Abstract
Objective
Distinguishing secondary hyperparathyroidism (sHPT) from eucalcemic primary hyperparathyroidism (EC-pHPT) is important. The objective of this study was to measure parathyroid hormone (PTH)-stimulated production of 1α,25-dihydroxyvitamin D (1,25[OH]2D) in early postmenopausal patients with idiopathic sHPT, who also fit the criteria for EC-pHPT, compared to age-matched controls.
Methods
In this pilot case-control study, postmenopausal women aged 44 to 55 years with normal serum calcium (Ca), glomerular filtration rate (GFR) ≥65 mL/min, and 25-hydroxyvitamin D (25[OH]D) ≥75 nmol/L (30 ng/mL) were given an 8 hour infusion of PTH(1-34), 12 pmol/kg/h. Patients (n = 5) had elevated PTH, normal 1,25(OH)2D, and no hypercalciuria. Controls (n = 5) had normal PTH. At baseline, 4, and 8 hours, serum Ca, creatinine (Cr), phosphorus (P), 1,25(OH)2D, fibroblast growth factor (FGF23), and 24,25(OH)2D as well as urine Ca, P, Cr, and cAMP/GFR were measured. The fractional excretion of calcium (FeCa) and tubular reabsorption of phosphorus (TMP)/GFR were calculated.
Results
Patients had lower 1,25(OH)2D levels (± SD) than controls at 4 (39.8 ± 6.9 versus 58.8 ± 6.7; P = .002) and 8 hours (56.4 ± 9.2 versus 105 ± 2.3; P = .003) of PTH infusion, attenuated after adjusting for higher body mass index (BMI) in patients (P = .05, .04), respectively. The 24,25(OH)2D levels were lower in patients than controls (1.9 ± 0.6 versus 3.4 ± 0.6, respectively; P = .007). No differences were seen in serum Ca or P, urine cAMP/GFR, TRP/GFR, FeCa, or PTH suppression at 8 hours (patients 50%, controls 64%).
Conclusion
Vitamin D sufficient patients who fit the criteria for EC-pHPT had reduced PTH-stimulated 1,25(OH)2D compared to controls, partially attributable to their higher BMI. Other causes of reduced 1,25(OH)2D production ruled out were excessive catabolism of vitamin D metabolites, elevated FGF23, and CYP27B1 mutation. Elevated BMI and idiopathic reduced PTH-stimulated 1,25(OH)2D production should be considered in the differential of sHPT.
INTRODUCTION
The phenotype of normocalcemia and elevated parathyroid hormone (PTH), with grossly normal renal function, has been estimated to be present in 0.6% of postmenopausal women (1) and 8.9% of postmenopausal women undergoing evaluation for osteoporosis (2). The primary differential in this phenotype is secondary hyperparathyroidism (sHPT) versus eucalcemic primary hyperparathyroidism (EC-pHPT) (3). It is important for the clinician to distinguish between these two diagnoses because the treatments differ. The most common causes of sHPT are chronic kidney disease (CKD) (4), aging (5-7), vitamin D deficiency (8,9), and hypercalciuria (10). Recently, elevated body mass index (BMI) has also been appreciated as a cause of sHPT through a reduction in the production of 1α,25-dihydroxyvitamin D (1,25[OH]2D) (11,12).
In CKD, the reduction in renal 1α-hydroxylation of 25-hydroxyvitamin D (25[OH]D) to active 1,25(OH)2D is one of the earliest changes leading to sHPT (13,14). In addition, in women, PTH has been shown to increase progressively with age after menopause, in the absence of overt CKD (15). In vitamin D deficiency, the reduction in 25(OH)D available to be converted to 1,25(OH)2D, similarly leads to sHPT (8,9). In hypercalciuria, the negative calcium balance resulting from renal calcium loss is presumed to stimulate PTH release (10). The mechanism of sHPT associated with elevated BMI is unclear (12,16).
EC-pHPT is defined as a normal serum calcium and elevated PTH in the absence of known causes of sHPT (17). Given the many potential causes for sHPT, in the evaluation of the middle-aged to elderly patient with normal serum calcium and elevated PTH, the effects of age, elevated BMI and mild CKD need to be carefully considered as possible causes of sHPT before diagnosing EC-hPHT. The definition of EC-pHTP does not require an elevated 1,25(OH)2D level. However, since PTH is the rate controlling factor in the production of 1,25(OH)2D (18), in the setting of young, healthy kidneys, an elevated PTH would be expected to be associated with an elevated 1,25(OH)2D level. A previous study comparing EC-pHPT to hypercalcemic pHPT (H-pHPT) showed that BMI was higher and renal function was lower in the EC-pHPT group, supporting the importance of BMI and GFR to both production of 1,25(OH)2D and serum calcium levels (16).
