Abstract
Plasticity constitutes the basis of behavioral changes as a result of experience. It refers to neural network shaping and re-shaping at the global level and to synaptic contacts remodeling at the local level, either during learning or memory encoding, or as a result of acute or chronic pathological conditions. ‘Plastic’ brain reorganization after central nervous system lesions has a pivotal role in the recovery and rehabilitation of sensory and motor dysfunction, but can also be “maladaptive”. Moreover, it is clear that brain reorganization it is not a “static” phenomenon but rather a very dynamic process. Spinal cord injury immediately initiates a change in brain state and starts cortical reorganization. In the long term, the impact of injury – with or without accompanying therapy – on the brain is a complex balance between supraspinal reorganization and spinal recovery. The degree of cortical reorganization after spinal cord injury is highly variable, and can range from no reorganization (i.e. “silencing”) to massive cortical remapping. This variability critically depends on the species, the age of the animal when the injury occurs, the time after the injury has occurred, and the behavioral activity and possible therapy regimes after the injury. We will briefly discuss these dependencies, trying to highlight their translational value. Overall, it is not only necessary to better understand how the brain can reorganize after injury with or without therapy, it is also necessary to clarify when and why brain reorganization can be either “good” or “bad” in terms of its clinical consequences. This information is critical in order to develop and optimize cost-effective therapies to maximize functional recovery while minimizing maladaptive states after spinal cord injury.
1. Introduction
The well-known somatotopic map of the sensorimotor cortex represents a dynamic equilibrium in the continuous interaction between the brain and the external world (Erzurumlu and Kind, 2001; Feldman and Brecht, 2005), a sort of competitive battle among different parts of the body to gain space in the cortical field: the more a part of the body is used, the more cortical space it gains in detriment of adjacent body parts (Elbert et al., 1995). This continuous cortical reorganization is the everyday life of the normal brain during sensorimotor learning (Holtmaat and Svoboda, 2009; Barnes and Finnerty, 2010), but it becomes particularly extreme after injuries that lead to massive deafferentation, e.g. stroke, peripheral injuries or spinal cord injury (Wall and Egger, 1971; Calford and Tweedale, 1988; Pons et al., 1991; Jain et al., 1997; Florence et al., 1998; Endo et al., 2007; Ghosh et al., 2010). In principle, cortical reorganization after deafferentation is neither “good” or “bad”: the good side of cortical reorganization can favor functional recovery (Hoffman et al., 2007; Lotze et al., 2006; Cramer et al., 2005; Curt et al 2002), but its bad side can be maladaptive and lead to phantom sensation and neuropathic pain (Flor et al., 1995; Lotze et al., 1999; Peyron et al., 2004; Wrigley et al., 2009; Gustin et al., 2012; Makin et al., 2013). It is therefore critical to fully understand the phenomenology and the mechanisms of cortical reorganization in order to design and optimize clinical strategies to manipulate it (Engineer et al., 2011).
In the present review we will focus on cortical reorganization after spinal cord injury, which is particularly challenging due to a number of factors. In fact, the degree of cortical reorganization after spinal cord injury is highly variable, and can range from no reorganization (i.e. “silencing”) to massive cortical remapping. This variability critically depends on the species, the age of the animal when the injury occurs, the time after the injury has occurred, and the behavioral activity and possible therapy regimes after the injury. We will briefly discuss these dependencies, trying to highlight their translational value for optimizing therapeutic interventions that both maximize functional recovery and minimize pain.
2. Cortical reorganization depends on species (Fig. 1)
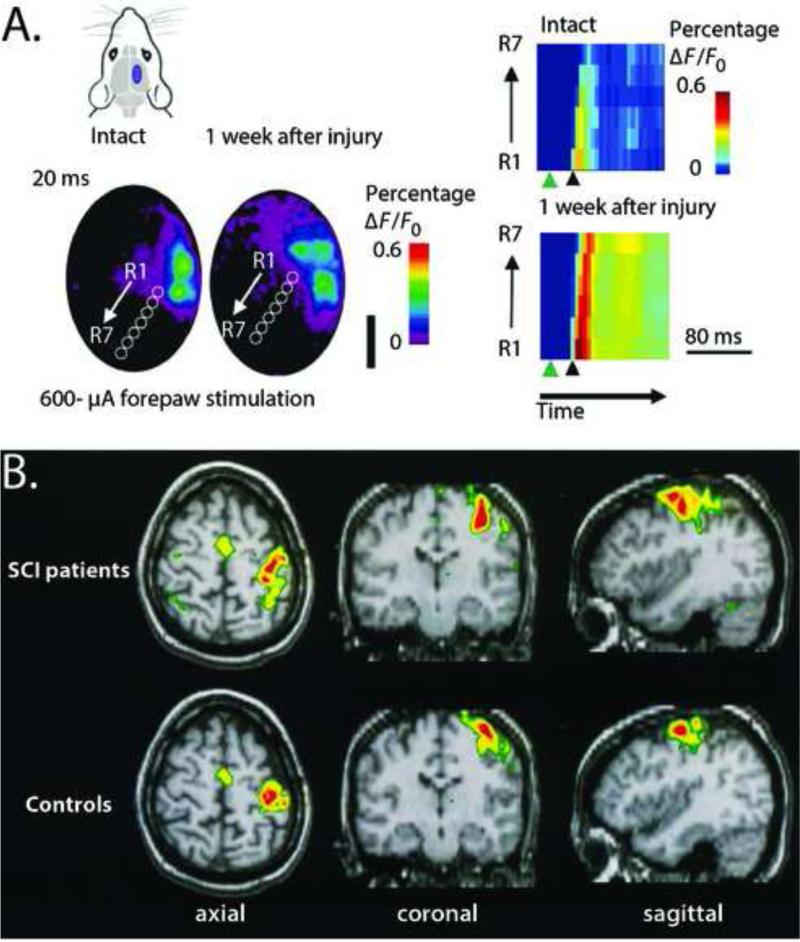
Figure 1.
Expansion of the intact cortex into the deafferented cortex after spinal cord injury occurs in several species from rat to human. A. Reorganization in the rat hindlimb cortex after bilateral, dorsal hemisection as measured by voltage sensitive dye (VSD) imagining. Left top left: purple region in the schematic representation of the rat head shows the region of the brain where VSD imaging was performed. Left bottom: seven regions of interest (ROIs), 300-μm in diameter, were defined (R1–R7 white circles). R1 was placed adjacent to the forelimb representation and subsequent ROIs were placed progressively caudo-medial at an angle of 35° from the midline. Right: activation of the voltage sensitive dye across the ROIs as a function of time in response to electrical stimulation of the forepaw. Activation begins nearest the forelimb representation and is greater one week after injury. Green arrowheads denote the time of stimulation. Black arrowhead denotes 20 ms after stimulation. Figure reproduced from Ghosh et al., (2010) with permission. B. Reorganization in the human cortex after spinal cord injury as measured by functional magnetic resonance imagining (fMRI). These data represent group results from nine paraplegic patients approximately 40 months after injury to the thoracic or lumbar region. Subjects were asked to repetitively perform finger-to-thumb opposition of the digits 2, 3, 4 and 5. Images show group averages. The activation patterns in the SCI patients (top row) is significantly enlarged compared to that of the controls (bottom row) with a medial and lateral expansion of the volume and an additional increase in activation in the contralateral premotor and parietal areas. Figure reproduced from Curt et al. (2002), with permission.
2.1 Cortical reorganization after spinal cord injury in humans
Cortical reorganization after spinal cord injury is commonly observed in patients. Mapping studies with transcranial magnetic stimulation (TMS) reveal enlargement of cortical sensorimotor areas that represent preserved muscles above the level of lesion in quadriplegic patients (Levy et al., 1990) and enhanced excitability of motor pathways targeting muscles rostral to the level of a spinal transection in paraplegic patients (Topka et al., 1991). PET studies confirm that patients with spinal cord injury exhibit expanded activation of cortical and subcortical brain areas during hand movements (Roelke Bruehlmeier et al., 1998; Curt et al., 2002). Intriguingly, EEG studies report reorganization of cortical motor activity to a more posterior – rather than more medial – location after spinal cord injury (Green et al., 1998), later confirmed with fMRI (Turner et al., 2003). fMRI studies also describe increased representation of non-impaired upper limb muscles in paraplegic patients (Curt et al., 2002), a medial-superior shift in cortical activation during tongue movements after cervical spinal cord injury (Mikulis et al., 2002), and a range of cortical reorganization patterns, from a relative stability of sensorimotor cortical topography in a tetraplegic patient with a rare late recovery (Corbetta et al., 2002), to abnormalities in brain motor system function during attempted and imagined movement after complete spinal cord injury (Cramer et al., 2005). Motor cortex reorganization after complete spinal cord injuries was also confirmed by combination of TMS and fMRI (Lotze et al., 2006). More recent works point toward a tight relationship between changes in cortical physiology and changes in cortical and cortico-spinal anatomy after spinal cord injury (Wrigley et al., 2009; Henderson et al., 2011; Freund et al., 2012; Freund et al., 2012). Finally, spinal cord injury not only affects evoked sensorimotor activity, but also slows down cortical spontaneous EEG activity (Tran et al., 2004; Boord et al., 2008; Wydenkeller et al., 2009). It is worth mention that an important literature exists on central nervous system plasticity after spinal cord injury in the context of breathing (Sharma et al., 2012; Hoh et al., 2013) and bladder function (Merrill et al., 2013; de Groat and Yoshimura, 2012). However, this plasticity is mostly subcortical (but see Zempleni et al., 2010), and will not be further discussed here. Overall, cortical reorganization appears as a complex phenomenon, not necessarily somatotopic, which has been associated with both functional recovery (Hoffman and Field-Fote, 2007; Jurkiewicz et al., 2007; Green et al., 2009), phantom sensations (Moore et al., 2000), and neuropathic pain (Ness et al., 1998; Boord et al., 2008; Wydenkeller et al., 2009; Wrigley et al., 2009; Gustin et al., 2012). Well-controlled studies in animal models are thus needed to decouple functional and maladaptive consequences of cortical reorganization after spinal cord injury.
2.2 Cortical reorganization after spinal cord injury in non-human primates
Research about cortical reorganization after spinal cord injury in non-human primates mainly focuses on the effects of dorsal column lesions. After cervical dorsal column section, neurons in the deafferented area 3b become initially unresponsive to stimulation of the hand, but after few weeks the area of cortical activation to spared inputs is greatly expanded, and after few months the deafferented hand cortical area becomes responsive to inputs from the face (Jain et al., 2000, 2008). This cortical reorganization is related to sprouting in the trigeminal-dorsal column complex in the brainstem (Jain et al., 2000; Kambi et al., 2014), and can also be observed at thalamic level (Jain et al., 2008). This reactivation of the deafferented hand cortex by inputs from the face seems more likely to contribute to phantom limb sensations than to functional recovery (Kaas et al., 2008), whereas the recovery of a near-normal cortical hand representation, possibly through alternate spinal afferents, seems to correlate with the recovery of hand use (Qi et al., 2014).
