Abstract
Background
Recently, the American Heart Association developed a set of 7 ideal health metrics that will be used to measure progress toward their 2020 goals for cardiovascular health. The objective of the present study was to examine how well these metrics predicted mortality from all causes and diseases of the circulatory system in a national sample of adults in the United States.
Methods and Results
We used data from 7622 adults ≥20 years of age who participated in the National Health and Nutrition Examination Survey from 1999 to 2002 and whose mortality through 2006 was determined via linkage to the National Death Index. For the dietary and glycemic metrics, we used alternative measures. During a median follow-up of 5.8 years, 532 deaths (186 deaths resulting from diseases of the circulatory system) occurred. About 1.5% of participants met none of the 7 ideal cardiovascular health metrics, and 1.1% of participants met all 7 metrics. The number of ideal metrics was significantly and inversely related to mortality from all causes and diseases of the circulatory system. Compared with participants who met none of the ideal metrics, those meeting ≥5 metrics had a reduction of 78% (adjusted hazard ratio, 0.22; 95% confidence interval, 0.10–0.50) in the risk for all-cause mortality and 88% (adjusted hazard ratio, 0.12; 95% confidence interval, 0.03–0.57) in the risk for mortality from diseases of the circulatory system.
Conclusion
The number of ideal cardiovascular health metrics is a strong predictor of mortality from all causes and diseases of the circulatory system.
Keywords: cardiovascular diseases, epidemiology, mortality, population, prevention, risk factors
Despite an impressive decline in the mortality rate from coronary heart disease and stroke since the 1960s, preliminary estimates for 2010 show that 595 444 people died of diseases of the heart, which remains the leading cause of death in the United States, and 129 180 died of cerebrovascular diseases.1 Consequently, more progress in reducing the mortality rate remains to be achieved. Reducing the mortality rate can be realized by lowering the incidence of cardiovascular disease, lowering the case-fatality rate of cardiovascular disease, or their combination.
In the recently released American Heart Association’s “Strategic Impact Goal Through 2020 and Beyond,”2 the AHA charted a new course by focusing on improving cardiovascular health in addition to decreasing cardiovascular disease mortality. The AHA developed 7 metrics that address cardiovascular health and created 3 states for each metric that reflected poor, intermediate, and ideal health. These metrics draw on the extensive body of epidemiological investigations that identified critical risk factors for cardiovascular disease and clinical trials that confirmed that lowering the level of some of these risk factors reduced the morbidity and mortality from cardiovascular disease. Furthermore, the AHA 2020 goals align more closely with the concept of primordial prevention3 and grew out of investigations that championed primordial prevention.4,5
Several studies have variably defined a low cardiovascular risk profile based on different risk factors and behaviors.6–10 However, at present, little is known about how well the new AHA 2020 goals predict the incidence and mortality from cardiovascular disease.11 Therefore, the objective of the present study was to examine the relationship between the AHA 2020 goals and mortality from all causes and diseases of the circulatory system in a recent cohort of US adults.
Methods
We used the public data files for the 2006 follow-up of participants of the 1999 to 2000 and 2001 to 2002 cycles of the National Health and Nutrition Examination Survey (NHANES). The samples in 1999 to 2000 and 2001 to 2002 were selected by use of a multistage, stratified sampling design and constitute representative samples of the noninstitutionalized civilian US population. Participants were interviewed at home and invited for a clinical examination. Details about the NHANES and its methods have been published.12 The study received approval from the National Center for Health Statistics Research Ethics Review Board, and participants were asked to sign an informed consent form.
The mortality status of participants ≥20 years of age through 2006 was determined from the National Death Index.13 Participants who were not deemed to have died as of December 31, 2006, were considered to be alive. The International Classification of Diseases, 10th revision, codes I00 to I99 were used to identify deaths from diseases of the circulatory system.
The AHA 2020 goals include the following 7 behaviors and risk factors: smoking status, body mass index, physical activity, healthy dietary score, total cholesterol, blood pressure, and fasting plasma glucose.2 Participants who had smoked 100 cigarettes during their lifetime and were still currently smoking were defined as showing evidence of poor health. Former smokers who had quit within the previous 12 months were defined as showing evidence of intermediate health. Former smokers who had quit for >12 months or participants who had never smoked were defined as showing evidence of ideal health. Body mass index was calculated from measured weight and height. Participants with a body mass index of ≥30, 25 to <30, and <25 kg/m2 had poor health, intermediate health, and ideal health, respectively. Participants were asked about the frequency and duration of participation in moderate and vigorous physical activity during the past 30 days. The weekly frequency of bouts of physical activity was calculated by multiplying the number of such bouts during the 30-day period by a factor of 7/30. For each participant, the weekly number of minutes of moderate activity (weekly frequency multiplied by the average duration for each bout) and the weekly number of minutes of vigorous activity (weekly frequency multiplied by the average duration for each bout multiplied by 2) were summed.14 Ideal health, intermediate health, and poor health were defined as ≥150, 1 to 149, and 0 minutes of moderate or vigorous activity per week, respectively.