In the evaluation of patients for low bone mass/osteoporosis, we have occasionally observed relatively young postmenopausal women with grossly normal GFR (>65 mL/min), normal serum calcium, elevated PTH, normal 25(OH)D, and normal 1,25(OH)2D. Given their young age, normal GFR, and elevated PTH, their 1,25(OH)2D levels were expected by us to be elevated, since PTH largely controls the production of 1,25(OH)2D in the setting of normal kidney function. The normal 1,25(OH)2D levels in these patients suggested to us an intrinsic reduction in the ability to produce 1,25(OH)2D in response to PTH, hence sHPT rather than EC-pHPT. We hypothesized that patients with this phenotype (age ≤55 years, GFR ≥65 mL/min, normal Ca, elevated PTH, absence of vitamin D deficiency and hypercalciuria (<4 mg/kg) (19) had sHPT due to a primary defect of 1α-hydroxylation of 25(OH)D to 1,25(OH)2D, independent of renal function and age. We further hypothesized that the reduced 1α-hydroxylation might be due to a mutation in the gene for 1α-hydroxylase, CYP27B1. The precedent for this possibility is the partial deficiency of the 1α-hydroxylase enzyme described in some patients with pseudovitamin D resistant rickets type I (VDDR-1), a disorder due to mutations in CYP27B1 (20).
To test our hypothesis, a pilot study was performed in patients with normal serum calcium and unexplained elevation of PTH, discovered during evaluation for low bone mass, and controls, comparing the production of 1,25(OH)2D during an 8 hour intravenous infusion of PTH(1-34). To evaluate for PTH resistance, changes in the fractional excretion of calcium (FeCa) and tubular reabsorption of phosphorus (TRP/GFR) with PTH(1-34) infusion were calculated. We report here results from 5 patients and 5 controls.
METHODS
Participants
Participants were studied at the University of Maryland Hospital (UMMS) General Clinical Research Center (GCRC) after signing institutional review board (IRB) approved informed consent, between 2006 and 2008. Participants in both groups, patients and controls, were postmenopausal females aged ≤55 years with GFR ≥65 mL/min. GFR was calculated using the National Kidney Foundation online calculator (http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm), which calculates GFR using both the CKD-Epidemiology Collaboration (CKD-EPI) and modification of diet in renal disease (MDRD) GFR equations, serum creatinine, age, sex, and race (Caucasian versus African American), with units expressed as mL/min/1.73 m2. GFR calculated from the CKD-EPI calculation was used for study inclusion (21). Patients were recruited from the metabolic bone clinic (MBC) at UMMS where they were referred for low bone mass or osteoporosis and found to have idiopathic secondary hyperparathyroidism (sHPT) during metabolic evaluation. Inclusion criteria for patients included: normal serum Ca, 25(OH)D≥30 ng/mL, elevated PTH >65 pg/mL, (the reference upper level), normal TSH and normal urine Ca (determined by 24-hour urine collection of <4 mg/kg) or fasting urine Ca/creatinine <0.2 [n = 1]). Normal serum Ca and elevation of PTH were documented in patients prior to the study for at least 6 months in all and up to 5 years in some. Although not required for participation, in four patients, after receiving oral calcitriol 0.5 μg for 2 weeks, PTH normalized and serum Ca remained normal (performed at least 3 months before PTH infusion). During the 3 year study period, 586 patients were seen in the MBC clinic for low bone mass, osteoporosis and parathyroid disorder. Of these, 45 (7.8%) had pHPT (hypercalcemia and either elevated or inappropriately normal PTH) and 31 (5.3%) had what we felt was unexplained sHPT (normal serum Ca, elevated PTH), although they would satisfy the criteria for EC-pHPT. Eight of the 31 patients with sHPT had GFR ≥65 mL/min, thereby meeting the criteria for inclusion as patients and were enrolled in the study; 5 patients agreed to the PTH infusion.
Controls were recruited from the UMMS MBC and by online advertising. Control inclusion criteria included: female, age≤55 years, GFR ≥65 mL/min, and normal serum Ca, 25(OH)D≥30 ng/mL, TSH, and PTH. Additional exclusion criteria for both groups included Paget disease of bone, active malignancy, nephrolithiasis, metabolic bone disease other than osteoporosis, diuretic and anticonvulsant use, diabetes mellitus, glucocorticoid use, known malabsorption syndrome, and symptoms suggesting malabsorption and smoking. Nine participants were enrolled in the control group based on history criteria; 4 were excluded by screening lab criteria (GFR <65 mL/min), 5 completed PTH infusion.
