Abstract
Anthocyanins are water-soluble polyphenolic compounds with a high nutraceutical value. Despite the fact that cultivated tomato varieties do not accumulate anthocyanins in the fruit, the biosynthetic pathway can be activated in the vegetative organs by several environmental stimuli. Little is known about the molecular mechanisms regulating anthocyanin synthesis in tomato. Here, we carried out a molecular and functional characterization of two genes, SlAN2 and SlANT1, encoding two R2R3-MYB transcription factors. We show that both can induce ectopic anthocyanin synthesis in transgenic tomato lines, including the fruit. However, only SlAN2 acts as a positive regulator of anthocyanin synthesis in vegetative tissues under high light or low temperature conditions.
Introduction
In plants, anthocyanins accumulate as water-soluble polyphenolic metabolites in the vacuole of many (sub)epidermal cell types, where they exert different functions depending on the tissue specificity. In vegetative tissues anthocyanins act as protective compounds after being synthesized in response to different environmental stimuli (e.g. UV irradiation and/or low temperature) and against pathogens, while in reproductive organs they exert an essential role in attracting pollinators and seed dispersers to assure the reproductive success [1]. Recently, a role for anthocyanins as reducing agents and signaling molecules involved in the modulation of ROS-signaling pathways emerged [2].
Given their physiological importance, the production of anthocyanins must be tightly regulated in plant cells and the regulatory network controlling their biosynthesis has been extensively studied in different species. Several transcription factors (TFs) and other proteins, active not only in vegetative but also in reproductive organs, have been identified (for reviews see [3,4]). In petunia (Petunia x hybrida) flowers, for example, different members of the R2R3-MYB family, including P. hybrida AN2 (PhAN2) [5], two different basic helix-loop-helix (bHLH) proteins, P. hybrida AN1 (PhAN1) [6] and P. hybrida JAF13 (PhJAF13) [7], and the WD-repeat (WDR) factor P. hybrida AN11 (PhAN11) [8], have been associated with the regulation of anthocyanin synthesis. Similarly, MYB, bHLH and WDR factors were found to be responsible of the anthocyanin pigmentation of some fleshy fruits, such as grapevine and apples [4]. In dicots, some of the TFs involved in the production of anthocyanins can individually activate specific early steps of the biosynthetic pathway; others can act in combination to activate transcription of the late biosynthetic structural genes (LBGs) [4]. The analysis of interactions between couples of these factors has brought to propose that a ternary complex constituted by MYB, bHLH and WDR proteins, which is known as MBW complex, is active in this sort of regulation [3], resulting in leaves, floral and fruit pigmentation [4]. Anthocyanins, as well as other plant secondary metabolites, are important phytonutrients and their beneficial effects on health have been demonstrated in a number of intervention studies both in human subjects and animal systems. Anthocyanins have anti-tumor and pro-apoptotic activities as well as anti-oxidative, anti-proliferative, anti-inflammatory, anti-neurodegenerative roles [9,10]. Moreover, anthocyanin-containing plant foods have been reported to prevent type-two diabetes, to reduce low-density lipoprotein (LDL) levels and to improve visual functions by inhibiting myopia and glaucoma [11–14]. For this reason, in the past two decades, there has been a growing interest in the identification of the genetic loci that regulate anthocyanin biosynthesis in major crops as targets for metabolic engineering or breeding programs.
Tomato (Solanum lycopersicum L.) is one of the most cultivated vegetable worldwide and its fruits represent a main component of the Mediterranean diet [15]. In many countries, tomato fruits and tomato-based food products are the largest dietary source of lycopene [16], a bioactive red linear carotene which is involved in preventing cardiovascular disease [17] and with chemopreventive effects on prostate cancer cells [18]. Lycopene is the most abundant carotenoid in the ripe fruit, followed by phytoene, phytofluene, β-carotene, ζ-carotene, δ-carotene, lutein, neurosporene and other minor compounds [19], most of which have a bioactive role in human health [20–22]. In addition to carotenoids, tomato fruits contain high amounts of soluble sugars, organic acids, amino acids, and minerals, which, together with hundreds of different volatiles, affect both the taste and the characteristic flavor [23]. Flavonols (mainly quercetin and kaempferol) and flavanones (naringenin), representing the major classes of flavonoids of tomato fruits [24], also contribute to their antioxidant properties. However, the concentration of flavonoids is considered sub-optimal and anthocyanins are generally not present [25–27]. Therefore, this species has been widely used as reference crop for metabolic engineering of the flavonoid pathway and to obtain anthocyanin-enriched tomatoes by using either breeding or transgenic approaches. Constitutive expression of MYB and bHLH regulators of the anthocyanin pathway from other species resulted in the formation of fruits with high levels of anthocyanins [26,28,29]. By a different strategy, interspecific crosses with Solanum wild species resulted in purple tomatoes, containing high amount of anthocyanins in the epidermis and the pericarp of the fruits [27,30,31].
Untill now the design of strategies for the breeding or engineering of anthocyanin-rich tomatoes has been limited by the poor knowledge of the regulators of the pathway in this species. In recent years, two members of the R2R3-MYB family have been identified and partially characterized [32–36]. These TFs are encoded by two paralog genes, S. lycopersicum Anthocyanin1 (SlANT1) and S. lycopersicum Anthocyanin2 (SlAN2), both mapping on chromosome 10 [36] and sharing high similarity with PhAN2 [32,35]. Constitutive expression of SlANT1 or SlAN2, induced by activation tagging [32] or transgenesis [36,37], caused anthocyanin accumulation in tomato plants, indicating that both MYB TFs activate anthocyanin biosynthetic genes. Interestingly, both SlANT1 and SlAN2 are possible candidates for the regulation of the fruit anthocyanin pigmentation in the Aft tomato accession [34–36]. Recently, other two MYB encoding genes, SlMYB7-like and SlMYB48-like have been identified as possible positive regulators of anthocyanin synthesis in tomato and targets of miR858, which acts as a negative regulator of the same pathway [38].
In this study, we present the functional characterization of SlANT1 and SlAN2 in tomato plants. We show that both proteins are able to induce anthocyanin production in the different organs of the plant and demonstrate that the triggering of anthocyanin synthesis by high light or cold in vegetative tissues is mediated by SlAN2, while SlANT1 does not play any role in this mechanism.
Materials and Methods
Plant material and growth conditions
The tomato variety Ailsa Craig (AC) (accession LA2838A, Tomato Genetic Resource Center, TGRC, University of California, USA), was used in all experiments, unless otherwise indicated. AC seeds were germinated in rockwool plugs (Grodan, Roermond, the Netherlands) and seedlings were transplanted after two weeks in plastic pots of 10 cm diameter containing a mixture of soil (Hawita Flor, Vechta, Germany) and pumice (70:30, by volume), and placed for other three weeks in a growth chamber (12-h/12-h photoperiod, irradiation intensity 50 μmol photons m−2 s−1, temperature 24°C, 50% relative humidity). For high light experiments, the plants were then transferred in a 14-h/10-h photoperiod, 28°C temperature, 70% to 80% relative humidity, and light intensity of approx. 300 μmol photons m−2 s−1. For low temperature experiments, the plants were placed in an incubator set at 15°C, with 14-h/10-h photoperiod, 50 μmol photons m−2 s−1 and 70% to 80% relative humidity. For RNA and anthocyanin extractions, tissues of apical leaves were sampled at the moment of plants transferring (T-0) and after 2, 4 and 7 days of cold or light treatments, frozen in liquid nitrogen and stored at -80°C until use.