In lieu of the AHA specific dietary criteria, we used the Healthy Eating Index (HEI) score as our healthy dietary score.15 The HEI includes 3 of the 5 primary criteria included in the AHA healthy dietary score: fruits and vegetables, whole grains, and sodium. Not included are sugar-sweetened beverages and fish consumption. Participants with an HEI score <50 were assigned to poor health; those with a score of >50 to <81 were assigned to intermediate health; and those with a score of ≥81 were assigned to ideal health.16 The index was determined from dietary information collected by a single 24-hour recall administered in person to participants attending the medical examination.
Serum total cholesterol and high-density lipoprotein cholesterol were measured enzymatically on a Hitachi 717 Analyzer or a Hitachi 912 Analyzer (Roche Diagnostics, Indianapolis, IN). Ideal health, intermediate health, and poor health were defined as <200 mg/dL, 200 to 239 mg/dL or treated to goal, and ≥240 mg/dL, respectively. Up to 4 attempts were made to measure blood pressure. The average of the last 2 measurements of blood pressure for participants who had 3 or 4 measurements, the last measurement for participants with only 2 measurements, and the only measurement for participants who had 1 measurement were used. Current use of antihypertensive medications was based on self-report. Ideal health, intermediate health, and poor health were defined as systolic blood pressure <120 mm Hg and diastolic blood pressure <80 mm Hg, systolic blood pressure of 120 to 139 mm Hg and diastolic blood pressure of 80 to 89 mm Hg or treated to goal, and systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg, respectively.
Although the AHA relied on fasting plasma glucose to determine hyperglycemia, we used hemoglobin A1c (HbA1c) concentrations for 2 reasons. First, recent (2010) recommendations from the American Diabetes Association allow the use of HbA1c to diagnose diabetes mellitus. Second, a sizeable percentage of participants in NHANES who have an examination are not fasting. Therefore, to maximize our sample size, we opted for concentration of HbA1c, which is not subject to fasting conditions. Participants with an HbA1c ≥6.5% had poor health; those with a concentration of 5.7 to <6.5% had intermediate health; and those with a concentration <5.7% had ideal health. Participants who reported being treated with insulin or oral medications to lower blood glucose and had a concentration <6.5% were deemed to have intermediate health.
We included several covariates in our analyses: age, sex, race or ethnicity (white, black, Mexican American, other), educational status (less than high school, high school graduate/General Educational Development or equivalent, more than school), alcohol use, self-reported health status, health insurance, history of cardiovascular disease, and history of cancer. We calculated alcohol use from several questions regarding the use of alcohol during a participant’s lifetime and 12-month period and recent use. Men who consumed an average of ≤2 drinks per day and women who consumed on average ≤1 drink per day were considered moderate drinkers, and men who consumed an average of >2 drinks per day and women who consumed on average >1 drink per day were considered excessive drinkers. Self-reported health status was determined from the question, “Would you say your health in general is excellent, very good, good, fair, or poor?” Health insurance coverage (yes/no) was derived from the question, “Are you covered by health insurance or some other kind of healthcare plan?” Participants who reported ever being told by a doctor or other health professional that they had congestive heart failure, coronary heart disease, angina pectoris, heart attack, or stroke were considered to have a history of cardiovascular disease.
Our analyses were limited to participants who were ≥20 years of age. We calculated mortality rates per 1000 person-years of follow-up. Adjustment for age or age and sex was performed by use of the direct method with the year 2000 US population. Differences in age-and sex-adjusted proportions were examined with t tests. Pearson correlations adjusted for age and sex between the ranks of continuous variables used to formulate the health metrics were calculated. Risk estimates for mortality were calculated by the use of Cox proportional hazards regression analysis for the individual and number of 2020 cardiovascular health goals. In these analyses, we adjusted for age (continuous), sex, race or ethnicity (white, black, Mexican American, other), educational attainment (less than high school, high school graduate or equivalent, and beyond high school), alcohol use (excessive alcohol use yes/no), self-reported health status (fair or poor health; good, very good, or excellent health), health insurance coverage (yes/no), and histories of cardiovascular disease (yes/no) and cancer (yes/no). Proportionality assumptions were examined with Schoenfeld residuals and were found to be met. Estimates were calculated with SAS and SUDAAN, the latter to account for the complex sampling design of the survey.