Somewhat similar results were obtained after localized cervical dorsal root lesions (rhizotomy), which cause both functional cortical reorganization (Darian-Smith and Brown, 2000) and sprouting in the brainstem (Darian-Smith, 2004, 2013). Intriguingly, in this model the reorganization was associated to functional recovery (Darian-Smith and Ciferri, 2006) and to neurogenesis within the spinal cord (Vessal et al., 2007) and in the sensorimotor cortex (Vessal and Darian-Smith, 2010). However, neurogenesis does not seem to occur when there is a direct trauma to the spinal cord with consequent glial scar formation (Vessal et al., 2007).
Cortical reorganization after dorsal column section is not limited to the primary somatosensory cortex, but also extends to the secondary somatosensory cortex and parietal ventral area (Tandon et al., 2009). In addition, major reorganization of the motor cortex may depend on damage to motor pathways or proprioceptive pathways. In fact, cervical spinal cord injury also produces long-term reorganization of the motor cortex paralleling recovery of finger dexterity (Schmidlin et al., 2004; Nishimura et al. 2007; Kambi et al., 2011), with increased expression of GAP-43 mRNA in the cortical areas involved in the functional recovery (Higo et al., 2009). At least part of the functional recovery from lesion of the corticospinal tract is mediated by reticulospinal systems (Zaaimi et al., 2012).
2.3 Cortical reorganization after spinal cord injury in rats
In rats, spontaneous cortical reorganization after spinal cord injury appears to be more limited and somewhat controversial compared to primates (at least in adult animals without any explicit therapeutic intervention). Despite early works showing thalamocortical reorganization after lesions of the dorsal columns or of the gracilis nucleus (Wall and Egger, 1971), Jain et al., (1995) reported absence of any cortical reorganization between 3 hours and 3 months after unilateral dorsal column section at thoracic level (T6-T8), with neurons in the deafferented hindlimb cortex becoming unresponsive to cutaneous stimulation of any part of the body. A similar absence of cortical reorganization was recently confirmed after complete thoracic spinal cord transection, when tested with classical electrophysiological techniques (Graziano et al., 2013). The same thoracic spinal cord transection, however, significantly affects gene expression and regulation of plasticity-related proteins in the sensorimotor cortex (Endo et al., 2007; Graziano et al., 2013), revealing a powerful molecular substrate for cortical reorganization. Indeed, using functional magnetic resonance imaging (fMRI), increased BOLD signals in response to forepaw stimuli are observed in the primary somatosensory cortex in response to stimulation of the intact forelimb after thoracic contusion (Hofstetter et al., 2003), thoracic transection (Endo et al., 2007) or thoracic bilateral dorsal section of the spinal cord (Ghosh et al., 2010). But increases in regional cerebral blood flow (rCBF) are also observed in unstimulated animals in brain structures associated with somatosensory processing – including hindpaw somatosenspry cortex and thalamus – after excitotoxic dorsal horn injury (Morrow et al., 2000; Paulson et al., 2005). Interestingly, long-term cortical reorganization after thoracic bilateral dorsal section, when assessed with voltage-sensitive dye imaging (VSD), appears to be temporally confined to the first week after injury (Ghosh et al., 2010). This discrepancy between the long-term cortical reorganization after spinal cord injury observed with functional imaging (particularly fMRI) and the limited reorganization observed with classical electrophysiological techniques might be simply due to differences in stimulus intensity among studies (light tactile stimuli in Jain et al., 1995 and Graziano et al., 2013; high-intensity electrical stimuli in Hofstetter et al., 2003, Endo et al., 2007 and Ghosh et al., 2010), could reflect differences in animal handling conditions after the injury, or could instead point toward an intriguing decoupling between metabolic and neuronal activity, which will need to be properly integrated to fully understand the mechanisms of long-term cortical reorganization after spinal cord injury.
Cortical reorganization after spinal cord injury in rats has been studied both in the context of functional recovery (Ghosh et al., 2009; Ghosh et al., 2010) and in the context of neuropathic pain (Endo et al., 2008). From the point of view of neuropathic pain, there is indeed evidence of increased fMRI activation at cortical level (Endo et al., 2008), but a critical role seem to be played by altered cortico-thalamic connectivity, as measured by fMRI resting state (Seminowicz et al., 2012), and altered subcortical processing. In particular, neuropathic pain after spinal cord injury has been linked to disruption of thalamic processing, both in lemniscal nuclei such as the VPL – mechanistically related with abnormal expression of sodium channels (Hains et al., 2005; Hains et al., 2006) – and paralemniscal nuclei such as the PO, with a critical role played by the zona incerta (Masri et al., 2009; Whitt et al., 2013). From the point of view of functional recovery, because most studies focus of on somatosensory reorganization, there is a need to better investigate the reorganization of the motor cortex (Oza and Giszter, 2014), possibly focusing both on the cortico-spinal tract, which is more related to fine movements, and in the cortico-reticulo-spinal tract, which is more related to posture and gross movements, including locomotion (see e.g. Bachmann et al., 2013). Intriguingly, the motor and sensory aspects of cortical reorganization after spinal cord injury seems to be tightly linked, as suggested by the beneficial effects induced by motor cortex stimulation on central pain (Lucas et al., 2011, Cha et al., 2013; Jiang et al., 2013).
3. Cortical reorganization depends on the age of the animal at the time of injury
The ability of the cortex to spontaneously reorganize is dependent on the age of the animal at the time of the lesion. In monkeys, dorsal column lesions within the first two weeks after birth lead to functional reorganization of the motor cortex in adulthood, uncovering the potential for motor reorganization due to loss of focal sensory inputs (Qi et al., 2010). In cats (McKinley and Smith, 1990) and rats (Jain et al., 1995), as described above, spinal cord injuries inflicted during adulthood are associated with little or no responsiveness in the deafferented cortex. However, cortical mappings of the somatosensory cortex of adult cats with spinal injuries inflicted within 6 weeks after birth demonstrate substantial cortical reorganization (McKinley and Swyter, 1989; Chau and McKinley, 1991; Casanova et al., 1991), with greater reorganization in kittens spinalized at 2 weeks compared to kittens spinalized at 6 weeks (McKinley and Smith, 1990). Some degree of cortical reorganization is also observed in adult rats spinally transected in the neonatal period (Jain et al., 2003). Interestingly, this cortical reorganization in adult animals spinalized as neonates is associated with an “infant lesion effect” (Bergman and Golberger, 1983a,b,c), which indicates sparing and greater recovery of function compared to animals spinalized during adulthood. For example, adult rats with neonatal transections at the T8/T9 levels can sometimes develop weight-supported stepping (Stelzner et al., 1975; Weber and Stelzner et al., 1977; Miya et al., 1997; Kao et al., 2006) associated with reorganization of the motor cortex, with low axial muscles being recruited from the rostral cortical axial representation that normally represents the neck and upper trunk (Giszter et al., 1998). Since the ability of these animals to maintain stance in the presence of controlled perturbations depended almost completely on the forelimbs (Giszter et al., 2007), it is likely that the sensory input from the forepaws is processed by this novel sensorimotor representation, activating the axial trunk musculature to improve balance by stabilizing the trunk and reducing the load on the hindlimbs. Speculatively, a possible critical role in the surprising motor recovery of adult rats transected as neonate could also be played by plasticity of the cortico-reticulo-spinal tract (Bachmann et al., 2013). Overall, the different response to injury in early development compared to adulthood (e.g. greater loss of neurons, possible preservation of connections that would normally be lost, etc.) seems to maximize the potential of cortical plasticity after spinal cord injury, suggesting that neonatal models might be particularly useful to unravel the key factors to maximize – or optimize – cortical reorganization in adulthood.
4. Cortical reorganization depends on the time after injury (Fig. 2)
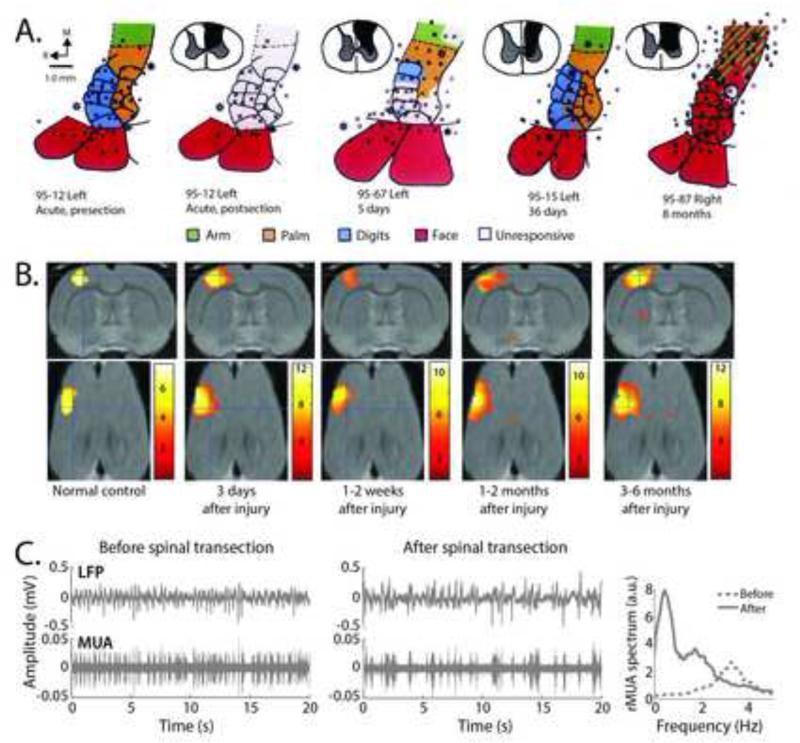
Figure 2.