Phylogenetic analysis
The identification of tomato regulatory anthocyanin genes was carried out using the BLAST search function tool of the Sol Genomics Network (SGN) [39,40] using the genomic sequences of petunia regulatory anthocyanin genes as query. The deduced amino acid sequences of 20 genes encoding R2R3-MYB proteins, 14 genes encoding bHLH factors and 12 genes encoding WDR proteins were aligned using the MUSCLE algorithm in the MEGA 6 package [41]. The results of the phylogenetic analysis was visualized by the Neighbor-Joining method [42] through MEGA 6. The statistical reliability of individual nodes was assessed by bootstrap analysis with 1000 replicates and the evolutionary distances were computed using the p-distance method. For the analysis of R2R3-MYB factors we used the MYB domain of the following proteins: SlANT1 (AAQ55181.1); SlAN2 (FJ705320.1); S. lycopersicum ANT1like, SlANT1like (ACT366161); S. lycopersicum AN2like, SlAN2like (ACT366117.1); Solanum tuberosum AN1, StAN1 (AAX53089.1); S. tuberosum AN2, StAN2 (AAX53091.1); PhAN2 (ABO21074.1); P. hybrida DPL, PhDPL (HQ116169); P. hybrida PHZ, PhPHZ (HQ116170); P. hybrida PH4, PhPH4 (BAP28594.1); P. hybrida ODO1, PhODO1 (Q50EX6.1); Nicotiana tabacum AN2, NtAN2 (ACO52470.1); Antirrhinum majus ROSEA1, AmROS1 (ABB83826.1); A. majus ROSEA2, AmROS2 (ABB83827.1); Arabidopsis thaliana MYB75, AtMYB75 (AAG42001.1); A. thaliana MYB113, AtMYB113 (NM_105308); A. thaliana MYB114, AtMYB114 (NM_105309); Zea mays C1, ZmC1 (AAA33482); Z. mays Pl, ZmPl (AAA19819); Malus domestica MYB10, MdMYB10 (ABB84753). The bHLH factors and their GenBank accession numbers are as follows: PhAN1 (AAG25927); PhJAF13 (AAC39455); A. thaliana GL3, AtGL3 (NP_680372); A. thaliana EGL3, AtEGL3 (NP_176552); A. thaliana TT8, AtTT8 (CAC14865); Z. mays IN1, ZmIN1 (AAB03841); Z. mays Lc, ZmLc (NP_001105339); M. domestica bHLH, MdbHLH (ADL36597); Citrus x sinensis MYC2, CsMYC2 (ABR68793); A. majus DELILA, AmDEL (AAA32663); N. tabacum AN1a, NtAN1a (HQ589208.1); N. tabacum AN1b, NtAN1b (HQ589209.1); S. lycopersicum AN1, SlAN1 (this study); S. lycopersicum JAF13, SlJAF13 (this study). The WDR factors and their GenBank accession numbers are as follows: PhAN11 (U94748.1); A. thaliana TTG1, AtTTG1 (NM_180739.2); Z. mays PAC1, ZmPAC1 (AY115485.1); S. lycopersicum AN11, SlAN11 (this study); Ipomoea purpurea WD40, IpWD40 (ABW69689.1); N. tabacum TTG2, NtTTG2 (ACN87316.1); S. tuberosum TTG1-like, StTTG1-like (XP_006347612.1); M. domestica TTG1-like, MdTTG1-like (XP_008343816.1); Gossypium hirsutum TTG1, GhTTG1 (AAK19614.1); G. hirsutum TTG3, GhTTG3 (AAM95645.1); Fragaria x ananassa TTG1, FaTTG1 (AFL02466.1); Prunus persica TTG1, PpTTG1 (ACQ65867.1).
These sequences were used in a second round of alignment and phylogenetic analysis, performed as described above, including 96 R2R3-MYB, 98 bHLH and 66 WDR amino acid sequences annotated in the tomato genome.
RNA-Seq in tomato tissues
Data from ILLUMINA RNA-Seq experiment were used to generate an expression heatmap of the genes involved in anthocyanin accumulation. The data are expressed as the average of FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) values obtained from two biological replicates of 7 tissues of S. lycopersicum cv. Moneymaker (accession LA2706, TGRC): root, stem, leaf, flower and fruit at mature green, breaker and ripe (10 days post breaker) developmental stages. The data were processed with the software Genesis 1.7.6 (Gene Expression Similarity Investigation Suite, [43]), and visualized with a log2 scale to reduce the saturation effect of highly expressed genes.
Promoter sequence analysis
The 2 kb nucleotide genomic sequences of SlANT1 and SlAN2 were obtained from the Sol Genomics Network [39,40]. Cis-acting promoter regulatory elements in SlANT1 and SlAN2 promoters were identified through the PlantCARE database [44].
Cloning of the MYB genes
AC genomic DNA was extracted from a single leaf using the “Wizard Genomic DNA Purification Kit” (Promega, Madison, WI, USA). SlANT1 and SlAN2 were amplified by PCR starting from AC genomic DNA using the “Phusion High-Fidelity DNA Polymerase” (New England Biolabs Inc., MA, USA) and the following pairs of primers: CACCATGAACAGTACATCTATGTC (forward) and TTAATCAAGTAGATTCCATAAGTCAA (reverse) for SlANT1; CACCATGAATACTCCTATGTGTGC (forward) and TTAATTAAGTAGATTCCATAAGTCAATATC (reverse) for SlAN2. The amplified sequences were cloned into pENTR/D-TOPO vector (Life Technologies, Carlsbad, CA, USA) and the entry clones were recombined with different destination vectors, as described below, via “Gateway Recombination Cloning Technology” (Life Technologies).
Expression analysis by quantitative RT-PCR (qPCR)
Total RNA was extracted from tomato plants using a “Spectrum Plant Total RNA Kit” (Sigma–Aldrich, St Louis, MO, USA). RNA was subjected to DNase treatment using a “TURBO DNA free Kit” (Life Technologies) and then reverse transcribed into cDNA with an “iScript cDNA Synthesis Kit” (Bio-Rad Laboratories, Hercules, CA, USA). qPCR was performed with an ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using the “iTaq Universal SYBR Green Supermix” (Bio-Rad) and the primers listed in S1 Table. S. lycopersicum elongation factor 1-alpha (SlEF1A) was used as reference gene. The relative quantification of each individual gene expression was performed using the geometric averaging method (geNorm) [45].
SlAN2 and SlANT1 ectopic expression in planta
The 35S:SlANT1 and 35S:SlAN2 constructs were produced by recombining the SlANT1 and SlAN2 entry clones with the Gateway compatible binary vector pK7WG2 [46] (http://gateway.psb.ugent.be/). Tomato plants ectopically expressing SlANT1 and SlAN2 were produced by Agrobacterium tumefaciens-mediated transformation [29]. For RNA extraction and qPCR analyses, leaves, petals, anthers, peel and flesh from fruits at the mature green stage [47] isolated from single representative transgenic lines [line T9002 with 35S:SlANT1 (T1 generation) and line R9009 with 35S:SlAN2 (T1 generation)] were used. The anthocyanin-rich phenotype of the T9002 and R9009 transgenic lines was inheritable.
Anthocyanin quantification
Anthocyanin extraction was performed as described by [48] starting from 0.5 mg of apical leaves. The total amount of anthocyanins was expressed as mg petunidin-3-(p-coumaroyl rutinoside)-5-glucoside per gram fresh weight, as described in [28]. Mean values were obtained from three independent replicates.
Protein localization assays in protoplasts
For localization in protoplasts, the entry clones of SlANT1 and SlAN2 were recombined with the Gateway destination vector p2FGW7 [46]. Arabidopsis mesophyll protoplasts were isolated from rosette leaves and transfected according to [49]. Fluorescence was imaged with a Nikon Eclipse Ti-5 video-confocal microscope using Endow GFP and DAPI filters.
Transactivation Assay
Transactivation assay was performed exploiting the Renilla reniformis (Rr) and Photinus pyralis (Pp) luciferase enzymes. The 35S:SlANT1 and 35S:SlAN2 effector constructs were produced by recombining the SlANT1 and SlAN2 entry clones with the vector p2GW7 [46]. The SlDFR promoter was amplified from AC genomic DNA using the primers pDFR_GWFW (CACCTTAGTGAAAGACCAACGTG) and pDFR_GWRV (TTTCAGAAATGAAAGGTAAAAAAGAGTC), cloned into pENTR/D-TOPO vector and then recombined with the reporter plasmid pPGWL7 containing the PpLuc gene. A RrLuc-overexpressing vector [50] was used to normalize luminescence values detected in protoplasts. Both effector and reporter plasmids were co-transformed in Arabidopsis mesophyll protoplasts, isolated as described above, and the relative levels of luciferase were measured, as described in [50]. Luminescence was measured with a Lumat LB 9507 Tube Luminometer (Berthold Technologies, NY, USA).