Intravenous Teriperatide Infusion Protocol
An IND was obtained (#76,579) to use teriparatide (Forteo) intravenously (IV) for this study. The Clinical Trials identifier was NCT0075444. After an overnight fast, teriparatide was infused IV at 12 pmol/kg/h over 8 hours. The infusion was prepared in our Investigational Drug Pharmacy by withdrawing 100 μg (0.4 mL) of teriparatide from a commercially available Forteo pen (with a syringe) and adding this to an IV bag containing 100 mL of saline and 4 mL of human serum albumin (HSA) 25%. The volume infused to participants was weight specific. The purpose of HSA was to prevent PTH from adhering to plastic infusion tubing. The final teriparatide concentration in infusions was 1 μg/ml and of HSA was 1%. This dose was chosen because in prior studies, 7 hours of infusion of PTH(1-34) resulted in an approximate doubling of the 1,25(OH)2D level (22). We chose an 8 hour infusion time to maximize stimulation of 1,25(OH)2D, the longest infusion time possible in our GCRC. Blood was collected at baseline, 4, and 8 hours for Ca, P, and 1,25(OH)2D; at baseline and 8 hours for PTH and 24,25-dihydroxyvitamin D; and at baseline for albumin and fibroblast growth factor 23 (FGF23). Participants remained fasting until the 4 hour samples were collected and then were given a standardized lunch containing 200 mg of phosphorus (P). Collected blood was immediately placed on ice; after 30 minutes, the samples were centrifuged for 10 minutes at 4°C and immediately frozen on dry ice and stored at –80°C until the assay was performed. Urine was collected at baseline, 4, and 8 hours for calcium (Ca), P, cAMP, and creatinine (Cr). Calculations were performed for FeCa, TRP/GFR, and urine (U) cAMP/GFR.
Laboratory Studies
Serum and urine chemistries, 25(OH)D (Diasorin kit), and PTH were performed at the clinical chemistry lab at UMMS. Assays for 1,25(OH)2D were performed in the laboratory of Dr Michael F Holick (Boston University Medical Center) (23); coefficient of variation (CV) for the assay was 8 to 10%. Urine for cAMP was immediately acidified after collection and analyzed at the ARUP Lab (Salt Lake City, UT, USA). Intact FGF23 was measured in all participants by ELISA (Johns Hopkins Bayview lab, Kainos kit) and a C-terminal FGF23 level assessment was conducted for 3 patients by the Mayo Medical Laboratory (Immunometric assay) in order to confirm the prior results. The 24,25(OH)2D assays were performed by Dr Ronald Horst (Iowa State University) (24). The entireCYP27B1 gene was sequenced using 10 primers (for 8 exons, 9 introns including promoter). PCR products were sequenced using ABI Big Dye v3.1 dye terminator, run on an ABI 3730 sequencer, and analyzed with Sequencer v4.5 software (Life Technologies Corp, Carlsbad, CA, USA).
Statistical Analyses
The patient and control group phenotypes were compared by the Student's paired t test. This study was performed prior to publication of reports of an effect of BMI on PTH (11) and 1,25(OH)2D (12), so we did not control for BMI in participant selection. Patients had higher BMI than controls. To evaluate the magnitude of effect of BMI on PTH and PTH-stimulated 1,25(OH)2D, we performed two analyses using data outside of this study. First, to evaluate the BMI/PTH relationship, we used our database of 1096 generally healthy Amish individuals (25), mean age 50.6±15.5 years, to perform a linear regression analysis between BMI and PTH (after adjusting PTH for serum creatinine and age). Secondly, since there were no published data on the relationship between BMI and PTH-stimulated 1,25(OH)2D, we used unpublished data from PTH infusion studies from one of our authors (MH), performed at the University of Pittsburgh (22) using PTH infusions concentrations of 12 pmol/kg/h and 1,25(OH)2D values at 7 hours (no results available for 8 hours) to perform linear regression analysis between BMI and PTH-stimulated 1,25(OH)2D.
RESULTS
Clinical characteristics of the patient group are shown in Table 1. All participants were Caucasian. Compared to controls, patients had higher PTH (expected per protocol), lower 24,25(OH)2D, and higher BMI (Table 2). The GFR values given in Table 1 are for the GFR-EPI calculation method (21), where GFR is expressed in mL/min/1.73 m2.
Table 1.
Characteristics of Patient Group
| Subject | Age (y) | GFRa | Cr | Ca (mg/dL) (8.6-10) | P (mg/dL) (2.7-4.5) | PTH (pg/mL) (12-65) | 25-D (ng/mL) | 1,25-D (pg/mL) (15-60) | 24-h Urine Ca (mg) or Ca/Cr | DXA T-Score |
|---|---|---|---|---|---|---|---|---|---|---|
| D103 | 49 | 89 | 0.88 | 9.4 | 3.5 | 107 | 33 | 56 | 182 | –1.3 |
| D106 | 52 | 88 | 0.77 | 9.1 | 4.1 | 91 | 33 | 42 | 193 | –2.4 |
| D109 | 54 | 87 | 0.70 | 9.3 | 4.2 | 100 | 33 | 50 | Ca/Cr 0.14 | –1.2 |
| D110 | 55 | 97 | 0.66 | 9.2 | 3.8 | 81 | 39 | 35 | 238 | –2.1 |
| D111 | 55 | 65 | 0.91 | 9.4 | 3.8 | 93 | 48 | 50 | Ca/Cr 0.11 | –2.2 |
Abbreviations: 1,25-D = 1α,25-dihydroxyvitamin D; 25-D = 25-hydroxyvitamin D; Ca = calcium; Cr = creatinine; DXA = dual-energy X-ray absorptiometry (lowest of spine, total hip or femoral neck); GFR = glomerular filtration rate.