The extent of reorganization after spinal cord injury is dependent on the time after injury. A. In primates, after unilateral, cervical dorsal column lesions, neurons in the deafferented cortex were unresponsive to sensory stimulation immediately after the lesion (95-12 acute, postsection). After 5 days, there was a significant enlargement of the intact regions above the level of the lesion (e.g. face) that then constricted by one month post-lesion. However, by 8 months post lesion, cells across the entire area 3b were responsive only to stimulation above the level of the lesion or the face (95-87 8 months). Figure reproduced from Jain et al. (1997), with permission. B. Similar results were found in the rat using fMRI. Group analysis of the responsiveness of cortex to electrical stimulation of the forepaw in 12 rats transected at T9. Coronal sections are along the top and horizontal sections are along the bottom. Significant enlargement of the fMRI signal, extending both medially and caudally (into the hindlimb cortex) from the original forelimb begins 3 days after injury and is then lost approximately 1-2 weeks after injury. By 1-2 months after injury, the expansion is reestablished and continues to enlarge by 3-6 months. Figure reproduced from Endo et al. (2007), with permission. C. Further studies in the rat show that urethane-induced delta oscillations (1-4Hz) before spinal transection (left panels showing local field potential, LFP, and multiunit activity, MUA) transitioned to slow-wave oscillations (<1Hz) immediately (i.e. within minutes) after spinal transection (middle panels), corresponding to a reduction in spontaneous activity as evidenced by a decrease mean amplitude of the rectified multi-unit activity (rMUA) and a decreased frequency of the rMUA spectrum (right panel). Figure reproduced from Aguilar et al. (2010), with permission.
Cortical reorganization after spinal cord injury dramatically depends on the time elapsed after the injury. In humans, functional improvements and the appearance of neuropathic pain, both of which have been separately associated to cortical reorganization, can take months-to-years to occur (Corbetta et al., 2002). In non-human primates, as commented above, cortical reorganization can take weeks-to-months to fully develop (Jain et al., 2000, 2008). Interestingly, cortical reorganization after dorsal column section is characterized by spatial shifts of digit activation sites that consist of an early moving away phase and a late returning phase, compared to the pre-lesion activation sites (Chen et al., 2012). Rat studies seem to confirm that cortical reorganization after spinal cord injury is not simply a progressive phenomenon, but can instead undergo several temporal phases. The classical long-term cortical expansion that can be observed with fMRI 1-3 months after spinal cord injury (Endo et al., 2007) is anticipated by an early expansion within the first week (Endo et al., 2007; Sydekum et al., 2014), which is also observed with VSD imaging (Ghosh et al., 2010). This early expansion of intact cortical representations is associated with an early decrease of spine density in deafferented cortical representations, both in rats (Kim et al., 2006) and mice (Ghosh et al., 2012). The relations between early and late cortical reorganization remain to be established.
In an effort to understand the early mechanisms of cortical reorganization, we recently investigated the electrophysiological changes occurring in the primary somatosensory cortex immediately (i.e. within 1-3 hours) after spinal cord injury. A complete thoracic spinal cord transection or hemisection in anesthetized rats immediately changes the state of the brain, decreasing cortical spontaneous activity as evidenced by a slowing of the frequency of anesthesia-induced oscillations (Aguilar et al., 2010; Yagüe et al., 2014). This deafferentation-dependent decrease of cortical spontaneous activity could in principle be mediated by decreased activity in primary somatosensory structures, ultimately mimicking a thalamo-cortical deafferentation (Rigas and Castro-Alamancos, 2007; Hirata and Castro-Alamancos, 2010; David et al., 2013), or by decreased activity in secondary structures regulating cortical synchrony and arousal at thalamic and brainstem, most likely involving a depression of the cholinergic system (Moruzzi and Magoun, 1949; Lindvall et al., 1974; Hobson et al., 1975; Foote et al., 1980; Aston-Jones and Bloom, 1981a,b; Satoh and Fibiger, 1986; Fox and Armstrong-James, 1986; Hallanger et al., 1987; Steriade et al., 1990; Aguilar and Castro-Alamancos, 2005; Ren et al., 2009). The latter hypothesis is supported by decreased anesthetic requirements in rats after spinal cord injury (Foffani et al., 2011), similar to the decreased requirements for general anesthesia in animals and humans after epidural anesthesia (reviewed in Foffani et al., 2011).
Because in the rat somatosensory cortex slower spontaneous activity correlates with increased somatosensory responses (Petersen et al., 2003; Sachdev et al., 2004; Hasenstaub et al., 2007; Reig and Sanchez-Vives, 2007), spinal cord transection or hemisection also immediately increases the cortical responses to stimuli delivered above the level of the lesion (Aguilar et al., 2010; Yagüe et al., 2014). But if cortical spontaneous activity is carefully monitored, cortical responses immediately after spinal cord transection still increase, even without a change in cortical state (Humanes-Valera et al., 2013; Yagüe et al., 2014). These increased responses could be due to a change in the equilibrium between excitation and inhibition at cortical and subcortical level. Intriguingly, even though the slower spontaneous activity seems to support the increased cortical responses immediately after spinal cord injury, it might not favor cortical reorganization in the long term. In fact, brainstem cholinergic activity is critical for cortical reorganization both in physiological conditions (Kilgard and Merzenich, 1998) and after peripheral or brain injury (Juliano et al., 1991; Conner et al., 2005). The decreased cortical spontaneous activity observed immediately after spinal cord transection could therefore at least partly explain the limited cortical reorganization observed after spinal cord injury in this rat model. Interestingly, in the hemisection model the decreased cortical spontaneous activity coexists with an immediate cortical hyperexcitability to preserved spinothalamic inputs (Yague et al., 2011), which could in turn contribute to the important functional, anatomical and behavioral changes observed after incomplete spinal cord injuries (Ghosh et al., 2009; Wasner et al., 2008; Densmore et al., 2010). In any case, these results suggest that to fully assess cortical reorganization after spinal cord injury it is necessary to monitor cortical spontaneous activity, and that cortical reorganization starts immediately after the lesion.
As reviewed so far, spinal cord injury by itself induces reorganization of supraspinal structures depending on the age of the animal at the time of the lesion and on the time elapsed after the lesion. In the following sections we explore how, in addition to the enhanced plasticity of supraspinal structures in response to lesions of the spinal cord itself, cortical reorganization can be further modulated by therapeutic interventions that had previously been thought to act solely at the level of the lesion or below. Pharmacotherapy and exercise represent the two best-studied therapeutic interventions for their impact on cortical reorganization.
5) Cortical reorganization can be promoted by pharmacological therapy after injury (Fig. 3)
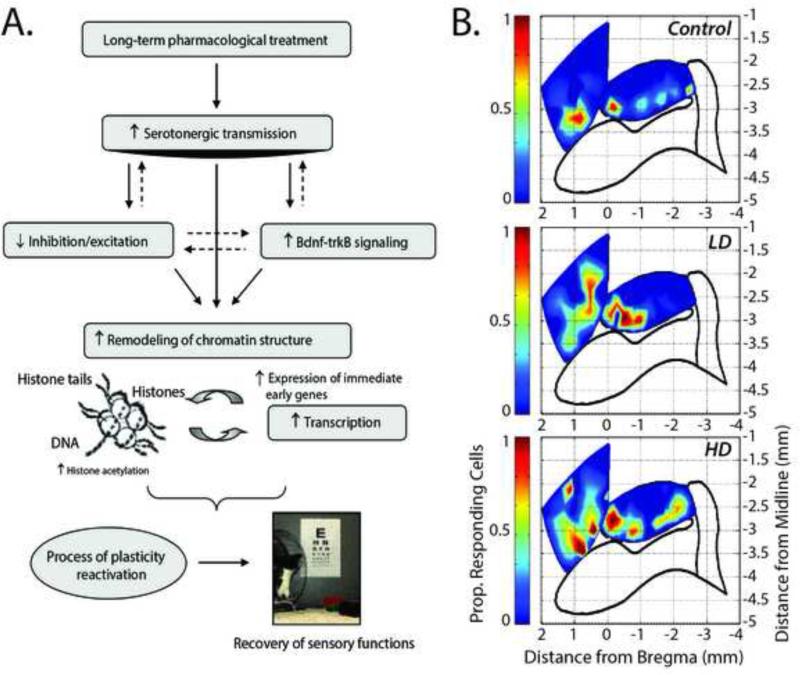
Figure 3.
Serotonin is known to promote adult cortical plasticity after injury. A. Studies have shown that pharmacotherapy after injury can increase BNDF-trkB signaling which increases the remodeling of chromatin structures ultimately lowering the threshold for cortical excitability, thus increasing the probability for sensory stimulation (in this case visual) to drive activity-dependent modification of synaptic transmission. Figure reproduced from Maya-Vetencourt et al. (2011), with permission. B. Similar results were observed after spinal cord injury. Top panel shows the responsiveness of the hindlimb sensory-motor cortex (HLSM, oval outlined in black) and forelimb motor cortex (quadrilateral located more rostrally) to tactile stimulation of the forepaw. Responsiveness is measured by the number of neurons that respond to the forepaw stimulation with increased firing rate. Middle panel shows expansion of the response into both the hindpaw sensory cortex and forepaw motor cortex after 8-weeks of a daily injection of a low dose (LD) of 5-HT agonists. Bottom panel shows greater expansion after 8-weeks of daily injection of a high dose (HD) of 5-HT agonists. Responses in the forepaw cortex (black outline more lateral from the hindpaw are not shown). Figure reproduced from Ganzer et al. (2013), with permission.
Spinal cord injury not only affects the ascending and descending sensorimotor pathways in the spinal cord, but also disrupts the descending monoamine projections from brainstem regions, particularly eliminating serotonin (5-HT) and modifying its receptors caudal to the site of injury (Clineschmidt et al., 1971; Yaksh and Wilson, 1979; Skagerberg and Björklund 1985; Basura et al., 2001; Garraway and Hochman, 2001; Hains et al., 2002). A large literature exist on the effects of spinal cord injury on the spinal serotonergic system, and encouraging functional improvements can be obtained with 5-HT agonists after spinal cord injury (Kim et al., 1999; Antri et al., 2002, 2003, 2005; Shumsky et al., 2005; Kao et al., 2006; Landry et al., 2006; Ung et al., 2008; Musienko et al., 2009; Courtine et al., 2009). However, the 5-HT system also plays a significant role above the level of the lesion, regulating cortical plasticity. During development, 5-HT contributes to the organization of sensory and motor systems by modulating experience dependent plasticity (Nishi and Azmitia, 1999; Lotto et al., 1999; Kirkwood, 2000). In adulthood, 5-HT can reverse deficits after injury or insult by reducing neuronal death and dendrite loss (Ramos et al., 2004), particulatly through phosphorylation of the cycloskeletal remodeling protein MAP2 (Azmitia et al., 1995), resulting in the sprouting of neurites (Fricker et al., 2005).