Virus Induced Gene Silencing (VIGS)
TRV-based T-DNA binary vectors pTRV1, pTRV2 and pTRV2/GATEWAY are from [51]. For both SlAN2 and SlANT1, a fragment of the cDNA was amplified using primers designed in order to introduce attB Gateway cloning sites: AN2_attB1: GGGGACAAGTTTGTACAAAAAAGCAGGCTTTGCATTGAAATTGAAGAAG, AN2_attB2: GGGGACCACTTTGTACAAGAAAGCTGGGTTCCATAAGTCAATATCAGTT, ANT1_attB1:GGGGACAAGTTTGTACAAAAAAGCAGGCTAGAAAAATCACCACCATTAAAT, ANT1_attB2:GGGGACCACTTTGTACAAGAAAGCTGGGTTTCCATAAGTCAATTTCAGCA. The fragment was cloned into the pTRV2 using the “Gateway Recombination Cloning Technology” (Life Technologies). Agrobacterium cultures were grown as described in [52], and cell concentration in the infiltration media was adjusted to an OD of 0.1. Tomato seedlings (Money Maker variety) were vacuum-infiltrated [53] with a 1:1 (v/v) mixture of two A. tumefaciens C58C1 strains, containing the pTRV1 and the pTRV2 binary vectors with the silencing fragment (or the empty pTRV2 as control) respectively. Infiltrated seedlings were plenty washed and kept in the dark for at least 12 hours and then grown under stressing conditions to promote anthocyanins accumulation: low temperature (17°C) and limiting soil (e.g. 3 plants in a 80x80x90 mm pot). The RNA was extracted from silenced and non-silenced tissues of three biological replicates and the expression levels of regulatory and target genes were measured as described above.
Results
Identification of tomato candidate anthocyanin regulatory genes
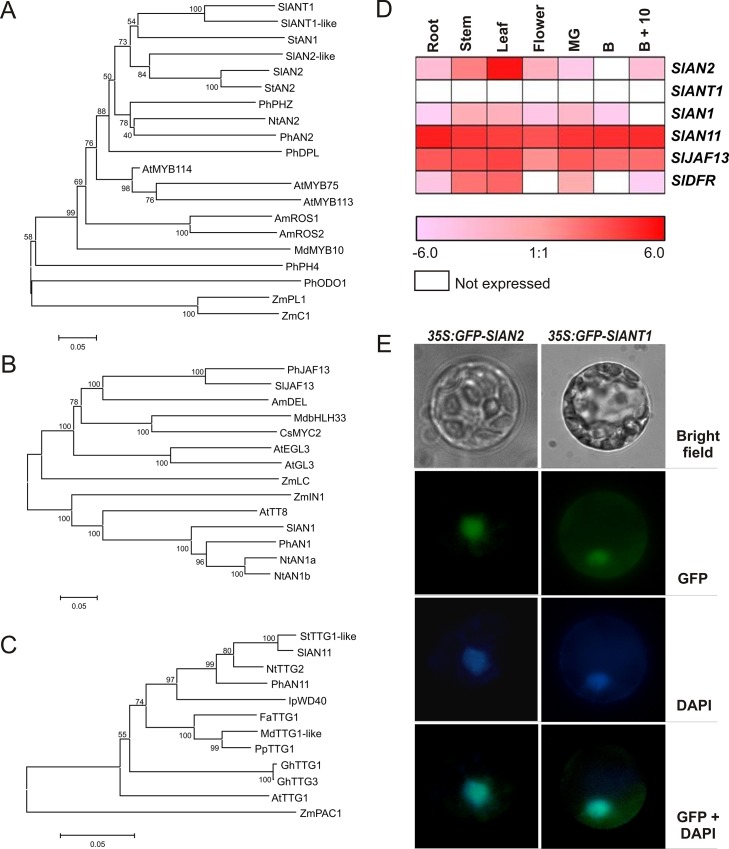
By analyzing the whole genome of tomato, all the annotated putative MYB proteins were compared with the main MYB regulatory factors involved in anthocyanin synthesis in other species (S1 Fig). Four different tomato MYB proteins, encoded by the genes Solyc10g086250, Solyc10g086260, Solyc10g086270, and Solyc10g086290, corresponding, respectively, to SlAN2 [33–35], SlANT1 [32,36], SlANT1like [35,54] and SlAN2like [35], grouped in one clade with MYB proteins of tobacco (N. tabacum), petunia and potato (S. tuberosum) involved in anthocyanin synthesis [5,55,56] (S1 Fig, Fig 1A). This clade included members from the Solanaceae family and was clearly separated from anthocyanin MYBs from other dicots, such as A. thaliana or A. majus, and monocots (S1 Fig, Fig 1A). This analysis confirms that SlANT1 and SlAN2 are indeed tomato MYB TFs involved in anthocyanin synthesis regulation.
Fig 1. Identification of possible tomato R2R3-MYB, bHLH, and WDR regulators of anthocyanin synthesis.
Evolutionary relationships of R2R3-MYB (A), bHLH (B) and WDR (C) proteins involved in anthocyanin pigmentation in different plant species. The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length (A = 4.04792561, B = 3.00531532, C = 0.83964559) is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 20 (A), 14 (B) and 12 (C) amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 790 (A), 364 (B) and 372 (C) positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41]. Expression heatmap (Log2 scale of FPKM values) of SlAN2, SlANT1, SlAN1, SlAN11, SlJAF13 and SlDFR genes in different tissues of tomato, analyzed by Illumina RNA-Seq (D). MG: Mature Green fruit; B: Breaker fruit; B+10: ripe fruit 10 days after breaker stage. Subcellular localization of GFP-SlANT1 and GFP-SlAN2 fusion proteins in transiently transformed A. thaliana mesophyll protoplasts (E). Pictures were taken with bright field, green fluorescent protein (GFP) and 4’6-diamidino-2-phenylindole (DAPI) filters.
To identify possible tomato regulators belonging to the bHLH and WDR families to be included in our analyses, a similar phylogenetic approach was followed. In this way we could identify two distinct bHLH factors, encoded by the genes Solyc09g065100 and Solyc08g081140, which group with the major plant bHLH factors involved in anthocyanin synthesis (S2 Fig). These two proteins show strong homology respectively to PhAN1 and PhJAF13 and were thus named SlAN1 and SlJAF13. The genes Solyc09g065100 and Solyc08g081140 likely correspond to the sequences mapped by [33] on chromosomes 9 and 8 of tomato and already named an1 and jaf13 for their homology with PhAN1 and PhJAF13, respectively. SlAN1 and SlJAF13 belong to two different clades of bHLH anthocyanin regulatory factors, the first one including A. thaliana TT8 and Z. mays IN1, and the second one A. thaliana GL3 and EGL3, A. majus DELILA, and Z. mays LC (S2 Fig, Fig 1B).
Finally, we identified a tomato anthocyanin-related WDR protein, encoded by the gene Solyc03g097340, by homology with the petunia PhAN11 (S3 Fig) and we named this protein SlAN11. Solyc03g097340 likely corresponds to the sequence mapped on chromosome 3 of tomato by [33] and already named an11 for its homology with PhAN11. SlAN11, as expected, groups with other dicot WDR proteins, such as PhAN11 and AtTTG1, while the Z. mays protein PAC1 is more distantly related (S3 Fig, Fig 1C).
The expression pattern of these tomato MYB, bHLH and WDR genes in plants grown in standard conditions was obtained from RNA-Seq data in 7 different tissues: root, stem, leaf, flower and fruit at mature green, breaker and ripe developmental stages (S2 Table; Fig 1D). The transcript of the LBG SlDFR, encoding a key enzyme in the anthocyanin biosynthetic pathway, was included in the analysis. In the heatmap of Fig 1D, SlAN2 shows the highest levels of expression in leaves, followed by stems. The expression pattern of SlAN1 and SlDFR is similar to SlAN2; SlAN11 and SlJAF13 transcripts are expressed in all analyzed organs, whereas SlANT1 expression was not detected in any tissue. An analysis carried out in a wider dataset of tomato tissues or developmental stages showed that the level of expression of SlANT1 is indeed always quite low in comparison to those of SlAN11 and SlJAF13 (S4 Fig).
The subcellular localization of the GFP fusions of SlANT1 and SlAN2 was assessed by expressing them under the control of the Cauliflower Mosaic Virus 35S promoter (35S) in transiently transformed protoplasts of A. thaliana. Both SlAN2 and SlANT1 localized into the cell nucleus (Fig 1E), consistently with their possible role as TFs.
