–1081. [Google Scholar]
- 19.Linearization of 100 μM pUC18 plasmid was carried out with 50 units of SmaI (Invitrogen Life Technologies) restriction enzyme in 100 mM Tris, pH = 7.6, 150 mM NaCl at 37 °C for 1 hr, followed by deactivation at 65 °C for 5 min
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