GFR is in mL/min/1.73 m2.
Table 2.
Baseline Laboratory Data on Patient Versus Control Groupsa
| Measurement | Patients (SD) | Controls (SD) | P value |
|---|---|---|---|
| Age (y) | 53.0 (2.5) | 51.8 (4.4) | 0.61 |
| Cr (mg/dL) | 0.78 (0.11) | 0.80 (0.10) | 0.79 |
| GFR (mL/min/1.73 m2) | 76.1 (11.4) | 76.1 (7.9) | 0.90 |
| BMI (kg/m2) | 31.5 (6.0) | 23.3 (3.5) | 0.03 |
| 25(OH)D (ng/mL) | 37.7 (2.63) | 42.7 (5.67) | 0.11 |
| 1,25(OH)2D (pg/mL) | 40.6 (5.6) | 46.2 (3.7) | 0.10 |
| PTH (pg/mL) | 71.8 (18.5) | 44.0 (14.3) | 0.03 |
| 24,25(OH)2D (ng/mL) | 1.9 (0.6) | 3.4 (0.6) | 0.007 |
Abbreviations: 1,25(OH)2D = 1α,25-dihydroxyvitamin D; 25(OH)D = 25-hydroxyvitamin D; BMI = body mass index; Cr = creatinine; GFR = glomerular filtration rate; PTH = parathyroid hormone.
n = 5 for each group.
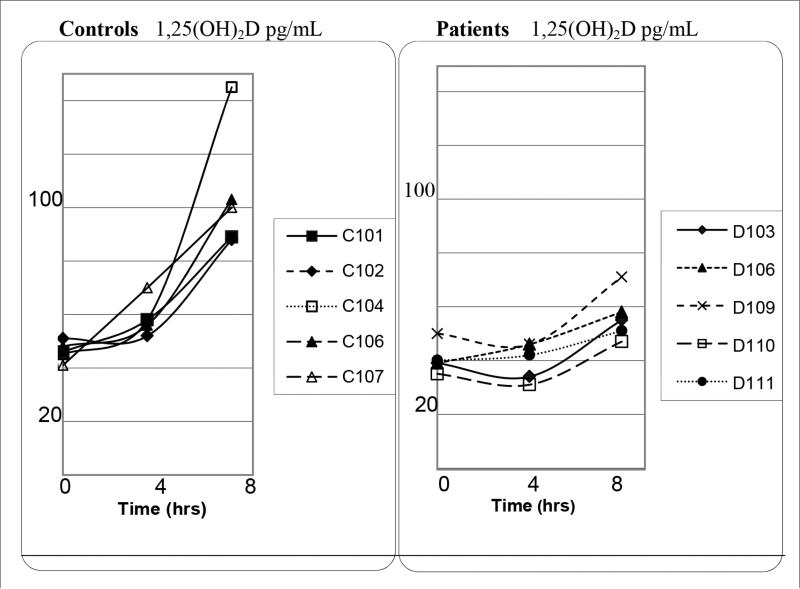
With PTH infusion, patients had lower 1,25(OH)2D levels (+SD) than controls at 4 hours (39.8 ± 6.9 versus 58.8 ± 6.7 pg/mL, respectively; P = .002) and 8 hours (56.4 ± 9.2 versus 105 ± 2.3 pg/mL, respectively; P = .003) of PTH infusion, as shown in Figure 1, which was attenuated after adjusting for higher BMI in patients (P = .05, .04).
Fig. 1.
Unadjusted 1α,25-dihydroxyvitamin D (1,25[OH]2D) levels during parathyroid hormone (PTH) (1-34) infusion in patients and controls. For the unadjusted difference between patients and controls at 4 hours (P = .002) and 8 hours (P = .003). After adjusting for body mass index (BMI), the difference between groups was attenuated (4 hours [P = .05] and 8 hours [P = .04]).
As shown in Table 3, serum Ca increased and P decreased with PTH infusion as expected. In patients and controls, Ca increased at 8 hours versus baseline (P = .02, P = .001), with no difference in magnitude of Ca increase between groups (P = .34). However, the decrease in serum P at 8 hours versus baseline was significant in patients (P = .004) but not controls (P = .27), with a significant difference between P change over time between groups (P = .03, P = .05 after adjusting for BMI). After 8 hours of PTH infusion, PTH was suppressed to a similar extent in controls (by 64%), and patients (59%), although the 8 hour PTH levels were higher in patients. At 4 hours, FeCa increased paradoxically in patients but decreased in controls; at baseline and 8 hours there was no difference (Table 3).