Expression profile of the MYB, bHLH, and WDR genes in lines ectopically expressing SlANT1 and SlAN2
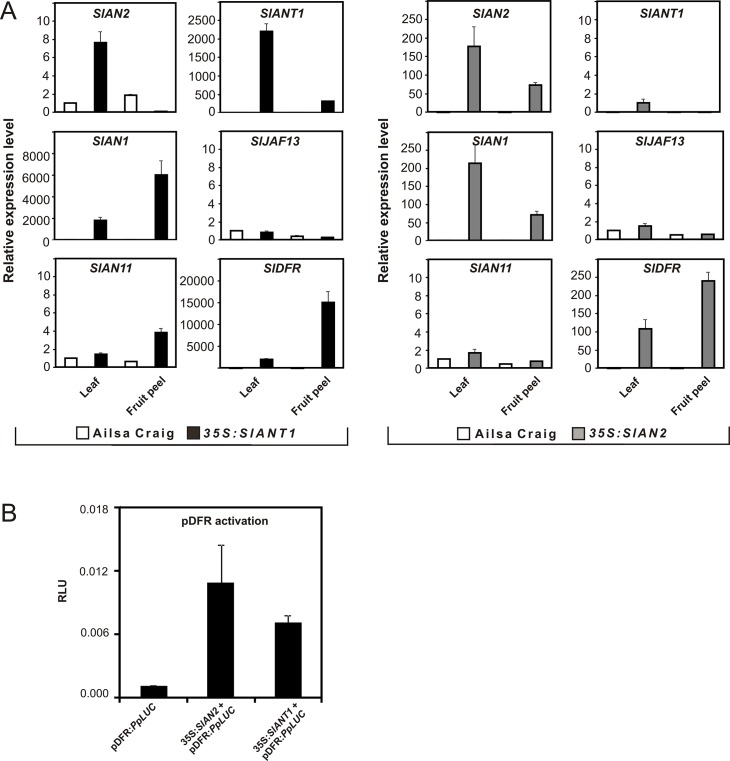
To study the effect of the ectopic expression of SlAN2 and SlANT1 in planta, we generated tomato lines expressing SlAN2 or SlANT1 under the control of the 35S promoter. Eleven independent transgenic lines for 35S:SlANT1 and 12 independent lines for 35S:SlAN2 were produced. All transgenic lines showed enhanced anthocyanin synthesis, resulting in a strong pigmentation of vegetative organs, flowers and fruits (Fig 2). This is in agreement with previous evidences obtained in tomato with SlANT1 [32,36] and SlAN2 [37]. Our results indicate that SlAN2 and SlANT1 ectopic expression results in similar phenotypes and indicate that the SlANT1 and SlAN2 proteins are equally able to activate the anthocyanin biosynthetic pathway in tomato. In leaves, anthocyanin accumulated both in leaflets and in leaf rachis; in flowers, pigmentation was mainly visible in the anthers (Fig 2). In both 35S:SlAN2 and 35S:SlANT1 plants, anthocyanins accumulated in fruit till the immature green stage resulting in intense pigmentation of the peel and in the surface of the locular cavities in immature green fruits (Fig 2). However, with fruit maturation, anthocyanin pigmentation of the peel progressively reduced in 35S:SlAN2 lines, while this was not observed in 35S:ANT1 fruits that remained strongly colored, although not homogeneously (Fig 2). This could be the consequence of prolonged anthocyanin synthesis in the fruits of 35S:SlANT1 plants, and/or prolonged persistence of the pigments till late ripening. In 35S:SlAN2 tomatoes instead, pigments present in the peel at the immature green stage, appeared to be diluted during further growth and ripening of the fruits, as if synthesis of anthocyanins arrested. Recently, Meng et al. [59] found that physiological changes in tomato fruit ripening were caused by overexpression of SlAN2. In particular, an increased ethylene synthesis as well as a reduction of carotenoid levels, including lycopene, with a consequent orange colour of the fruits at ripening, were found associated to SlAN2 overexpression. These changes may reflect a possible peculiar role of this TF as a regulator of fruit ripening, in addition to trigger of anthocyanin synthesis.
Fig 2. 35S:SlANT1 and 35S:SlAN2 transgenic tomato lines.
Phenotype of 35S:SlAN2 and 35S:SlANT1 lines compared to the control non-transformed plants (Ailsa Craig). Details of immature green fruits, section of the immature green fruits, red ripened fruits, section of the red fruits, leaves, and flowers are shown. 35S:SlANT1 line T9002 and 35S:SlAN2 line R9009 were chosen for the phenotypic analysis.
The intense anthocyanin pigmentation observed in many organs of the transgenic lines demonstrated that the ectopic expression of a single endogenous R2R3-MYB factor (SlAN2 or SlANT1) was sufficient to activate the anthocyanin biosynthetic pathway. This indicated that the other regulators of the pathway were already expressed where anthocyanin accumulated or that their expression was induced by SlAN2 and SlANT1. To verify these hypotheses we examined by qPCR the expression pattern of the putative regulatory genes in the transgenic lines. Representative plants of single 35S:SlANT1 and 35S:SlAN2 lines, characterized by a strong anthocyanin phenotype, were selected for the analysis. In both transgenic lines, the expression of either SlANT1 or SlAN2 resulted in strong induction of the bHLH gene SlAN1 and of the LBG gene SlDFR in leaves and fruit peel (Fig 3A). Furthermore, in leaves, small activation effects on SlAN2 in the SlANT1 transgenic line, and on SlANT1 in the SlAN2 transgenic line were also observed (Fig 3A). On the contrary, the expression levels of the other bHLH gene, SlJAF13, and of the WDR gene SlAN11 appeared to be similar in wild type and transgenic plants (Fig 3A).
Fig 3. Effect of the overexpression of SlANT1 and SlAN2 on other genes of the anthocyanin pathway.
Quantitative analysis of transcript levels of SlAN2, SlANT1, SlAN1, SlAN11, SlJAF13 and SlDFR in leaves and peel from green fruits of the 35S:SlANT1 and 35S:SlAN2 lines in comparison with control Ailsa Craig plants (A). Expression levels are shown as relative units, with the value of AC leaves set to one. A sample composed of two biological replicates was analyzed for each plant tissue and data are means of two technical replicates ± SD. 35S:SlANT1 line T9002 and 35S:SlAN2 line R9009 were chosen for the qPCR analysis. Transient transformation experiment in Arabidopsis mesophyll protoplasts showing that both SlAN2 and SlANT1 activated the SlDFR promoter (B). Protoplasts were transfected with the reporter plasmid containing the SlDFR promoter driving firefly luciferase (PpLuc) gene alone (first histogram) or in combination with the effector plasmid containing either the full length SlAN2 or SlANT1 coding sequence (second or third histograms, respectively). A 35S:Renilla-luciferase (RrLuc) plasmid was used as an internal control. Data are expressed as Relative Luc Activity (RLU) (PpLuc/RrLuc) and are means of eight biological replicates ± SE.
A transactivation assay carried out in A. thaliana protoplasts showed that both SlANT1 and SlAN2 can activate the promoter of SlDFR (Fig 3B).