To assess cortical reorganization in response to 5-HT pharmacotherapy after spinal cord injury without confounding effects from spared fibers left after partial lesions, we recently studied the effect of different 5-HT agonists and doses on cortical reorganization in adult rats after a thoracic (T8/9) spinal cord transection (Ganzer et al., 2013), assessed by single-neuron responses to light, cutaneous stimuli. Combinations of 5-HT receptor agonists induce expansion of the intact forelimb somatosensory (FLS) cortex into the deafferented hindlimb sensorimotor (HLSM) cortex and into the intact forelimb motor (FLM) cortex. The magnitude of this expansion is dose-dependent and positively correlates with behavioral recovery, and its topographic organization is in good agreement with the important overlap observed between the somatosensory cortex and motor cortex in the rat (Sievert et al., 1986; Morales-Botello et al., 2012). An intriguing possibility is that the expansion of forelimb somatosensory function into forelimb motor cortex may be due, in part, as a way to maintain the correct sensorimotor overlap, likely useful for locomotion in the rat.
As reviewed in the previous sections, spinal cord injury alone (i.e. without therapy) induces a more plastic state in the brain, altering the transcriptional activities of genes and the expression of proteins associated with cortical plasticity (Endo et al., 2007; Graziano et al., 2013). It is possible that this increased plastic state of the brain after spinal cord injury returns the sensorimotor cortex to a state reminiscent of that during development, when the actions of 5-HT are associated with plasticity rather than modulation of sensory input (Bennett-Clarke et al., 1994, 1995; Inaba et al., 2009; Jones et al., 2009; Kojic et al., 1997; Normann and Clark, 2005). This idea is not completely new. For example, the 5-HT1A receptor was implicated in the restoration of visual function in amblyopic rats by reinstating ocular dominance plasticity (Maya-Vetencourt et al., 2008, 2011), and the 5-HT2A receptor was shown to facilitate the delivery of AMPA receptors to the postsynaptic membrane as well as other late-LTP mechanisms during reorganization of the whisker cortex after visual deprivation (Jitsuki et al., 2011). Therefore, activation of 5-HT receptors within the sensorimotor cortex after spinal cord injury, combined with improved behavioral outcome from its actions below the level of the lesion, are likely to facilitate cortical reorganization by restructuring connections that could be relevant for behavioral recovery.
The increased plastic state of the brain after spinal cord injury also suggests other alternative or complementary pharmacotherapy approaches to promote (or control) cortical reorganization after spinal cord injury. For example, spinal cord injury in rats produces upregulation in dorsal column nuclei of extracellular chondroitin sulfate proteoglycans (CSPGs) (Massey et al., 2006), which are known to limit plasticity after CNS injuries (McKeon et al., 1991; Pindzola et al., 1993; Silver and Miller, 2004), including spinal cord injury (Davies et al., 1999; Tang et al., 2003; Jones et al., 2003). The limiting effects of CSPGs on plasticity can be overcome by both chondroitinase ABC (ChABC) and BDNF (Tropea et al., 2003): after cervical dorsal column section, ChABC treatment favors sprouting both in the spinal cord (Barrit et al., 2006; Lee et al., PNAS 2010) and in the cuneate nucleus (Massey et al., 2006), and provides neuroprotection to corticospinal neurons (Carter et al., 2008); similarly increased spinal sprouting is obtained with ChABC treatment after unilateral cervical spared-root lesion (Cafferty et al., 2008); ChABC was also shown to promote cortical reorganization after cervical dorsal column lesion in monkeys (Bowes et al., 2012). More in general, pharmacological therapies designed to reduce neuroinflammation, induce neuroprotection and affect functional and anatomical plasticity at the level of the spinal cord – including therapies targeting the Nogo signaling system (Endo et al., 2009) – are also likely to affect cortical reorganization, possibly contributing to functional outcome after spinal cord injury.
6) Cortical reorganization can be promoted by exercise therapy after injury (Fig. 4)
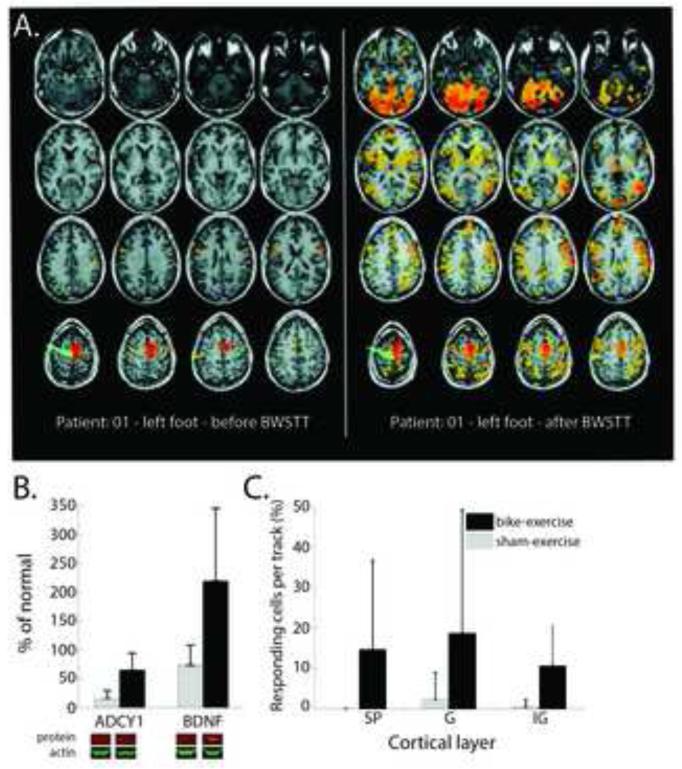
Figure 4.
Exercise promotes cortical reorganization after spinal cord injury. A. Robotic locomotor therapy that improved functional outcome also enhanced motor representation of the foot. Images are an example from one patient. The red-orange areas show regions of brain activation in response to voluntary ankle plantar flexion and toe flection as measure by fMRI. Figure reproduced from Winchester et al. (2005), with permission. B. Similar results were found for spinalized rats after 8-weeks of passive bike exercise below the level of the lesion. The exercise increased the levels of adenylate cyclase 1 (ADY1) and brain-derived neurotrophic factor (BDNF) in the cortex. C. Bike exercise also increased the probability that cells in the deafferented hindlimb cortex would respond to tactile stimulation of the forelimbs across all cortical layers: supragranular (SP), granular (G) and infragranular (IG). Figure reproduced from Graziano et al. (2013), with permission.
It is well established that exercise favors brain plasticity, mainly via activation of the BDNF system (Neeper et al., 1995, 1996; Gomez-Pinilla et al., 2002; Vaynman and Gomez-Pinilla, 2005; Vaynman et al., 2003; 2004; 2006; Ding et al., 2011). Both after peripherial injuries and traumatic brain injuries, exercise induces cortical plasticity that is related to functional recovery (Friel et al., 2000; Florence et al., 2001; Ramanathan et al., 2006; Griesbach et al., 2009). The same relation between exercise, cortical reorganization and functional recovery holds after spinal cord injury. It is important to note that a clear distinction between the effects of exercise and of use-dependent training is often problematic, except when exercise therapy is passively delivered below the level of a complete spinal lesion (in this case there are obviously no use-dependent training effects). In humans, intensive task-specific rehabilitative training with robotic locomotor threrapy after incomplete spinal cord injury can promote supraspinal plasticity in the motor centers known to be involved in locomotion (Winchester et al., 2005). Similarly, a longitudinal fMRI study in patients with cervical spinal cord injury showed that improvement in function after exercise therapy is associated with the extent of motor cortex activation (Jurkiewicz et al., 2007). In addition, a case report on intensive, bimanual training of a C6 motor-complete spinal injury resulted in functional improvement and an increased representation of the involved muscles in the cortex (Hoffman et al., 2007). In adult rats spinalized as neonates, treadmill training induces a novel organization in both the somatosensory cortex (Kao et al., 2009; 2011) and the motor cortex (Giszter et al., 1998), which directly correlates with the number of weight-supported steps these animals are able to take while locomoting on the treadmill (Giszter et al., 2008; Kao et al. 2011). Interestingly, exercise produces a prophylactic neuroprotective effect on the brain for subsequent spinal cord injuries in adult rats (Gomez-Pinilla et al., 2012).
Similarly to pharmacotherapy, exercise interventions also have the capacity to promote locomotor recovery and plasticity at many levels of the sensorimotor system, both in the spinal cord and in the brain. After spinal cord injury, exercise can be administered both passively (Murphy et al., 1999) or actively (Ilha et al. 2011; Nessler et al., 2005; Timoszyk et al. 2005; Dobkin et al., 2006). In animal studies, exercise is associated with the upregulation of neurotrophic factors within the spinal cord (Keeler et al. 2012; Liu et al. 2010, 2012), which can encourage plasticity through a wide variety of molecular mechanisms (for reviews see: BDNF: Weishaupt et al., 2012; Neurotrophins: Ebadi et al., 1997; GDNF: Bohn 2004). In humans, passive bicycling exercise is a common non-invasive therapy that can reduce spasticity (De Mello et al., 2004; Kiser et al., 2005), increase bone density (Hangartner et al., 1994; Lauer et al., 2011) and reduce lower limb blood pooling (Phillips et al., 1998). In addition, locomotor training, usually with body-weight support on a treadmill, has also an impact on functional recovery (Wernig et al., 1995; Van Hedel and Dietz, 2010; Wessels et al., 2010; Barbeau, 2003; Harkema et al., 2012a,b). It has been generally assumed that this functional recovery is due to activation and/or relearning in the spinal circuits (Dietz and Harkema, 2004; Edgerton et al., 2004; Harkema et al., 2008; Harkema et al., 2012b).
To determine whether exercise therapies targeted below the level of the lesion could have an impact in supraspinal centers, we recently assessed whether passive bicycling exercise to the hindlimbs after complete thoracic transection of the spinal cord induced any effects on the sensorimotor cortex (Graziano et al., 2013). Somewhat surprisingly, passive bicycling exercise of the paralyzed hindlimbs promotes the upregulation of plasticity-related proteins BDNF and ADCY1 within the sensorimotor cortex, accompanied by significant expansion of the forepaw somatosensory responses into the deafferented hindlimb cortex (Graziano et al., 2013). Even though the neuroendocrine and other systemic mechanisms likely mediating these effects need to be further clarified, the possible causal relationships between increased BDNF and cortical reorganization seem clearer. In fact, we already mentioned that the limiting action of CSPGs on plasticity can be overcome not only by ChABC but also – and synergistically – by BDNF (Tropea et al., 2003). This finding might well explain why exercise, by increasing BDNF levels in the brain (Graziano et al., 2013), can favor cortical reorganization (Graziano et al., 2013) and functional recovery (Wang et al., 2011) after spinal cord injury.