Table 3.
Results for Patients and Controls with PTH(1-34) Infusion
| Parameter | Patients (SD) | Controls (SD) | P valuea | P valueb |
|---|---|---|---|---|
| Ca (mg/dL) | ||||
| t = 0 h | 9.2 (0.34) | 9.0 (0.26) | .45 | .88 |
| t = 4 h | 9.3 (0.40) | 9.3 (0.18) | .69 | .80 |
| t = 8 h | 9.7 (0.24) | 9.8 (0.23) | .90 | .81 |
| P (mg/dL) | ||||
| t = 0 h | 4.0 (0.33) | 3.5 (0.22) | .02 | .06 |
| t = 4 h | 3.5 (0.32) | 3.3 (0.25) | .26 | .29 |
| t = 8 h | 3.4 (0.40) | 3.3 (0.30) | .73 | .90 |
| PTH (pg/min) | ||||
| t = 8 h | 29.6 (11.5) | 16.2 (5.6) | .05 | .74 |
| TMP/GFR | ||||
| t = 0 h | 3.44 (0.34) | 2.52 (1.43) | .23 | .08 |
| t = 4 h | 2.54 (0.11) | 2.50 (0.35) | .84 | .37 |
| t = 8 h | 2.62 (0.26) | 2.62 (0.38) | .98 | .99 |
| FeCa | ||||
| t = 0 h | 0.66 (0.16) | 0.61 (0.19) | .61 | .93 |
| t = 4 h | 1.05 (0.42) | 0.31 (0.53) | .04 | .29 |
| t = 8 h | 0.57 (0.30) | 0.36 (0.52) | .45 | .75 |
Abbreviations: Ca = calcium; FeCa = fractional excretion of calcium; P = phosphorus; PTH = parathyroid hormone;
TMP/GFR = tubular reabsorption of phosphorus/glomerular filtration rate ratio.
Bold numbers indicate statistical significance P <.05.
BMI adjusted.
Intact FGF23 levels from Johns Hopkins Bayview were undetectable in controls and in three patients; in 2 patients, intact FGF23 was normal (mean 27 ± 30 RU/mL, normal <180 RU/mL). Because undetectable FGF23 levels were unexpected, C-terminal FGF23 was repeated at the Mayo Clinic Lab in three patients (including the two who had detectable values in the JHH Bayview assay) and was normal in all three (92 ± 32 RU/mL; normal <180 RU/ mL), ruling out excessive FGF23 as the cause of reduced 1,25(OH)2D production. Due to funding limits, C-terminal FGF23 was not performed in all participants.
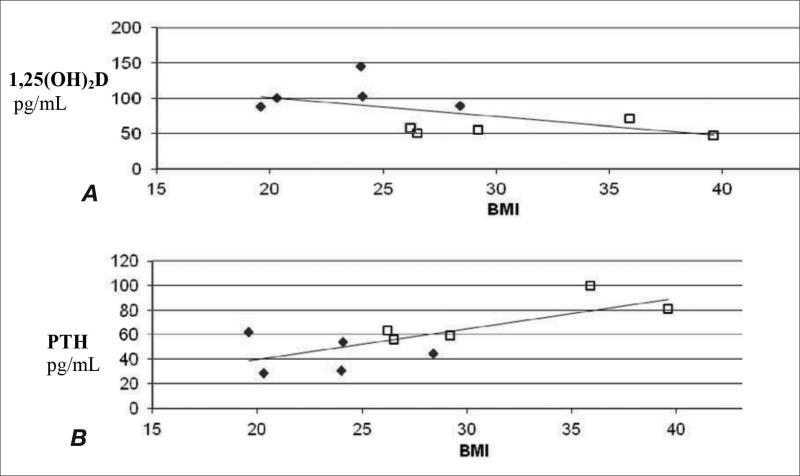
There was a trend toward correlation between 8 h PTH-stimulated 1,25(OH)2D and BMI (Fig. 2A) and a significant correlation between PTH and BMI (Fig. 2B) from the current study. To evaluate the effect of BMI on PTH values in a larger population, as described above, linear regression analysis of BMI versus PTH (adjusted for age and serum creatinine) performed in our Amish database (20) (n = 1096) showed a weak but significant inverse correlation of –0.1 (P = .001), similar to the correlation of –0.11 previously reported (11). Although BMI has recently been reported to be inversely associated with 1,25(OH)2D levels (12), no information is available on the effect of BMI on PTH-stimulated 1,25(OH)2D. Using data from PTH infusion studies conducted by MH on healthy, young individuals (n = 14, mean age 28.7 ± 1.05 years, BMI mean 26.2 ± 3.9, range 22.5 to 32.6) given a similar PTH infusion dose as in the current study of 12 pmol/kg/h (n = 10) and similar dose of 16 pmol/kg/h (n = 4), linear regression analysis showed no correlation between BMI and PTH-stimulated 1,25(OH)2D after 7 hours of infusion (data not shown). We did not combine 1,25(OH)2D data from these 14 subjects with data from the current study (Fig. 2A) because of the different type of PTH used (Forteo in the current study versus generic lyophilized PTH[1-34] powder with no stabilizers in the prior study), the age difference, and PTH infusion time (8 hours in current study versus 7 hours in the prior study).