Together, these results indicate that the MYB factors SlAN2 and SlANT1 can induce the transcription of SlDFR by activating transcription of the bHLH gene SlAN1. This is in agreement with previous observations that MYB proteins in other species regulate the transcription of their bHLH partners and subsequently form together with the bHLH protein and the WDR one (which is expressed in all plant parts, [8]) a MBW complex that activates the structural genes [6,60]
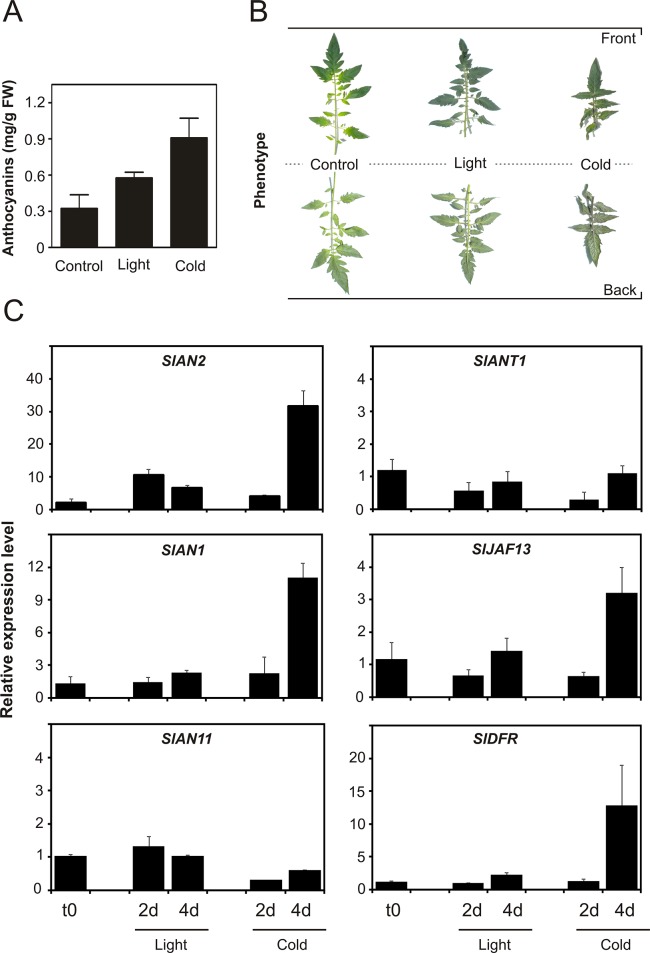
SlAN2 is involved in the up-regulation of the anthocyanin pathway upon light and cold stress
To investigate whether SlANT1 and SlAN2 are modulated by environmental factors, we analyzed their expression in vegetative tissues of 4-week-old tomato plants that were exposed for 7 days to high light or low temperature conditions. These factors are known to be major triggers of anthocyanin synthesis and accumulation in plant green parts [1]. Anthocyanin synthesis was activated during the exposure to high light or low temperature, as confirmed by both the analysis of the anthocyanin content of the leaves at the end of the treatments (Fig 4A) and the phenotype of the leaves themselves (Fig 4B). qPCR analysis, carried out after 2d and 4d of light or low temperature, showed the induction of SlAN2 following both treatments (Fig 4C). Moreover, a slight induction of SlAN1 and SlDFR was observed as a consequence of the light treatment, particularly after 4d (Fig 4C). More pronounced was the activation of the same genes, as well as of SlJAF13, during the low temperature treatment (Fig 4C). SlAN11 expression was not particularly affected by high light or cold (Fig 4C), suggesting that there was not a direct correlation with the activation of the pathway and probably that basal SlAN11 expression levels were sufficient to induce anthocyanin synthesis. SlANT1 is expressed at very low levels at standard growth conditions, as seen in Figs 1D and 3A. Moreover no activation was detected upon light or cold induction (Fig 4C). This indicates that SlANT1 does not contribute to the activation of the anthocyanin biosynthetic pathway neither under standard growth condition, nor upon stress conditions by high light and low temperature.
Fig 4. Induction of anthocyanin synthesis in tomato plants under high light and low temperatures conditions.
Anthocyanin content in leaves from Ailsa Craig plants treated for 7 days with high light (approx. 300 μmol photons m−2 s−1) or low temperature (15°C) compared to untreated control plants (A) and phenotypes of the same leaves (B). Quantitative analysis of transcript levels of selected anthocyanin genes in vegetative tissues subjected to 2 and 4 days of high light or low temperature treatments (C). Expression levels are shown as relative units, with the value of one of the biological replicates of control untreated samples set to one. Data are means of three biological replicates ± SD.
The analysis of a 2 kb nucleotide genomic sequence upstream of SlAN2 indicated the presence of several cis-acting elements involved in light responsiveness, such as GATA-motifs, Box 4 and I-box elements and many others (S5 Fig). Specific cold responsive elements were not found. However, cold-responsive genes can also be regulated through cis-acting abscisic acid response elements (ABREs) [61] and some ABREs were identified in the promoter region of SlAN2 (S5 Fig). Furthermore, jasmonate responsive elements and defense and stress-responsive elements, compatible with other developmental and environmental triggers of anthocyanin synthesis [1,62], were found (S5 Fig). The presence of all these cis-acting elements, particularly the high number of light responsive elements (LREs), was a further indication of the involvement of SlAN2 in the regulation of anthocyanin synthesis, particularly when induced by light. However, the analysis carried out on the promoter sequence of SlANT1 highlighted the presence of similar categories of regulatory elements (LREs, ABREs, defense, stress and jasmonate responsive elements) (S6 Fig). Moreover, additional and specific cis-acting regulatory elements, potentially involved in other hormonal and developmental mechanisms controlling anthocyanin synthesis, such as gibberellin and drought [1,62], were found (S6 Fig). It is thus possible that SlANT1 transcription is induced in conditions different from those controlling SlAN2, even if we cannot exclude that light conditions different from the ones tested in our experimental set-up (for example higher) could also activate SlANT1 transcription, due to the high number of LREs identified in its promoter.
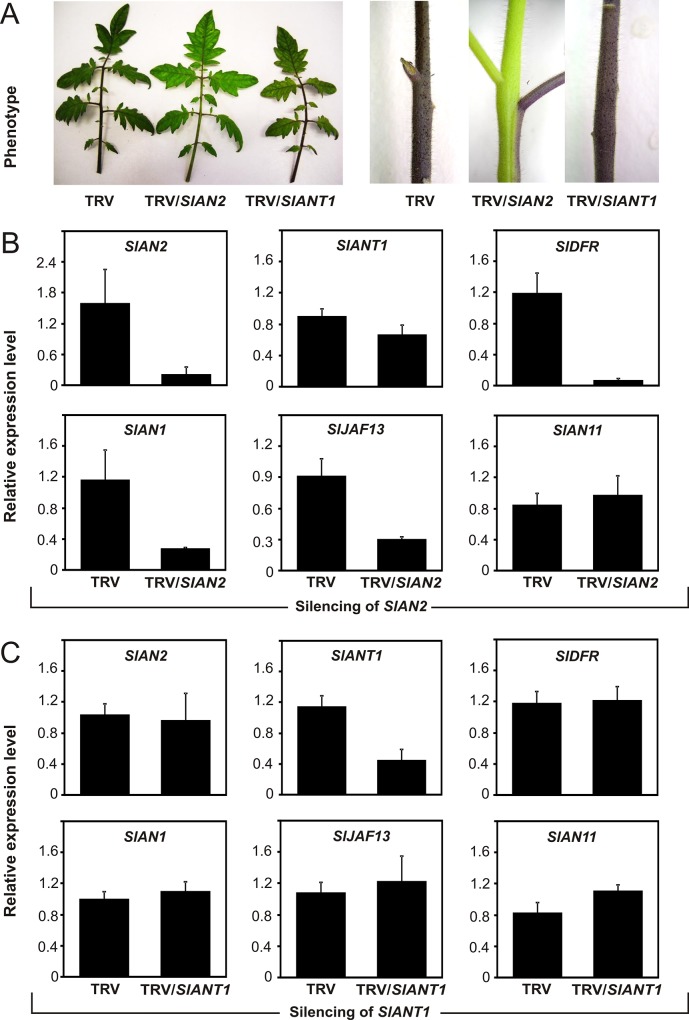
SlAN2 plays a major role in the activation of anthocyanin biosynthesis
To further elucidate the respective contribution of each of the two MYB genes in the activation of the anthocyanin pathway, tomato seedlings were grown under stress conditions (low temperature plus limiting soil) to promote strong anthocyanin accumulation and Virus Induced Gene Silencing (VIGS) of SlAN2 or SlANT1 was carried out. As shown in Fig 5A, the silencing of SlAN2 caused a strong reduction of anthocyanin accumulation both in leaves and in the stem, as compared to control plants. qPCR analysis showed a strong down-regulation of SlAN2 mRNA in the silenced tissues, confirming the effectiveness of the VIGS, as well as a strong repression of the expression of SlDFR (Fig 5B). Furthermore, silenced SlAN2 tissues showed significant reduction of the bHLHs SlAN1 and SlJAF13 transcripts providing evidence that SlAN2 is involved in the transcriptional regulation of these genes. Interestingly, the expression in petals of the petunia bHLH factor PhAN1 is similarly down-regulated in mutants for the MYB protein PhAN4 [6]. On the contrary, the expression of SlAN11 was not altered in silenced SlAN2 tissues, further confirming that SlAN11 is expressed independently from SlAN2. SlANT1 was not significantly affected by the silencing of SlAN2 (Fig 5B), suggesting that this gene did not play an important role in the anthocyanin accumulation observed in the not silenced plants. This was further confirmed by the silencing of SlANT1 itself. In tomato seedlings growing in the same stressing conditions and accumulating elevated quantities of anthocyanins, the reduced expression of SlANT1 obtained by VIGS and confirmed by qPCR did not result in loss of pigmentation (Fig 5A) or in changes in the expression of the genes analyzed (Fig 5C). All these results, together with those shown in Fig 4, suggest that SlAN2, together with SlAN1 and probably SlJAF13, is involved in the regulation of anthocyanin biosynthesis in vegetative tissues of tomato plants upon light and cold induction and that SlDFR is a target gene of these regulators. Furthermore, the role of SlANT1 in the regulation of pigment accumulation seems to be at most marginal.