7) Combined therapies to manipulate cortical reorganization after spinal cord injury
There is substantial evidence that 5-HT and BDNF interact at the cellular level (Nibuya et al., 1995; Russo-Neustadt et al., 1999, 2000; Ivy et al., 2003; Garcia et al., 2003; Mattson et al., 2004), which can potentially lead to an amplification of their plastic effects when administered in combination. Since exercise increases the levels of BDNF (see above), exercise combined with 5-HT pharmacotherapy could potentially promote the rewiring of sensorimotor cortical circuits and locomotor recovery after spinal cord injury more effectively than either alone. However, the interaction might not necessarily be always functionally synergic, due to the complex state-dependent modulatory action of 5-HT throughout sensorimotor systems (Eccles, 1964; Proudfit and Anderson 1973; Waterhouse et al., 1986; Bassant et al., 1990; Lopez-Garcia and King, 1996; Sheibani and Farazifard, 2006). From a translational viewpoint, the complexity of this interaction highlights the importance of better understanding the mechanisms underlying combination therapies to maximize functional recovery after spinal cord injury. For example, the somewhat limited clinical improvement achieved by conventional locomotor training after incomplete spinal cord injury – either with or without partial body-weight-support (Morawietz and Moffat, 2013) – could be enhanced by proper concomitant therapies, such as pharmacotherapy and/or epidural stimulation (Courtine et al., 2009; Angeli et al., 2014), or even deep brain stimulation (Bachmann et al., 2013), with a likely critical role played by plastic changes in supraspinal circuits.
In the context of promoting/controlling cortical reorganization after spinal cord injury in humans, an important clinical role could be played by non-invasive brain stimulation (NIBS), a growing family of techniques that share the common characteristic to be able to change cortical excitability and induce plastic changes in the brain. Most of these techniques are easy to use, safe, can focally stimulate the nervous system and can be easily combined with exercise and pharmacotherapy. Commonly used NIBS techniques include repetitive transcranial magnetic stimulation (rTMS; Hallet, Neuron 2007) and related techniques, transcranial direct current stimulation (tDCS; Nitsche and Paulus 2000; Priori, 2003) and related techniques, and more recently transcranial focused ultrasound brain stimulation (tFUS; Legon et al., 2014) and transcranial static magnetic field stimulation (tSMS; Oliviero et al., 2011). Few studies have already assessed the use of NIBS in patients with spinal cord injury, both to maximize functional outcome (Belci et al., 2004) and to reduce neuropathic pain (Fregni et al., 2006). Moreover, tDCS has been tested to improve the efficacy of visual illusion treatment of neuropathic pain (Soler et al., 2010). It is important to remark the need to gain deeper knowledge about the mechanisms and timing of cortical reorganization after spinal cord injury in order optimize NIBS-based treatment. In any case, NIBS offer promising tools to manipulate cortical reorganization after spinal cord injury. Future clinical studies should demonstrate if this manipulation, possibly combined with other therapies, is clinically relevant or not.
8) Conclusions
Plasticity constitutes the basis of behavioral changes as a result of experience. It refers to neural network shaping and re-shaping at the global level and to synaptic contacts remodeling at the local level, either during learning or memory encoding, or as a result of acute or chronic pathological conditions. ‘Plastic’ brain reorganization after central nervous system lesions has a pivotal role in the recovery and rehabilitation of sensory and motor dysfunction, but can also be “maladaptive”. Moreover, it is clear that brain reorganization it is not a “static” phenomenon but rather a very dynamic process. Spinal cord injury immediately initiates a change in brain state and starts cortical reorganization. In the long-term, the impact of injury – with or without accompanying therapy – on the brain is a complex balance between supraspinal reorganization and spinal recovery (e.g. the reduction of the spinal edema and of the inflammation slowly allows for an increase of the brain-to-spine and spine-to-brain connections). Therefore, it is not only necessary to better understand how the brain can reorganize after injury with or without therapy, it is also necessary to clarify when and why brain reorganization can be either “good” or “bad” in terms of its clinical consequences. This information is critical in order to develop and optimize cost-effective therapies to maximize functional recovery while minimizing neuropathic pain after spinal cord injury.
Reorganization in the brain after SCI lesion plays a pivotal role in recovery
Brain reorganization can also be maladaptative
After spinal cord injury, this reorganization is a dynamic phenomenon
The extent of reorganization is highly variable depending on species, age and therapy regimes
Understanding reorganization can optimize cost-effective
Acknowledgements
KM was supported by R01 NS05741 from the National Institutes of Health (USA), grant 89500 from Shriners Hospital for Children (USA) and grant P113 from International Foundation for Research in Paraplegia (Switzerland). AO was supported by grant TRA-173 by the Spanish Ministry of Health and Social Policies. JA was supported by grant SAF2012-40109 from the Spanish Government, Ministerio de Economía y Competitividad. GF was supported by grant PI11/02451 from Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (Spain) co-funded by FEDER, and by grant P120 from International Foundation for Research in Paraplegia (Switzerland).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A, Foffani G. Spinal cord injury immediately changes the state of the brain. J Neurosci. 2010;30:7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar JR, Castro-Alamancos MA. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci. 2005;25:10990–11002. doi: 10.1523/JNEUROSCI.3229-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Antri M, Mouffle C, Orsal D, Barthe JY. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur J Neurosci. 2003;18:1963–1972. doi: 10.1046/j.1460-9568.2003.02916.x. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC, Rubinstein VJ, Strafaci JA, Rios JC, Whitaker-Azmitia PM. 5-HT1A agonist and dexamethasone reversal of para-chloroamphetamine induced loss of MAP-2 and synaptophysin immunoreactivity in adult rat brain. Brain Res. 1995;677:181–192. doi: 10.1016/0006-8993(95)00051-q. [DOI] [PubMed] [Google Scholar]
- Bachmann LC, Matis A, Lindau NT, Felder P, Gullo M, Schwab ME. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med. 2013;5:208ra146. doi: 10.1126/scitranslmed.3005972. [DOI] [PubMed] [Google Scholar]
- Barbeau H. Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. doi: 10.1177/0888439002250442. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010;16:186–198. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassant MH, Ennouri K, Lamour Y. Effects of iontophoretically applied monoamines on somatosensory cortical neurons of unanesthetized rats. Neuroscience. 1990;39:431–439. doi: 10.1016/0306-4522(90)90279-d. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, Goshgarian HG. Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol. 2001;169:255–263. doi: 10.1006/exnr.2001.7682. [DOI] [PubMed] [Google Scholar]
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord. 2004;42:417–419. doi: 10.1038/sj.sc.3101613. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Lane RD, Rhoades RW. Fenfluramine depletes serotonin from the developing cortex and alters thalamocortical organization. Brain Res. 1995;702:255–260. doi: 10.1016/0006-8993(95)00867-5. [DOI] [PubMed] [Google Scholar]
- Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW. Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat's somatosensory cortex. J Neurosci. 1994;14:7594–7607. doi: 10.1523/JNEUROSCI.14-12-07594.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MC. Motoneurons crave glial cell line-derived neurotrophic factor. Exp Neurol. 2004;190:263–275. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46:118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- Boord P, Siddall PJ, Tran Y, Herbert D, Middleton J, Craig A. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord. 2008;46:118–123. doi: 10.1038/sj.sc.3102077. [DOI] [PubMed] [Google Scholar]
- Bowes C, Massey JM, Burish M, Cerkevich CM, Kaas JH. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci U S A. 2012;109:2595–2600. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Infant lesion effect: III. Anatomical correlates of sparing and recovery of function after spinal cord damage in newborn and adult cats. Brain Res. 1983;285:137–154. doi: 10.1016/0165-3806(83)90047-0. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Infant lesion effect: II. Sparing and recovery of function after spinal cord damage in newborn and adult cats. Brain Res. 1983;285:119–135. doi: 10.1016/0165-3806(83)90046-9. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Goldberger ME. Infant lesion effect: I. Development of motor behavior following neonatal spinal cord damage in cats. Brain Res. 1983;285:103–117. doi: 10.1016/0165-3806(83)90045-7. [DOI] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci. 2008;28:11998–12009. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci. 2008;28:14107–14120. doi: 10.1523/JNEUROSCI.2217-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova C, McKinley PA, Molotchnikoff S. Responsiveness of reorganized primary somatosensory (SI) cortex after local inactivation of normal SI cortex in chronic spinal cats. Somatosens Mot Res. 1991;8:65–76. doi: 10.3109/08990229109144730. [DOI] [PubMed] [Google Scholar]
- Cha M, Ji Y, Masri R. Motor cortex stimulation activates the incertothalamic pathway in an animal model of spinal cord injury. J Pain. 2013;14:260–269. doi: 10.1016/j.jpain.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CW, McKinley PA. Chronological observations of primary somatosensory cortical maps in kittens following low thoracic (T12) spinal cord transection at 2 weeks of age. Somatosens Mot Res. 1991;8:355–376. doi: 10.3109/08990229109144758. [DOI] [PubMed] [Google Scholar]
- Chen LM, Qi HX, Kaas JH. Dynamic reorganization of digit representations in somatosensory cortex of nonhuman primates after spinal cord injury. J Neurosci. 2012;32:14649–14663. doi: 10.1523/JNEUROSCI.1841-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clineschmidt BV, Pierce JE, Lovenberg W. Tryptophan hydroxylase and serotonin in spinal cord and brain stem before and after chronic transection. J Neurochem. 1971;18:1593–1596. doi: 10.1111/j.1471-4159.1971.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Conner JM, Chiba AA, Tuszynski MH. The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron. 2005;46:173–179. doi: 10.1016/j.neuron.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Burton H, Sinclair RJ, Conturo TE, Akbudak E, McDonald JW. Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc Natl Acad Sci U S A. 2002;99:17066–17071. doi: 10.1073/pnas.262669099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128:2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Lastra L, Lacourse MG, Cohen MJ. Brain motor system function after chronic, complete spinal cord injury. Brain. 2005;128:2941–2950. doi: 10.1093/brain/awh648. [DOI] [PubMed] [Google Scholar]