Fig. 2.
Correlations of body mass index (BMI) with parathyroid hormone (PTH) levels and 1α,25-dihydroxyvitamin D (1,25[OH]2D). A. 1,25(OH)2D after 8 hours of PTH infusion versus BMI in patients and controls. B. Baseline PTH levels versus BMI in patients. Patients are notated as open squares and controls are notated as closed diamonds. n=5 in each group.
Treatment with calcitriol in 4 patients (the 5th was not followed clinically after study) showed normalization of PTH with retention of eucalcemia in all during 3-12 months of follow up (data not shown).Sequencing of CYP27B1 revealed an intronic variant of unknown significance between exons 1 and 2 in one patient, which was not present in the other 4 patients or 5 controls. There was no association of genotype of any of the 8 SNPs of CYP27B1 with the patient phenotype.
DISCUSSION
In this pilot study, idiopathic secondary hyperparathyroidism (sHPT) was associated with reduced PTH-stimulated 1,25(OH)2D production in 5 vitamin D sufficient, postmenopausal Caucasian women aged ≤55 years with low bone mass compared to 5 age and sex-matched controls. The difference in PTH-stimulated 1,25(OH)2D production was partially attenuated after adjusting for higher BMI in the patients group. Although we found that in a young, healthy population (<30 years of age), BMI did not significantly affect PTH-stimulated 1,25(OH)2D production, the effect of older age may have contributed to the difference in our current study. PTH has been shown to increase in healthy postmenopausal women over the age of 55 (11), and reduced production of 1,25(OH)2D may be the cause. BMI has been recently reported to significantly affect 1,25(OH)2D, such that for every increase of 1 kg/m2 in BMI, 1,25(OH)2D decreased by 2.1 pg/mL (0.9 pmol/L) (12). We are unaware of any prior studies on the effect of BMI on PTH-stimulated 1,25(OH)2D in postmenopausal women.
The higher BMI in our patient group may have led to an overestimation of GFR by the CKD-EPI equation, as shown previously (26). Although the serum Cr in both groups appeared grossly normal, calculation of their GFR by the MDRD method revealed that they had mild renal insufficiency, CKD Stage II. Among the numerous stimuli to PTH secretion in CKD, reduced production of 1,25(OH)2D is one of the first to occur (14). Although sHPT is well known to occur with overt CKD, stage III and worse (GFR< 60), it can occur with more mildly reduced GFR below 70 mL/min (13). In fact, a large cross-sectional study of >1800 patients (mean age 71 years) reported sHPT in 12% with a GFR >80 mL/min and 17% with a GFR 40 to 89 mL/min (14). Since we did measure creatinine clearance, we cannot rule out the possibility that our patient group had slightly lower GFR than controls, due to their higher BMI, and that this could have contributed to their reduced 1,25(OH)2D production.
The patient group in this study satisfied the criteria for EC-pHPT, which as currently defined (3), does not specify a 1,25(OH)2D level. However, a lower 1,25(OH)2D level has been reported in EC-pHPT patients compared to hypercalcemic pHPT patients (16). In this study, Cr was lower and BMI was higher in EC-pHPT than pHPT patients, supporting the concept that reduced renal function and elevated BMI may be important for the EC-pHPT phenotype. Of note, in that study, 40% of patients with EC-pHPT had hypercalcuria or nephrolithiasis, which are known causes of sHPT, suggesting that these patients may have started with sHPT, and developed “tertiary” HPT over time, although this was felt to be unlikely by the authors (16). In our study, we excluded patients with hypercalciuria, since this is a known cause of sHPT. Our findings that PTH normalized with calcitriol supported the diagnosis of sHPT rather than EC-pHPT in our patients group. A larger trial of calcitriol in patients with this phenotype is needed to evaluate the response to calcitriol as a possible discriminator between EC-pHPT and sHPT.