Fig 5. Virus Induced Gene Silencing of SlAN2 and SlANT1 in vegetative tissues of tomato plants.
Phenotype of SlAN2 and SlANT1 silenced leaves and stems (TRV/SlAN2, TRV/SlANT1, respectively) compared to non-silenced controls (TRV) (A). Quantitative analysis of transcript levels for SlAN2, SlAN1, SlAN11, SlDFR, SlANT1 and SlJAF13 in SlAN2 silenced tomato plants (TRV/SlAN2) (B) and in SlANT1 silenced tomato plants (TRV/SlANT1) (C) compared to non-silenced controls (TRV). Expression levels are shown as relative units, with the value of one of the biological replicates of control TRV samples set to one. Data are means of three biological replicates ± SD.
Discussion
In recent years, several attempts were made to increase the nutritional value of tomato fruits by inducing the synthesis of anthocyanins. These flavonoids are indeed normally absent in tomato fruits, although some lines, like Aft, produce small amounts of anthocyanins in the fruit skin [25]. Another line, atv is instead characterized by high anthocyanin levels in vegetative tissues [25]. The cross between Aft and atv results in much higher anthocyanin levels in the fruit [30,31]. The product of the atv locus is presently unknown, while Aft probably encodes a MYB gene, either SlANT1 [36] or SlAN2 [34,35]. Attempts to increase the anthocyanin content in tomato fruits were made by expressing anthocyanin regulatory genes from different plants species. The most successful case are the lines obtained by [28] by expressing two snapdragon genes, Del, a bHLH-type TF, and Ros1, an R2R3 MYB-type TF, obtaining tomato fruits characterized by an intense purple coloration both in the peel and flesh. Recently, two different tomato R2R3 MYB-type TFs involved in anthocyanin synthesis have been identified and their ectopic expression resulted in increased anthocyanin pigmentation in tomato plants [32–36], as confirmed by the results presented here (Fig 2).
In this work we analyzed the role of SlAN2 and SlANT1 within one experimental setting and compared their roles in the induction of pigmentation in different plant parts under growth conditions that result in anthocyanin accumulation. Our results indicate that the SlAN2 protein is as efficient as SlANT1 in inducing anthocyanin synthesis in tomato fruits when their expression is driven by the 35S promoter. Both 35S:SlANT1 and 35S:SlAN2 plants displayed high expression of SlDFR, encoding a key enzyme of the anthocyanin biosynthetic pathway. This is confirmed by the transactivation of the SlDFR promoter in Arabidopsis protoplasts by the two tomato MYBs (Fig 3B). The nuclear localization of both R2R3-MYB factors is in line with the possible involvement of these proteins as TFs (Fig 1C). All these results indicate that the two MYB proteins are functionally active and apparently interchangeable. However, the level of expression of SlANT1 is low in tomato plants and does not get induced upon exposure to stimuli that result, instead, in higher expression of SlAN2. This is based on (i) the analysis of Illumina RNA-Seq data (Fig 1D), (ii) qPCR data (Figs 3–5), and (iii) publicly available microarray data (S4 Fig). These observations suggest different roles for the two MYB factors: SlAN2 is transcriptionally activated by high light or low temperatures, whereas, surprisingly, SlANT1 does not respond to these stimuli excluding its involvement in the strong accumulation of pigment under these growth conditions. Silencing of SlAN2 by VIGS results in reduced anthocyanin biosynthesis and transcription of SlDFR, SlAN1 and SlJAF13, whereas no changes were observed following silencing of SlANT1 (Fig 5). Overall, our results indicate that SlAN2 induces anthocyanin accumulation in tomato in response to high light and low temperature through the control of the expression of SlAN1 and SlJAF13 (Fig 5B). Expression of the WDR gene SlAN11 seems instead to not require SlAN2 (Fig 5B), implying that its possible role in the regulation of anthocyanin synthesis is independent from light and temperature. In other plant species the WDR factors involved in anthocyanin synthesis are constitutionally expressed in all plant parts [8] and contribute to the activation/stabilization of the transcription complex by interacting with it [63].
A role of SlANT1 in the synthesis of anthocyanins in tomato is so far not proven. The homology shared by the SlANT1 protein with other MYB regulators of the anthocyanin pathway, the strong induction of anthocyanin accumulation by this protein when ectopically expressed in transgenics (Fig 2), the presence in its promoter region of several LREs and other regulatory elements compatible with anthocyanin synthesis (S6 Fig), and its ability to activate the SlDFR promoter in transient assays in Arabidopsis protoplasts (Fig 3B) seem however to indicate that SlANT1 is able to participate to the MBW complex and activate the same target genes induced by SlAN2. It was shown that SlANT1 expression is responsive to nitrogen depletion [64], while a survey using Genevestigator [65] of 194 different perturbations by microarray analysis reveals that changes in expression of SlANT1 do not exceed +1.28 fold/-1.2 fold, while expression of SlDFR displays changes of +498 fold/-7.7 fold under the same experimental conditions. This suggests that SlANT1 is poorly responding to environmental clues.
During the evolution of the tomato clade (Solanum genus, section Lycopersicon) [66] at least 13 tomato species have evolved and occupied various habitats of the western coast of South America, from central Ecuador to northern Chile, including the Galapagos Islands [67]. Only two of them accumulate lycopene in the fruits (S. lycopersicum and S. pimpinellifolium), other two produce yellow to orange fruits (S. galapagense and S. cheesmaniae), while the others (S. arcanum, S. chilense, S. corneliomulleri, S. pennellii, S. peruvianum, S. huaylasense, S. chmielewskii, S. habrochaites and S. neorickii) produce green mature fruits which in some cases accumulate anthocyanin pigments to various degrees (S7 Fig). Moreover, as cultivated tomato is a domesticated species, some characters could have gone through very strong selection operated by growers and this is very likely to have involved characters like pigmentation patterns. It is therefore not excluded that the SlANT1 allele of tomato is a “relique” allele which acquired its low expression level and/or insensitivity to environmental stimuli during speciation/domestication processes. In a previous paper [68], nucleotide and amino acid polymorphisms in ANT1 gene were detected between AC and the Aft genotype, derived from S. chilense. These polymorphisms were not accompanied by differences in ANT1 transcription rate, at least in the fruit peel [68]. However, the same authors could not exclude the existence of differences in the promoter regions of Aft and S. lycopersicum ANT1 genes leading them to respond differently to the same environmental factor [68]. Furthermore, we used the coding sequences of SlAN2 and SlANT1 to identify their orthologs in the S. pennellii genome database available on SGN [69,40]. As in the case of the Aft-AN2 allele [54], that derives from S. chilense, the ortholog of SlAN2 in S. pennelii, SpAN2 (annotated as Sopen10g035640) is conserved (S8 Fig). To the contrary, there is no annotation for the ortholog of SlANT1 in S. pennellii (SpANT1), since point mutations generate a premature stop codon in the sequence with respect to SlANT1 and Aft-ANT1 [54,68] (S9 Fig). These data suggest that a low selective pressure acted on ANT1 during speciation/domestication processes. However only a systematic characterization of these genes in all the wild tomato species would help to elucidate this aspect. Nevertheless, a role for SlANT1 MYB factor in the activation of pigmentation in domesticated tomato under other conditions than those studied here, in different tissues or at different developmental stages of the plant cannot be excluded at this stage.
SlAN2 is therefore the main MYB regulator of anthocyanin biosynthesis in tomato plants in response to stimuli like light and cold.
Supporting Information
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 30.36267216 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 116 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 786 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 37.06676035 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 112 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 1083 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 21.14733530 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 78 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 2873 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
Expression of SlANT1 (Solyc10g08260.1, yellow dots), SlJAF13 (Solyc08g0811420.2, two probe sets represented by blue and green dots) and SlAN11 (Solyc03g097340.1, red dots) in 6 developmental stages and 15 anatomical parts of tomato plants. A large dataset of microarray analyses was selected and queried using Genevestigator.