- Curt A, Alkadhi H, Crelier GR, Boendermaker SH, Hepp-Reymond MC, Kollias SS. Changes of non-affected upper limb cortical representation in paraplegic patients as assessed by fMRI. Brain. 2002;125:2567–2578. doi: 10.1093/brain/awf250. [DOI] [PubMed] [Google Scholar]
- Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002;19:43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- Curt A, Bruehlmeier M, Leenders KL, Roelcke U, Dietz V. Differential effect of spinal cord injury and functional impairment on human brain activation. J Neurotrauma. 2002;19:43–51. doi: 10.1089/089771502753460222. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Primary afferent terminal sprouting after a cervical dorsal rootlet section in the macaque monkey. J Comp Neurol. 2004;470:134–150. doi: 10.1002/cne.11030. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Brown S. Functional changes at periphery and cortex following dorsal root lesions in adult monkeys. Nat Neurosci. 2000;3:476–481. doi: 10.1038/74852. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Ciferri M. Cuneate nucleus reorganization following cervical dorsal rhizotomy in the macaque monkey: its role in the recovery of manual dexterity. J Comp Neurol. 2006;498:552–565. doi: 10.1002/cne.21088. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Lilak A, Alarcon C. Corticospinal sprouting occurs selectively following dorsal rhizotomy in the macaque monkey. J Comp Neurol. 2013;521:2359–2372. doi: 10.1002/cne.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RC, Leresche N, Crunelli V. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33:19599–19610. doi: 10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima VM, de Mello DF. [Nursing care given to children aged less than one year at a basic district health unit]. Rev Bras Enferm. 2004;57:531–533. doi: 10.1590/s0034-71672004000500002. [DOI] [PubMed] [Google Scholar]
- De Mello MT, Esteves AM, Tufik S. Comparison between dopaminergic agents and physical exercise as treatment for periodic limb movements in patients with spinal cord injury. Spinal Cord. 2004;42:218–221. doi: 10.1038/sj.sc.3101575. [DOI] [PubMed] [Google Scholar]
- Densmore VS, Kalous A, Keast JR, Osborne PB. Above-level mechanical hyperalgesia in rats develops after incomplete spinal cord injury but not after cord transection, and is reversed by amitriptyline, morphine and gabapentin. Pain. 2010;151:184–193. doi: 10.1016/j.pain.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 1985;96:1954–1960. 2004. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- Ding Q, Ying Z, Gomez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–780. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurotrophins and their receptors in nerve injury and repair. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Eccles JC. Presynaptic Inhibition in the Spinal Cord. Prog Brain Res. 1964;12:65–91. doi: 10.1016/s0079-6123(08)60618-4. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain. 2008;138:292–300. doi: 10.1016/j.pain.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Tominaga T, Brene S, Olson L. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain. 2007;130:2951–2961. doi: 10.1093/brain/awm237. [DOI] [PubMed] [Google Scholar]
- Endo T, Tominaga T, Olson L. Cortical changes following spinal cord injury with emphasis on the Nogo signaling system. Neuroscientist. 2009;15:291–299. doi: 10.1177/1073858408329508. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 470:101–104. doi: 10.1038/nature09656. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Kind PC. Neural activity: sculptor of 'barrels' in the neocortex. Trends Neurosci. 2001;24:589–595. doi: 10.1016/s0166-2236(00)01958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brecht M. Map plasticity in somatosensory cortex. Science. 2005;310:810–815. doi: 10.1126/science.1115807. [DOI] [PubMed] [Google Scholar]
- Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Florence SL, Boydston LA, Hackett TA, Lachoff HT, Strata F, Niblock MM. Sensory enrichment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. Eur J Neurosci. 2001;13:1755–1766. doi: 10.1046/j.0953-816x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB, Kaas JH. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- Foffani G, Humanes-Valera D, Calderon-Munoz F, Oliviero A, Aguilar J. Spinal cord injury immediately decreases anesthetic requirements in rats. Spinal Cord. 2011;49:822–826. doi: 10.1038/sc.2011.11. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Armstrong-James M. The role of the anterior intralaminar nuclei and N-methyl D-aspartate receptors in the generation of spontaneous bursts in rat neocortical neurones. Exp Brain Res. 1986;63:505–518. doi: 10.1007/BF00237474. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, Castro AW, Souza DR, Riberto M, Freedman SD, Nitsche MA, Pascual-Leone A. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122:197–209. doi: 10.1016/j.pain.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ. Disability, atrophy and cortical reorganization following spinal cord injury. Brain. 2011;134:1610–1622. doi: 10.1093/brain/awr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund P, Wheeler-Kingshott CA, Nagy Z, Gorgoraptis N, Weiskopf N, Friston K, Thompson AJ, Hutton C. Axonal integrity predicts cortical reorganisation following cervical injury. J Neurol Neurosurg Psychiatry. 2012;83:629–637. doi: 10.1136/jnnp-2011-301875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res Mol Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Friel KM, Heddings AA, Nudo RJ. Effects of postlesion experience on behavioral recovery and neurophysiologic reorganization after cortical injury in primates. Neurorehabil Neural Repair. 2000;14:187–198. doi: 10.1177/154596830001400304. [DOI] [PubMed] [Google Scholar]
- Ganzer PD, Moxon KA, Knudsen EB, Shumsky JS. Serotonergic pharmacotherapy promotes cortical reorganization after spinal cord injury. Exp Neurol. 2013;241:84–94. doi: 10.1016/j.expneurol.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia C, Chen MJ, Garza AA, Cotman CW, Russo-Neustadt A. The influence of specific noradrenergic and serotonergic lesions on the expression of hippocampal brain-derived neurotrophic factor transcripts following voluntary physical activity. Neuroscience. 2003;119:721–732. doi: 10.1016/s0306-4522(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Hochman S. Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol. 2001;86:2183–2194. doi: 10.1152/jn.2001.86.5.2183. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, Mueggler T, Baltes C, Rudin M, Weber B, Schwab ME. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci. 2010;13:97–104. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Peduzzi S, Snyder M, Schneider R, Starkey M, Schwab ME. Heterogeneous spine loss in layer 5 cortical neurons after spinal cord injury. Cereb Cortex. 2012;22:1309–1317. doi: 10.1093/cercor/bhr191. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci. 2009;29:12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter S, Davies MR, Ramakrishnan A, Udoekwere UI, Kargo WJ. Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates. J Neurophysiol. 2008;100:839–851. doi: 10.1152/jn.00866.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Davies MR, Graziani V. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J Neurophysiol. 2007;97:2663–2675. doi: 10.1152/jn.00308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ, Davies M, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol. 1998;80:3021–3030. doi: 10.1152/jn.1998.80.6.3021. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Zhuang Y. Brain and spinal cord interaction: protective effects of exercise prior to spinal cord injury. PLoS One. 2012;7:e32298. doi: 10.1371/journal.pone.0032298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Foffani G, Knudsen EB, Shumsky J, Moxon KA. Passive exercise of the hind limbs after complete thoracic transection of the spinal cord promotes cortical reorganization. PLoS One. 2013;8:e54350. doi: 10.1371/journal.pone.0054350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Wang S, Stein JF, Pereira EA, Kringelbach ML, Liu X, Brittain JS, Aziz TZ. Neural signatures in patients with neuropathic pain. Neurology. 2009;72:569–571. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JB, Sora E, Bialy Y, Ricamato A, Thatcher RW. Cortical sensorimotor reorganization after spinal cord injury: an electroencephalographic study. Neurology. 1998;50:1115–1121. doi: 10.1212/wnl.50.4.1115. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32:14874–14884. doi: 10.1523/JNEUROSCI.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex. 2010;20:1409–1419. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- Hains BC, Everhart AW, Fullwood SD, Hulsebosch CE. Changes in serotonin, serotonin transporter expression and serotonin denervation supersensitivity: involvement in chronic central pain after spinal hemisection in the rat. Exp Neurol. 2002;175:347–362. doi: 10.1006/exnr.2002.7892. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128:2359–2371. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Alterations in burst firing of thalamic VPL neurons and reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol. 2006;95:3343–3352. doi: 10.1152/jn.01009.2005. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hangartner TN, Rodgers MM, Glaser RM, Barre PS. Tibial bone density loss in spinal cord injured patients: effects of FES exercise. J Rehabil Res Dev. 1994;31:50–61. [PubMed] [Google Scholar]
- Harkema SJ, Ferreira CK, van den Brand RJ, Krassioukov AV. Improvements in orthostatic instability with stand locomotor training in individuals with spinal cord injury. J Neurotrauma. 2008;25:1467–1475. doi: 10.1089/neu.2008.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93:1588–1597. doi: 10.1016/j.apmr.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2012;93:1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci. 2007;27:9607–9622. doi: 10.1523/JNEUROSCI.2184-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ, Siddall PJ. Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J Neurosci. 2012;31:2630–2637. doi: 10.1523/JNEUROSCI.2717-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo N, Nishimura Y, Murata Y, Oishi T, Yoshino-Saito K, Takahashi M, Tsuboi F, Isa T. Increased expression of the growth-associated protein 43 gene in the sensorimotor cortex of the macaque monkey after lesioning the lateral corticospinal tract. J Comp Neurol. 2009;516:493–506. doi: 10.1002/cne.22121. [DOI] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 2010;103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007;87:208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Field-Fote EC. Cortical reorganization following bimanual training and somatosensory stimulation in cervical spinal cord injury: a case report. Phys Ther. 2007;87:208–223. doi: 10.2522/ptj.20050365. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schweinhardt P, Klason T, Olson L, Spenger C. Numb rats walk -a behavioural and fMRI comparison of mild and moderate spinal cord injury. Eur J Neurosci. 2003;18:3061–3068. doi: 10.1111/j.1460-9568.2003.03062.x. [DOI] [PubMed] [Google Scholar]
- Hoh DJ, Mercier LM, Hussey SP, Lane MA. Respiration following spinal cord injury: evidence for human neuroplasticity. Respir Physiol Neurobiol. 2013;189:450–464. doi: 10.1016/j.resp.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Humanes-Valera D, Aguilar J, Foffani G. Reorganization of the intact somatosensory cortex immediately after spinal cord injury. PLoS One. 2013;8:e69655. doi: 10.1371/journal.pone.0069655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha J, Centenaro LA, Broetto Cunha N, de Souza DF, Jaeger M, do Nascimento PS, Kolling J, Ben J, Marcuzzo S, Wyse AT, Gottfried C, Achaval M. The beneficial effects of treadmill step training on activity-dependent synaptic and cellular plasticity markers after complete spinal cord injury. Neurochem Res. 2011;36:1046–1055. doi: 10.1007/s11064-011-0446-x. [DOI] [PubMed] [Google Scholar]