In our patients, excessive catabolism of vitamin D metabolites was ruled out as the cause of reduced PTH-stimulated 1,25(OH)2D by their lower 24,25(OH)2D levels. We speculate that lower levels of 24,25(OH)2D in patients may be due to reduced activation of CYP24A1 from reduced 1,25(OH)2D production over time, since 1,25(OH)2D is a more potent stimulator of CYP24A1 than 25(OH)D (27). We found no evidence of excessive FGF23 or PTH resistance in patients to explain their reduced 1α-hydroxylation. Although fasting serum P levels and FeCa at 4 hours were higher in patients than controls, these levels, as well as TRP/GFR and serum Ca, were the same in both groups at baseline and after 8 hours of PTH infusion, and the differences at 4 hours resolved after adjusting for higher patient BMI. Reduced renal reabsorption of Ca has been previously noted in EC-pHPT, although the cause is unknown (16). Since the difference in FeCa in our study resolved after adjusting for BMI, factors associated with elevated BMI appear to be mediating this effect on urine Ca. Since 1,25(OH)2D plays a role in renal Ca reabsorption (28), the lower 1,25(OH)2D levels in our patients may have been the cause of their transient increase in FeCa.
Our findings of reduced PTH-stimulated 1,25(OH)2D production in postmenopausal women with low bone mass are similar to those reported over 30 years ago in elderly women with osteoporosis (5), except for the important difference that our patients were more than 20 years younger. An inherent reduction in 1,25(OH)2D production may be a true cause of secondary osteoporosis; however, whether normalizing PTH in sHPT in patients with osteoporosis improves BMD is currently unknown.
Prior studies have reported sHPT with normal renal function in 0.6 to 8.9% (1,2) of postmenopausal women. What proportion of these women have reduced PTH-stimulated production of 1,25(OH)2D is unknown. Our findings suggest that two underappreciated contributors of apparently idiopathic sHPT are occult CKD and elevated BMI-associated reduction in 1,25(OH)2D production. Clinicians need to carefully consider the effects of high BMI and subtle renal insufficiency when evaluating patients with a normal serum Ca and elevated PTH. In all four of our patients treated with calcitriol, PTH normalized and serum Ca remained normal, supporting sHPT as the diagnosis. A larger study of calcitriol, preferably with long-term follow-up, in patients who satisfy the criteria for EC-pHPT will be needed to determine if response to calcitriol could be a useful test to distinguish EC-pHPT from sHPT.
The main limitation of this study was the small sample size, which is true of pilot studies. In addition, we did not closely evaluate Ca intake in participants and we did not measure serum magnesium. The strengths of this study include the use of PTH infusion to stimulate 1,25(OH)2D production, exclusion of patients with known hypercalciuria and nephrolithiasis to give a more consistent phenotype, and measurement of 1,25(OH)2D in an accurate assay. We excluded both excessive catabolism of vitamin D metabolites by 24-hydroxylase and overtly elevated FGF23 as potential causes of sHPT. Although the inadvertent mismatch of our groups on BMI was a limitation, it allowed us to determine that the higher BMI in the patient group partially explained their sHPT. However, after adjusting for BMI, a significant difference remained between patients and controls, so other unknown factors affecting PTH-stimulated 1,25(OH)2D production appear to be present. Larger studies, matching patient and control groups for BMI, should be performed to further clarify the effect of BMI on 1,25(OH)2D in postmenopausal women. We found no convincing evidence that a mutation in CYP27B1 was the cause.
CONCLUSION
In conclusion, we have studied Vitamin D sufficient postmenopausal woman with low bone mass, normal serum calcium, and elevated PTH, who satisfy the current criteria for ECpHPT, and found preliminary evidence in a pilot study that these patients may have a variant of sHPT due to reduced PTH-stimulated 1,25(OH)2D production. The reduced 1,25(OH)2D production was partially, but not completely, attributable to elevated BMI. We propose that elevated BMI should be considered as a potential contributor to sHPT in the evaluation of middle-aged women with eucalcemic PTH elevation. Whether or not a trial of calcitriol to determine PTH suppressibility would help discriminate EC-pHPT from sHPT warrants further study.