(TIF)
2 kb nucleotide genomic sequence of SlAN2 promoter (A). Light, abscisic acid, defense and stress and methyl jasmonate responsive elements are highlighted with different colors. Legend of the different responsive elements (B). The analysis was carried out with the PlantCARE Software. Only a sub-set of the cis-acting responsive elements identified was reported.
(TIF)
2 kb nucleotide genomic sequence of SlANT1 promoter (A). Light, abscisic acid, defense and stress, gibberellin, drought and methyl jasmonate responsive elements are highlighted with different colors. Legend of the different responsive elements (B). The analysis was carried out with the PlantCARE Software. Only a sub-set of the cis-acting responsive elements identified was reported.
(TIF)
The pictures are available on the TGRC website (http://tgrc.ucdavis.edu/index.aspx). A: S. arcanum, accession LA2813 (photo by Scott Peacock); B: S. chilense, accession LA2965 (photo by Scott Peacock); C: S. corneliomulleri, accession LA3157 (photo by Scott Peacock); D: S. pennellii, accession LA1656 (photo by Rick, Charles M.); E: S. peruvianum, accession LA2958 (photo by Scott Peacock); F: S. huaylasense, accession LA1981 (photo by Rick, Charles M.); G: S. chmielewskii, accession LA3663 (photo by Scott Peacock); H: S. habrochaites, accession LA1986 (photo by Scott Peacock); I: S. neorickii, accession. LA2190 (photo by Rick, Charles M.).
(TIF)
Red shading indicates identical sequences.
(TIF)
The red box indicates the mutated codon that produce a premature stop in SpANT1. Red shading indicates identical sequences.
(TIF)
(DOCX)
Normalized expression (FPKM) of SlAN2, SlANT1, SlAN1, SlAN11, SlJAF13 and SlDFR in different tissues of tomato, analyzed by Illumina RNA-Seq. MG: Mature Green fruit; B: Breaker fruit; B+10: ripe fruit 10 days after breaker stage. Data are the average of two independent biological replicates.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Work supported by the Italian Ministry of Agriculture (Alisal and Nutrisol projects) and by the European Commission (Traditom project, contract n. 634561). The work benefited from the networking activities of COST action FA1106 (Quality Fruit).
References
- 1. Gould KS. Nature's Swiss army knife: The diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol. 2004; 5: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M. Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci. 2013; 14: 3540–3555. 10.3390/ijms14023540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koes R, Verweij CW, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005; 5: 236–242. [DOI] [PubMed] [Google Scholar]
- 4. Petroni K, Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011; 181: 219–229. 10.1016/j.plantsci.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 5. Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, et al. Molecular analysis of the anthocyanin2 gene of Petunia and its role in the evolution of flower color. Plant Cell 1999; 11: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spelt C, Quattrocchio F, Mol JN, Koes R. Anthocyanin1 of petunia encodes a basic helix-loop-helix protein that directly activates transcription of structural anthocyanin genes. Plant Cell 2000; 12: 1619–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quattrocchio F, Wing JF, van der Woude K, Mol JN, Koes R. Analysis of bHLH and MYB domain proteins: species specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J. 1998; 13: 475–488. [DOI] [PubMed] [Google Scholar]
- 8. de Vetten N, Quattrocchio F, Mol J, Koes R. The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Gene Dev. 1997; 11: 1422–1434. [DOI] [PubMed] [Google Scholar]
- 9. Buer CS, Imin N, Djordjevic MA. Flavonoids: new roles for old molecules. J Integr Plant Biol. 2010; 52: 98–111. 10.1111/j.1744-7909.2010.00905.x [DOI] [PubMed] [Google Scholar]
- 10. Spencer JP. The impact of fruit flavonoids on memory and cognition. Br J Nutr. 2010; 104: S40–S47. 10.1017/S0007114510003934 [DOI] [PubMed] [Google Scholar]
- 11. Tsuda T, Horio F, Uchida K, Aoki H, Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr. 2003; 133: 2125–2130. [DOI] [PubMed] [Google Scholar]
- 12. Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2010; 56: 159–170. [DOI] [PubMed] [Google Scholar]
- 13. Ashida H, Horio F, Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007; 74: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 14. Ohguro L, Ohguro H, Nakazawa M. Effects of anthocyanins in blackcurrant on retinal blood flow circulation of patients with normal tension glaucoma. A pilot study. Hirosaki Med J. 2007; 59: 23–32. [Google Scholar]
- 15. Borghesi E, Gonzalez-Miret ML, Escudero-Gilete ML, Malorgio F, Heredia FJ, Melendez-Martinez AJ. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J Agric Food Chem. 2011; 59: 11676–11682. 10.1021/jf2021623 [DOI] [PubMed] [Google Scholar]
- 16. Mangels AR, Holden JM, Beecher GR, Forman MR, Lanza E. Carotenoid content of fruits and vegetables: an evaluation of analytic data. J Am Diet Assoc. 1993; 93: 284–296. [DOI] [PubMed] [Google Scholar]
- 17. Burton-Freeman BM, Sesso HD. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv Nutr. 2014: 5: 457–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013; 14: 14620–14646. 10.3390/ijms140714620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fantini E, Falcone G, Frusciante S, Giliberto L, Giuliano G. Dissection of tomato lycopene biosynthesis through virus-induced gene silencing. Plant Physiol. 2013; 163: 986–998. 10.1104/pp.113.224733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med. 2002; 227: 845–851. [DOI] [PubMed] [Google Scholar]
- 21. Rao AV, Rao LG. Carotenoids and human health. Pharmacol Res. 2007; 55: 207–216. [DOI] [PubMed] [Google Scholar]
- 22. Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014; 6: 466–488. 10.3390/nu6020466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petro-Turza M. Flavor of tomato and tomato products. Food Rev Int. 1987; 2: 309–351. [Google Scholar]
- 24. Torres CA, Davies NM, Yañez JA, Andrews PK. Disposition of selected flavonoids in fruit tissues of various tomato (Lycopersicon esculentum Mill.) Genotypes. J Agric Food Chem. 2005; 53: 9536–9543. [DOI] [PubMed] [Google Scholar]
- 25. Jones CM, Mes P, Myers JR. Characterization and inheritance of the anthocyanin fruit (Aft) tomato. J Hered. 2003; 94: 449–456. [DOI] [PubMed] [Google Scholar]
- 26. Bovy A, Schijlen E, Hall RD. Metabolic engineering of flavonoids in tomato (Solanum lycopersicum): the potential for metabolomics. Metabolomics 2007; 3: 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mes PJ, Boches P, Myers JR. Characterization of tomatoes expressing anthocyanin in the fruit. J Am Soc Hort Sci. 2008; 133: 262–269. [Google Scholar]
- 28. Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, et al. Enrichment of tomato fruit with health promoting anthocyanins by expression of select transcription factors. Nat Biotech. 2008; 26: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 29. Zuluaga DL, Gonzali S, Loreti E, Pucciariello C, Degl'Innocenti E, Guidi L, et al. Arabidopsis thaliana MYB75/PAP1 transcription factor induces anthocyanin production in transgenic tomato plants. Funct Plant Biol. 2008; 35: 606–618. [DOI] [PubMed] [Google Scholar]
- 30. Gonzali S, Mazzucato A, Perata P. Purple as a tomato: towards high anthocyanin tomatoes. Trends Plant Sci. 2009; 14: 237–241. 10.1016/j.tplants.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 31. Povero G, Gonzali S, Bassolino L, Mazzucato A, Perata P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J Plant Physiol. 2011; 168: 270–279. 10.1016/j.jplph.2010.07.022 [DOI] [PubMed] [Google Scholar]
- 32. Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N, et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell 2003; 15: 1689–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Jong WS, Eannetta NT, De Jong DM, Bodis M. Candidate gene analysis of anthocyanin pigmentation loci in the Solanaceae . Theor Appl Genet. 2004; 108: 423–432. [DOI] [PubMed] [Google Scholar]
- 34. Boches P, Myers J. The anthocyanin fruit tomato gene (Aft) is associated with a DNA polymorphism in a MYB transcription factor. Hort Science 2007; 42: 856. [Google Scholar]
- 35.Boches PS. Breeding Tomato for Increased Fruit Phenolics. PhD Thesis, Oregon State University. 2009.