- Inaba M, Maruyama T, Yoshimura Y, Hosoi H, Komatsu Y. Facilitation of low-frequency stimulation-induced long-term potentiation by endogenous noradrenaline and serotonin in developing rat visual cortex. Neurosci Res. 2009;64:191–198. doi: 10.1016/j.neures.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003;75:81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC, Kaas JH. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature. 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- Jain N, Diener PS, Coq JO, Kaas JH. Patterned activity via spinal dorsal quadrant inputs is necessary for the formation of organized somatosensory maps. J Neurosci. 2003;23:10321–10330. doi: 10.1523/JNEUROSCI.23-32-10321.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Florence SL, Kaas JH. Limits on plasticity in somatosensory cortex of adult rats: hindlimb cortex is not reactivated after dorsal column section. J Neurophysiol. 1995;73:1537–1546. doi: 10.1152/jn.1995.73.4.1537. [DOI] [PubMed] [Google Scholar]
- Jain N, Florence SL, Qi HX, Kaas JH. Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc Natl Acad Sci U S A. 2000;97:5546–5550. doi: 10.1073/pnas.090572597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Qi HX, Collins CE, Kaas JH. Large-scale reorganization in the somatosensory cortex and thalamus after sensory loss in macaque monkeys. J Neurosci. 2008;28:11042–11060. doi: 10.1523/JNEUROSCI.2334-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Ji Y, Voulalas PJ, Keaser M, Xu S, Gullapalli RP, Greenspan J, Masri R. Motor Cortex Stimulation Suppresses Cortical Responses to Noxious Hindpaw Stimulation After Spinal Cord Lesion in Rats. Brain Stimul. 2013 doi: 10.1016/j.brs.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsuki S, Takemoto K, Kawasaki T, Tada H, Takahashi A, Becamel C, Sano A, Yuzaki M, Zukin RS, Ziff EB, Kessels HW, Takahashi T. Serotonin mediates cross-modal reorganization of cortical circuits. Neuron. 2011;69:780–792. doi: 10.1016/j.neuron.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KA, Srivastava DP, Allen JA, Strachan RT, Roth BL, Penzes P. Rapid modulation of spine morphology by the 5-HT2A serotonin receptor through kalirin-7 signaling. Proc Natl Acad Sci U S A. 2009;106:19575–19580. doi: 10.1073/pnas.0905884106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci U S A. 1991;88:780–784. doi: 10.1073/pnas.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair. 2007;21:527–538. doi: 10.1177/1545968307301872. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Qi HX, Burish MJ, Gharbawie OA, Onifer SM, Massey JM. Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp Neurol. 2008;209:407–416. doi: 10.1016/j.expneurol.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambi N, Halder P, Rajan R, Arora V, Chand P, Arora M, Jain N. Large-scale reorganization of the somatosensory cortex following spinal cord injuries is due to brainstem plasticity. Nat Commun. 2014;5:3602. doi: 10.1038/ncomms4602. [DOI] [PubMed] [Google Scholar]
- Kambi N, Tandon S, Mohammed H, Lazar L, Jain N. Reorganization of the primary motor cortex of adult macaque monkeys after sensory loss resulting from partial spinal cord injuries. J Neurosci. 2011;31:3696–3707. doi: 10.1523/JNEUROSCI.5187-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Jacob-Vadakot S, Himes BT, Murray M, Moxon KA. Role of the 5-HT2C receptor in improving weight-supported stepping in adult rats spinalized as neonates. Brain Res. 2006;1112:159–168. doi: 10.1016/j.brainres.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Knudsen EB, Murray M, Moxon KA. Functional role of exercise-induced cortical organization of sensorimotor cortex after spinal transection. J Neurophysiol. 2011;106:2662–2674. doi: 10.1152/jn.01017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Murray M, Moxon KA. Exercise induces cortical plasticity after neonatal spinal cord injury in the rat. J Neurosci. 2009;29:7549–7557. doi: 10.1523/JNEUROSCI.2474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol. 2006;198:401–415. doi: 10.1016/j.expneurol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, Simansky KJ. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. J Neurosci. 1999;19:6213–6224. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A. Serotonergic control of developmental plasticity. Proc Natl Acad Sci U S A. 2000;97:1951–1952. doi: 10.1073/pnas.070044697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser TS, Reese NB, Maresh T, Hearn S, Yates C, Skinner RD, Pait TG, Garcia-Rill E. Use of a motorized bicycle exercise trainer to normalize frequency-dependent habituation of the H-reflex in spinal cord injury. J Spinal Cord Med. 2005;28:241–245. doi: 10.1080/10790268.2005.11753818. [DOI] [PubMed] [Google Scholar]
- Kojic L, Gu Q, Douglas RM, Cynader MS. Serotonin facilitates synaptic plasticity in kitten visual cortex: an in vitro study. Brain Res Dev Brain Res. 1997;101:299–304. doi: 10.1016/s0165-3806(97)00083-7. [DOI] [PubMed] [Google Scholar]
- Landry ES, Lapointe NP, Rouillard C, Levesque D, Hedlund PB, Guertin PA. Contribution of spinal 5-HT1A and 5-HT7 receptors to locomotor-like movement induced by 8-OH-DPAT in spinal cord-transected mice. Eur J Neurosci. 2006;24:535–546. doi: 10.1111/j.1460-9568.2006.04917.x. [DOI] [PubMed] [Google Scholar]
- Lauer RT, Smith BT, Mulcahey MJ, Betz RR, Johnston TE. Effects of cycling and/or electrical stimulation on bone mineral density in children with spinal cord injury. Spinal Cord. 2011;49:917–923. doi: 10.1038/sc.2011.19. [DOI] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon W, Sato TF, Opitz A, Mueller J, Barbour A, Williams A, Tyler WJ. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat Neurosci. 2014;17:322–329. doi: 10.1038/nn.3620. [DOI] [PubMed] [Google Scholar]
- Levy WJ, Jr., Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- Levy WJ, Jr., Amassian VE, Traad M, Cadwell J. Focal magnetic coil stimulation reveals motor cortical system reorganized in humans after traumatic quadriplegia. Brain Res. 1990;510:130–134. doi: 10.1016/0006-8993(90)90738-w. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Nobin A, Stenevi U. The adrenergic innervation of the rat thalamus as revealed by the glyoxylic acid fluorescence method. J Comp Neurol. 1974;154:317–347. doi: 10.1002/cne.901540307. [DOI] [PubMed] [Google Scholar]
- Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Keeler BE, Zhukareva V, Houle JD. Cycling exercise affects the expression of apoptosis-associated microRNAs after spinal cord injury in rats. Exp Neurol. 2010;226:200–206. doi: 10.1016/j.expneurol.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Pre-and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. Eur J Neurosci. 1996;8:2188–2197. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci Lett. 1999;269:87–90. doi: 10.1016/s0304-3940(99)00422-x. [DOI] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci. 1999;2:501–502. doi: 10.1038/9145. [DOI] [PubMed] [Google Scholar]
- Lotze M, Laubis-Herrmann U, Topka H. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restor Neurol Neurosci. 2006;24:97–107. [PubMed] [Google Scholar]
- Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain. 2011;152:1398–1407. doi: 10.1016/j.pain.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen-Berg H. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun. 2013;4:1570. doi: 10.1038/ncomms2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manduch M, Bezuhly M, Anastakis DJ, Crawley AP, Mikulis DJ. Serial fMRI of adaptive changes in primary sensorimotor cortex following thumb reconstruction. Neurology. 2002;59:1278–1281. doi: 10.1212/wnl.59.8.1278. [DOI] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. J Neurophysiol. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Maya-Vetencourt JF, Tiraboschi E, Spolidoro M, Castren E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur J Neurosci. 2011;33:49–57. doi: 10.1111/j.1460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley PA, Smith JL. Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal cord transection in cats. J Neurosci. 1990;10:1429–1443. doi: 10.1523/JNEUROSCI.10-05-01429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley PA, Smith JL. Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal cord transection in cats. J Neurosci. 1990;10:1429–1443. doi: 10.1523/JNEUROSCI.10-05-01429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley PA, Swyter E. Progressive changes in somatosensory cortical maps in 6-week-old kittens cord-transected at T12. Brain Res. 1989;484:378–382. doi: 10.1016/0006-8993(89)90385-5. [DOI] [PubMed] [Google Scholar]
- Merrill L, Girard B, Arms L, Guertin P, Vizzard MA. Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr Pharm Des. 2013;19:4411–4422. doi: 10.2174/1381612811319240008. [DOI] [PubMed] [Google Scholar]
- Mikulis DJ, Jurkiewicz MT, McIlroy WE, Staines WR, Rickards L, Kalsi-Ryan S, Crawley AP, Fehlings MG, Verrier MC. Adaptation in the motor cortex following cervical spinal cord injury. Neurology. 2002;58:794–801. doi: 10.1212/wnl.58.5.794. [DOI] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. J Neurosci. 1997;17:4856–4872. doi: 10.1523/JNEUROSCI.17-12-04856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Dunbar C, Kostyk SK, Gehi A, Corkin S. Referred phantom sensations and cortical reorganization after spinal cord injury in humans. Proc Natl Acad Sci U S A. 2000;97:14703–14708. doi: 10.1073/pnas.250348997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Arch Phys Med Rehabil. 2013;94:2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Morrow TJ, Paulson PE, Brewer KL, Yezierski RP, Casey KL. Chronic, selective forebrain responses to excitotoxic dorsal horn injury. Exp Neurol. 2000;161:220–226. doi: 10.1006/exnr.1999.7246. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- Musienko P, van den Brand R, Maerzendorfer O, Larmagnac A, Courtine G. Combinatory electrical and pharmacological neuroprosthetic interfaces to regain motor function after spinal cord injury. IEEE Trans Biomed Eng. 2009;56:2707–2711. doi: 10.1109/TBME.2009.2027226. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Ness TJ, San Pedro EC, Richards JS, Kezar L, Liu HG, Mountz JM. A case of spinal cord injury-related pain with baseline rCBF brain SPECT imaging and beneficial response to gabapentin. Pain. 1998;78:139–143. doi: 10.1016/S0304-3959(98)00153-5. [DOI] [PubMed] [Google Scholar]