Abbreviations
- 1,25(OH)2D
1α,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- BMI
body mass index
- CKD
chronic kidney disease
- EC-pHPT
eucalcemic primary hyperparathyroidism
- H-pHPT
hypercalcemic pHPT
- PTH
parathyroid hormone
- sHPT
secondary hyperparathyroidism
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Lundgren E, Rastad J, Thrufjell E, Akerstrom G, Ljunghall S. Population –based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121:287–294. doi: 10.1016/s0039-6060(97)90357-3. [DOI] [PubMed] [Google Scholar]
- 2.Marques TF, Vasconcelos R, Diniz E, Régo D, Griz L, Bandeira F. Normocalcemic primary hyperparathyroidism in clinical practice: an indolent condition or a silent threat. Arq Bras Endocrinol Metabol. 2011;55:314–317. doi: 10.1590/s0004-27302011000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism. Arq Bras Endocrinol Metabol. 2010;54:106–109. doi: 10.1590/s0004-27302010000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yumita S, Suzuki M, Akiba T, Akizawa T, Seino Y, Kurokawa K. Levels of serum 1,25(OH)2D in patients with pre-dialysis chronic renal failure. Tohoku J Exp Med. 1996;180:45–56. doi: 10.1620/tjem.180.45. [DOI] [PubMed] [Google Scholar]
- 5.Slovik DM, Adams JS, Neer RM, Holick MF, Potts JT. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Eng J Med. 1981;305:372–374. doi: 10.1056/NEJM198108133050704. [DOI] [PubMed] [Google Scholar]
- 6.Bouillon R, Carmeliet G, Boonen S. Ageing and calcium metabolism. Baillieres Clin Endocrinol Metab. 1997;11:341–365. doi: 10.1016/s0950-351x(97)80332-1. [DOI] [PubMed] [Google Scholar]
- 7.Zung A, Chalew SA. Effect of age on the response to parathyroid hormone. Metabolism. 1997;46:1246–1251. doi: 10.1016/s0026-0495(97)90225-0. [DOI] [PubMed] [Google Scholar]
- 8.Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25:585–591. doi: 10.1016/j.beem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Soubervielle JC, Lawson-Body E, Hammadi B, Sarfati E, Kahan A, Cormier C. The use in clinical practice of parathyroid hormone normative values established in vitamin D-sufficient subjects. J Clin Endocrinol Metab. 2003;88:3501–3504. doi: 10.1210/jc.2003-030097. [DOI] [PubMed] [Google Scholar]
- 10.Hess B, Jaeger P. The tale of parathyroid function in idiopathic hypercalciuria. Scanning Microsc. 7:403–408. 993. [PubMed] [Google Scholar]
- 11.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Determinants of plasma PTH and their implication for defining a reference interval. Clin Endocrinol (Oxf) 2011;74:37–43. doi: 10.1111/j.1365-2265.2010.03894.x. [DOI] [PubMed] [Google Scholar]
- 12.Konradsen S, Ag H, Lindberg F, Hexeberg S, Jorde R. Serum 1,25-dihydroxyvitamin D is inversely associated with body mass index. Eur J Nutr. 2008;47:87–91. doi: 10.1007/s00394-008-0700-4. [DOI] [PubMed] [Google Scholar]
- 13.Gal-Moscovici A, Sprague SM. Role of vitamin D deficiency in chronic kidney disease. J Bone Miner Res. 2007;22(Supp 2):V91–V94. doi: 10.1359/jbmr.07s203. [DOI] [PubMed] [Google Scholar]
- 14.Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 15.Khosla S, Atkinson EJ, Melton LJ, Riggs BL. Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study. J Clin Endocrinol Metab. 1997;82:1522–1527. doi: 10.1210/jcem.82.5.3946. [DOI] [PubMed] [Google Scholar]
- 16.Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: Evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88:4641–4648. doi: 10.1210/jc.2002-021404. [DOI] [PubMed] [Google Scholar]
- 17.Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007;92:3001–3005. doi: 10.1210/jc.2006-2802. [DOI] [PubMed] [Google Scholar]
- 18.Jones G, Strugnell SA, DeLuca HE. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 19.Sella S, Cattelan C, Realdi G, Giannini S. Bone disease in primary hypercalciuria. Clin Cases Miner Bone Metab. 2008;5:118–126. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JT, Lin C-J, Burridge SM, Fu GK, Labuda M, Portale AA, Miller WL. Genetics of Vitamin D 1-alpha-hydroxylase deficiency in 17 families. Am J Hum Genet. 1998;63:1694–1702. doi: 10.1086/302156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz MJ, Tedesco MB, Sereika SM, et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2Vitamin D. J Bone Miner Res. 2005;20:1792–1803. doi: 10.1359/JBMR.050602. [DOI] [PubMed] [Google Scholar]
- 23.Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of 1,25-dihydroxyvitamin D. J Nutr Biochem. 1990;1:320–327. doi: 10.1016/0955-2863(90)90068-v. [DOI] [PubMed] [Google Scholar]
- 24.Pettifor JM, Bikle DD, Cavaleros M, Zachen D, Kamdar MC, Ross FP. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Ann Intern Med. 1995;122:511–513. doi: 10.7326/0003-4819-122-7-199504010-00006. [DOI] [PubMed] [Google Scholar]
- 25.Streeten EA, McBride DJ, Lodge AL, et al. Reduced incidence of hip fracture in the Old Order Amish. J Bone Miner Res. 2004;19:308–313. doi: 10.1359/JBMR.0301223. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal N, Porter AC, Tang IY, Becker BN, Akkina SK. Creatinine-based estimations of kidney function are unreliable in obese kidney donors. J Transplant. 2012:872–894. doi: 10.1155/2012/872894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashiro K, Abe T, Oue N, Yasui W, Ryoji M. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol Cell Endocrinol. 2004;226:27–32. doi: 10.1016/j.mce.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Hoenderop JG, Dardenne O, Van Abel M, et al. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J. 2002;16:1398–1406. doi: 10.1096/fj.02-0225com. [DOI] [PubMed] [Google Scholar]