- 36. Schreiber G, Reuveni M, Evenor D, Oren-Shamir M, Ovadia R, Sapir-Mir R, et al. Anthocyanin1 from Solanum chilense is more efficient in accumulating anthocyanin metabolites than its Solanum lycopersicum counterpart in association with the anthocyanin fruit phenotype of tomato. Theor Appl Genet. 2011; 124: 295–308. 10.1007/s00122-011-1705-6 [DOI] [PubMed] [Google Scholar]
- 37. Meng X, Wang J-R, Wang G-D, Liang X-Q, Li X-D, Meng QW. An R2R3-MYB gene, LeAN2, positively regulated the thermo-tolerance in transgenic tomato. J Plant Physiol. 2015; 175: 1–8. 10.1016/j.jplph.2014.09.018 [DOI] [PubMed] [Google Scholar]
- 38. Jia X, Shen J, Liu H, Li F, Ding N, Gao C, et al. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta. 2015; 242: 283–293. 10.1007/s00425-015-2305-5 [DOI] [PubMed] [Google Scholar]
- 39. Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012; 485: 635–641. 10.1038/nature11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fernandez-Pozo N, Menda N, Edwards JD, Saha S, Tecle IY, Strickler SR, et al. The Sol Genomics Network (SGN)—from genotype to phenotype to breeding. Nucleic Acids Res 2015; 43: D1036–D1041. 10.1093/nar/gku1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987; 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 43. Sturn A, Quackenbush J, Trajanoski Z. Genesis: cluster analysis of microarray data. Bioinformatics. 2002; 18: 207–208. [DOI] [PubMed] [Google Scholar]
- 44. Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, et al. PlantCARE: a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002; 30:325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002; 3: RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karimi M, Inzé D, Depicker A. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 2002; 7: 193–195. [DOI] [PubMed] [Google Scholar]
- 47. Giovannoni JJ. Genetic regulation of fruit development and ripening. Plant Cell. 34; 16: S170–S180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006; 140: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007; 2: 1565–1572. [DOI] [PubMed] [Google Scholar]
- 50. Weits DA, Giuntoli B, Kosmacz M, Parlanti S, Hubberten HM, Riegler H, et al. Plant cysteine oxidases control the oxygen-dependent branch of the N-end-rule pathway. Nat Commun. 2014; 6; 5: 3425 10.1038/ncomms4425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y, Schiff M, Dinesh‐Kumar SP. Virus‐induced gene silencing in tomato. Plant J. 2002; 31(6): 777–786. [DOI] [PubMed] [Google Scholar]
- 52. Orzaez D, Mirabel S, Wieland WH, Granell A. Agroinjection of tomato fruits. A tool for rapid functional analysis of transgenes directly in fruit. Plant Physiol. 2006; 140: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ekengren SK, Liu Y, Schiff M, Dinesh-Kumar SP, Martin GB. A MAPK cascade, NPR1, and TGA transcription factors play a role in Pto-mediated disease resistance in tomato. Plant J. 2003; 6: 905–917. [DOI] [PubMed] [Google Scholar]
- 54.Povero G. Physiological and genetic control of anthocyanin pigmentation in different species. PhD Thesis, Vrije Universiteit of Amsterdam. 2011.
- 55. Jung CS, Griffiths HM, De Jong DM, Cheng S, Bodis M, Kim TS, et al. The potato developer (D) locus encodes an R2R3 MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin. Theor Appl Genet. 2009; 120: 45–57. 10.1007/s00122-009-1158-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pattanaik S, Kong Q, Zaitlin D, Werkman JR, Xie CH, Patra B, et al. Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta. 2010; 231: 1061–1076. 10.1007/s00425-010-1108-y [DOI] [PubMed] [Google Scholar]
- 57. Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985; 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 58. Nei M, Kumar S. Molecular Evolution and Phylogenetics New York: Oxford University Press; 2000. [Google Scholar]
- 59. Meng X, Yang D, Li X-D, Zhao S, Sui N, Meng Q. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol Biochem 2015; 89: 24–30. 10.1016/j.plaphy.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 60. Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001; 13: 2099–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR. Abscisic Acid Induces CBF Gene Transcription and Subsequent Induction of Cold-Regulated Genes via the CRT Promoter Element. Plant Physiol. 2004; 135: 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, Perata P. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol. 2008; 179: 1004–1016. 10.1111/j.1469-8137.2008.02511.x [DOI] [PubMed] [Google Scholar]
- 63. Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004; 39: 366–380. [DOI] [PubMed] [Google Scholar]
- 64. Løvdal T, Olsen KM, Slimestad R, Verheul M, Lillo C. Synergetic effects of nitrogen depletion, temperature, and light on the content of phenolic compounds and gene expression in leaves of tomato. Phytochem. 2010; 71: 605–613. [DOI] [PubMed] [Google Scholar]
- 65. Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, et al. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008; 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peralta IE, Knapp S, Spooner DM. New species of wild tomatoes (Solanum section Lycopersicon: Solanaceae) from Northern Peru. Syst Botany 2005; 30: 424–434. [Google Scholar]
- 67. Taylor IB. Biosystematics of the tomato In: Atherton JG, Rudich J, editors. The tomato crop: a scientific basis for improvement. London: Chapman and Hall; 1986. pp. 1–34. [Google Scholar]
- 68. Sapir M, Oren-Shamir M, Ovadia R, Reuveni M, Evenor D, Tadmor Y, et al. Molecular aspects of Anthocyanin fruit tomato in relation to high pigment-1. J Hered 2008; 99: 292–303. 10.1093/jhered/esm128 [DOI] [PubMed] [Google Scholar]
- 69. Bolger A, Scossa F, Bolger ME, Lanz C, Maumus F, Tohge T, et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat Genet 2014; 46: 1034–1038. 10.1038/ng.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 30.36267216 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 116 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 786 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 37.06676035 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 112 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 1083 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
The evolutionary history was inferred using the Neighbor-Joining method [42]. The optimal tree with the sum of branch length = 21.14733530 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches [57]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The evolutionary distances were computed using the p-distance method [58] and are in the units of the number of amino acid differences per site. The analysis involved 78 amino acid sequences. All ambiguous positions were removed for each sequence pair. There were a total of 2873 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 [41].
(TIF)
Expression of SlANT1 (Solyc10g08260.1, yellow dots), SlJAF13 (Solyc08g0811420.2, two probe sets represented by blue and green dots) and SlAN11 (Solyc03g097340.1, red dots) in 6 developmental stages and 15 anatomical parts of tomato plants. A large dataset of microarray analyses was selected and queried using Genevestigator.
(TIF)
2 kb nucleotide genomic sequence of SlAN2 promoter (A). Light, abscisic acid, defense and stress and methyl jasmonate responsive elements are highlighted with different colors. Legend of the different responsive elements (B). The analysis was carried out with the PlantCARE Software. Only a sub-set of the cis-acting responsive elements identified was reported.
(TIF)
2 kb nucleotide genomic sequence of SlANT1 promoter (A). Light, abscisic acid, defense and stress, gibberellin, drought and methyl jasmonate responsive elements are highlighted with different colors. Legend of the different responsive elements (B). The analysis was carried out with the PlantCARE Software. Only a sub-set of the cis-acting responsive elements identified was reported.
(TIF)
The pictures are available on the TGRC website (http://tgrc.ucdavis.edu/index.aspx). A: S. arcanum, accession LA2813 (photo by Scott Peacock); B: S. chilense, accession LA2965 (photo by Scott Peacock); C: S. corneliomulleri, accession LA3157 (photo by Scott Peacock); D: S. pennellii, accession LA1656 (photo by Rick, Charles M.); E: S. peruvianum, accession LA2958 (photo by Scott Peacock); F: S. huaylasense, accession LA1981 (photo by Rick, Charles M.); G: S. chmielewskii, accession LA3663 (photo by Scott Peacock); H: S. habrochaites, accession LA1986 (photo by Scott Peacock); I: S. neorickii, accession. LA2190 (photo by Rick, Charles M.).
(TIF)
Red shading indicates identical sequences.
(TIF)
The red box indicates the mutated codon that produce a premature stop in SpANT1. Red shading indicates identical sequences.
(TIF)
(DOCX)
Normalized expression (FPKM) of SlAN2, SlANT1, SlAN1, SlAN11, SlJAF13 and SlDFR in different tissues of tomato, analyzed by Illumina RNA-Seq. MG: Mature Green fruit; B: Breaker fruit; B+10: ripe fruit 10 days after breaker stage. Data are the average of two independent biological replicates.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.