- Nessler JA, Timoszyk W, Merlo M, Emken JL, Minakata K, Roy RR, de Leon RD, Edgerton VR, Reinkensmeyer DJ. A robotic device for studying rodent locomotion after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng. 2005;13:497–506. doi: 10.1109/TNSRE.2005.858432. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Azmitia EC. Agonist-and antagonist-induced plasticity of rat 5-HT1A receptor in hippocampal cell culture. Synapse. 1999;31:186–195. doi: 10.1002/(SICI)1098-2396(19990301)31:3<186::AID-SYN3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt. 2000;3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann C, Clark K. Selective modulation of Ca(2+) influx pathways by 5-HT regulates synaptic long-term plasticity in the hippocampus. Brain Res. 2005;1037:187–193. doi: 10.1016/j.brainres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Mordillo-Mateos L, Arias P, Panyavin I, Foffani G, Aguilar J. Transcranial static magnetic field stimulation of the human motor cortex. J Physiol. 2011;589:4949–4958. doi: 10.1113/jphysiol.2011.211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza CS, Giszter SF. Plasticity and alterations of trunk motor cortex following spinal cord injury and non-stepping robot and treadmill training. Exp Neurol. 2014;256:57–69. doi: 10.1016/j.expneurol.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Gorman AL, Yezierski RP, Casey KL, Morrow TJ. Differences in forebrain activation in two strains of rat at rest and after spinal cord injury. Exp Neurol. 2005;196:413–421. doi: 10.1016/j.expneurol.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Schneider F, Faillenot I, Convers P, Barral FG, Garcia-Larrea L, Laurent B. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63:1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Kiratli BJ, Sarkarati M, Weraarchakul G, Myers J, Franklin BA, Parkash I, Froelicher V. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23:641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Proudfit HK, Anderson EG. Influence of serotonin antagonists on bulbospinal systems. Brain Res. 1973;61:331–341. doi: 10.1016/0006-8993(73)90537-4. [DOI] [PubMed] [Google Scholar]
- Qi HX, Jain N, Collins CE, Lyon DC, Kaas JH. Functional organization of motor cortex of adult macaque monkeys is altered by sensory loss in infancy. Proc Natl Acad Sci U S A. 2010;107:3192–3197. doi: 10.1073/pnas.0914962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HX, Reed JL, Gharbawie OA, Burish MJ, Kaas JH. Cortical neuron response properties are related to lesion extent and behavioral recovery after sensory loss from spinal cord injury in monkeys. J Neurosci. 2014;34:4345–4363. doi: 10.1523/JNEUROSCI.4954-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci U S A. 2006;103:11370–11375. doi: 10.1073/pnas.0601065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AJ, Rubio MD, Defagot C, Hischberg L, Villar MJ, Brusco A. The 5HT1A receptor agonist, 8-OH-DPAT, protects neurons and reduces astroglial reaction after ischemic damage caused by cortical devascularization. Brain Res. 2004;1030:201–220. doi: 10.1016/j.brainres.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Reig R, Sanchez-Vives MV. Synaptic transmission and plasticity in an active cortical network. PLoS One. 2007;2:e670. doi: 10.1371/journal.pone.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Zhang L, Lu Y, Yang H, Westlund KN. Central lateral thalamic neurons receive noxious visceral mechanical and chemical input in rats. J Neurophysiol. 2009;102:244–258. doi: 10.1152/jn.90985.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. Thalamocortical Up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci. 2007;27:4261–4272. doi: 10.1523/JNEUROSCI.0003-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt A, Beard RC, Cotman CW. Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology. 1999;21:679–682. doi: 10.1016/S0893-133X(99)00059-7. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Beard RC, Huang YM, Cotman CW. Physical activity and antidepressant treatment potentiate the expression of specific brain-derived neurotrophic factor transcripts in the rat hippocampus. Neuroscience. 2000;101:305–312. doi: 10.1016/s0306-4522(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Ebner FF, Wilson CJ. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol. 2004;92:3511–3521. doi: 10.1152/jn.00347.2004. [DOI] [PubMed] [Google Scholar]
- Satoh K, Fibiger HC. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986;253:277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res. 2004;1017:172–183. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Jiang L, Ji Y, Xu S, Gullapalli RP, Masri R. Thalamocortical asynchrony in conditions of spinal cord injury pain in rats. J Neurosci. 2012;32:15843–15848. doi: 10.1523/JNEUROSCI.2927-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H, Alilain WJ, Sadhu A, Silver J. Treatments to restore respiratory function after spinal cord injury and their implications for regeneration, plasticity and adaptation. Exp Neurol. 2012;235:18–25. doi: 10.1016/j.expneurol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheibani V, Farazifard R. Dorsal raphe nucleus stimulation modulates the response of layers IV and V barrel cortical neurons in rat. Brain Res Bull. 2006;68:430–435. doi: 10.1016/j.brainresbull.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Shumsky JS, Kao T, Amato N, Simansky K, Murray M, Moxon KA. Partial 5-HT(1A) receptor agonist activity by the 5-HT(2C) receptor antagonist SB 206,553 is revealed in rats spinalized as neonates. Exp Neurol. 2005;191:361–365. doi: 10.1016/j.expneurol.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Sievert CF, Neafsey EJ. A chronic unit study of the sensory properties of neurons in the forelimb areas of rat sensorimotor cortex. Brain Res. 1986;381:15–23. doi: 10.1016/0006-8993(86)90684-0. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Skagerberg G, Bjorklund A. Topographic principles in the spinal projections of serotonergic and non serotonergic brainstem neurons in the rat. Neuroscience. 1985;15:445–480. doi: 10.1016/0306-4522(85)90225-8. [DOI] [PubMed] [Google Scholar]
- Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, Navarro X, Pascual-Leone A. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain. 2010;133:2565–2577. doi: 10.1093/brain/awq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzner DJ, Ershler WB, Weber ED. Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol. 1975;46:156–177. doi: 10.1016/0014-4886(75)90039-4. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydekum E, Ghosh A, Gullo M, Baltes C, Schwab M, Rudin M. Rapid functional reorganization of the forelimb cortical representation after thoracic spinal cord injury in adult rats. Neuroimage. 2014;87:72–79. doi: 10.1016/j.neuroimage.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Tandon S, Kambi N, Lazar L, Mohammed H, Jain N. Large-scale expansion of the face representation in somatosensory areas of the lateral sulcus after spinal cord injuries in monkeys. J Neurosci. 2009;29:12009–12019. doi: 10.1523/JNEUROSCI.2118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Topka H, Cohen LG, Cole RA, Hallett M. Reorganization of corticospinal pathways following spinal cord injury. Neurology. 1991;41:1276–1283. doi: 10.1212/wnl.41.8.1276. [DOI] [PubMed] [Google Scholar]
- Tran Y, Boord P, Middleton J, Craig A. Levels of brain wave activity (8-13 Hz) in persons with spinal cord injury. Spinal Cord. 2004;42:73–79. doi: 10.1038/sj.sc.3101543. [DOI] [PubMed] [Google Scholar]
- Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J Neurosci. 2003;23:7034–7044. doi: 10.1523/JNEUROSCI.23-18-07034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J Neurosci. 2003;23:7034–7044. doi: 10.1523/JNEUROSCI.23-18-07034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Lee JS, Schandler SL, Cohen MJ. An fMRI investigation of hand representation in paraplegic humans. Neurorehabil Neural Repair. 2003;17:37–47. doi: 10.1177/0888439002250443. [DOI] [PubMed] [Google Scholar]
- Ung RV, Landry ES, Rouleau P, Lapointe NP, Rouillard C, Guertin PA. Role of spinal 5-HT2 receptor subtypes in quipazine-induced hindlimb movements after a low-thoracic spinal cord transection. Eur J Neurosci. 2008;28:2231–2242. doi: 10.1111/j.1460-9568.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- van Hedel HJ, Dietz V. Rehabilitation of locomotion after spinal cord injury. Restor Neurol Neurosci. 2010;28:123–134. doi: 10.3233/RNN-2010-0508. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. doi: 10.1016/j.brainres.2005.11.062. [DOI] [PubMed] [Google Scholar]
- Vessal M, Aycock A, Garton MT, Ciferri M, Darian-Smith C. Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. Eur J Neurosci. 2007;26:2777–2794. doi: 10.1111/j.1460-9568.2007.05871.x. [DOI] [PubMed] [Google Scholar]
- Vessal M, Darian-Smith C. Adult neurogenesis occurs in primate sensorimotor cortex following cervical dorsal rhizotomy. J Neurosci. 2010;30:8613–8623. doi: 10.1523/JNEUROSCI.5272-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD, Egger MD. Formation of new connexions in adult rat brains after partial deafferentation. Nature. 1971;232:542–545. doi: 10.1038/232542a0. [DOI] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasner G, Lee BB, Engel S, McLachlan E. Residual spinothalamic tract pathways predict development of central pain after spinal cord injury. Brain. 2008;131:2387–2400. doi: 10.1093/brain/awn169. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Interaction of serotonin with somatosensory cortical neuronal responses to afferent synaptic inputs and putative neurotransmitters. Brain Res Bull. 1986;17:507–518. doi: 10.1016/0361-9230(86)90218-2. [DOI] [PubMed] [Google Scholar]
- Weber ED, Stelzner DJ. Behavioral effects of spinal cord transection in the developing rat. Brain Res. 1977;125:241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Weishaupt N, Blesch A, Fouad K. BDNF: the career of a multifaceted neurotrophin in spinal cord injury. Exp Neurol. 2012;238:254–264. doi: 10.1016/j.expneurol.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Wessels M, Lucas C, Eriks I, de Groot S. Body weight-supported gait training for restoration of walking in people with an incomplete spinal cord injury: a systematic review. J Rehabil Med. 2010;42:513–519. doi: 10.2340/16501977-0525. [DOI] [PubMed] [Google Scholar]
- Whitt JL, Masri R, Pulimood NS, Keller A. Pathological activity in mediodorsal thalamus of rats with spinal cord injury pain. J Neurosci. 2013;33:3915–3926. doi: 10.1523/JNEUROSCI.2639-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester P, McColl R, Querry R, Foreman N, Mosby J, Tansey K, Williamson J. Changes in supraspinal activation patterns following robotic locomotor therapy in motor-incomplete spinal cord injury. Neurorehabil Neural Repair. 2005;19:313–324. doi: 10.1177/1545968305281515. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ, Henderson LA. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex. 2009;19:224–232. doi: 10.1093/cercor/bhn072. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, Middleton JW, Henderson LA, Siddall PJ. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141:52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Wydenkeller S, Maurizio S, Dietz V, Halder P. Neuropathic pain in spinal cord injury: significance of clinical and electrophysiological measures. Eur J Neurosci. 2009;30:91–99. doi: 10.1111/j.1460-9568.2009.06801.x. [DOI] [PubMed] [Google Scholar]
- Yague JG, Foffani G, Aguilar J. Cortical hyperexcitability in response to preserved spinothalamic inputs immediately after spinal cord hemisection. Exp Neurol. 2011;227:252–263. doi: 10.1016/j.expneurol.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Yagüe JG, Humanes-Valera D, Aguilar J, Foffani G. Functional reorganization of the forepaw cortical representation immediately after thoracic spinal cord hemisection in rats. Exp Neurol. 2014;257C:19–24. doi: 10.1016/j.expneurol.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Wilson PR. Spinal serotonin terminal system mediates antinociception. J Pharmacol Exp Ther. 1979;208:446–453. [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain. 2012;135:2277–2289. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni MZ, Michels L, Mehnert U, Schurch B, Kollias S. Cortical substrate of bladder control in SCI and the effect of peripheral pudendal stimulation. Neuroimage. 2010;49:2983–2994. doi: 10.1016/j.neuroimage.2009.10.064. [DOI] [PubMed] [Google Scholar]