Results
Of the 9471 participants ≥20 years of age who had an examination, 10 were ineligible for the follow-up study and 8005 had sufficient information for the cardiovascular health metrics. Additional exclusions for incomplete information of other study variables reduced the sample size to 7622. The sample included 3635 men, 3987 women, 3857 whites, 1329 blacks, 1814 Mexican Americans, and 622 of another race or ethnicity. The median age was 43 years.
The age- and sex-adjusted prevalence of ideal health for the individual health factors ranged from 10.9% for the HEI dietary score to 81.3% for HbA1c (Table 1). Without adjustment, 1.5% of participants had no ideal health criteria, 8.4% had 1, 19.9% had 2, 27.4% had 3, 22.1% had 4, 14.0% had 5, 5.5% had 6, and 1.1% had 7 (after adjustment for age and sex: 1.5%, 8.6%, 20.3%, 27.5%, 21.9%, 13.8%, 5.4%, and 1.0%, respectively). The interrelationships between the dichotomized health metrics and correlations for continuous variables are shown in Tables 2 and 3.
Table 1.
Selected Age- and Sex-Adjusted Baseline Characteristics Among Adults ≥20 Years of Age by Mortality Status, National Health and Nutrition Examination Survey 1999 to 2002
| Characteristics | Deceased (n=532) | Survivors (n=7090) |
|---|---|---|
| Age, y* | 67.0 (64.7– 69.3) | 44.2 (43.5–44.9) |
| Men, %† | 66.5 (54.2–76.8) | 47.7 (46.6–48.9) |
| Whites, % | 57.8 (50.7–64.5) | 73.3 (69.5–76.8) |
| Education beyond high school, % | 34.2 (25.1–44.5) | 54.3 (51.3–57.3) |
| Excessive alcohol use, % | 8.7 (5.6–13.3) | 12.7 (9.6–6.7) |
| Poor or fair health, % | 44.7 (34.8–55.1) | 14.4 (12.8–16.3) |
| Has health insurance, % | 83.7 (70.4–91.7) | 82.8 (80.9–84.5) |
| History of cardiovascular disease, % | 34.0 (24.5–45.0) | 7.1 (6.3–7.9) |
| History of cancer, % | 13.4 (9.8–17.9) | 7.2 (6.4–8.1) |
| Body mass index, kg/m2 | 30.1 (28.2–31.9) | 28.0 (27.7–28.3) |
| Physically active, min/wk‡ | 7.3 (3.9–13.0) | 33.2 (27.7–39.7) |
| Healthy Eating Index score, % | 58.7 (56.2–61.3) | 63.9 (63.1–64.7) |
| Total cholesterol, mg/dL | 204.8 (192.2–217.4) | 203.5 (201.6–205.4) |
| Systolic blood pressure, mm Hg | 122.5 (119.4–125.5) | 122.6 (121.9–123.4) |
| Diastolic blood pressure, mm Hg | 70.2 (68.0–72.5) | 72.5 (71.9–73.1) |
| Hemoglobin A1c, % | 5.8 (5.5–6.1) | 5.4 (5.4–5.5) |
| Not currently smoking, % | 68.6 (55.3–79.4) | 73.2 (70.7–75.5) |
| Body mass index <25 kg/m2, % | 27.4 (18.5–38.6) | 34.8 (33.1–36.6) |
| Physically active, % | 27.0 (18.7–37.3) | 42.6 (39.6–45.7) |
| Healthy Eating Index ≥80, % | 4.3 (2.5–7.3) | 11.2 (9.6–13.1) |
| Total cholesterol <200 mg/dL, % | 42.9 (36.5–49.5) | 45.1 (43.4–46.7) |
| Optimal blood pressure, % | 36.4 (28.9–44.6) | 40.7 (38.6–42.9) |
| Hemoglobin A1c <5.7%, % | 66.0 (55.4–75.1) | 82.0 (79.9–83.9) |
| No. of AHA 2020 metrics for ideal health | ||
| 0 | 4.7 (1.6–12.8) | 1.5 (1.1–1.9) |
| 1 | 9.6 (6.3–14.3) | 8.4 (7.3–9.7) |
| 2 | 28.6 (21.4–37.1) | 19.9 (18.5–21.4) |
| 3 | 28.0 (20.2–37.4) | 27.6 (26.0–29.2) |
| 4 | 24.0 (16.0–34.4) | 22.1 (20.9–23.4) |
| ≥5 | 5.2 (1.7–14.6) | 20.5 (18.5–22.8) |
Values are mean or percent (95% confidence interval) as appropriate.
Unadjusted.
Adjusted for age.
Means represent back-transformed means of Box-Cox transformation.
Table 2.
Age- and Sex-Adjusted Percentages of Ideal Cardiovascular Health Metrics among Participants ≥20 Years of Age by Ideal Cardiovascular Health Metric Status, National Health and Nutrition Examination Survey 1999–2002
| Ideal Cardiovascular Health Metric Status | Ideal Cardiovascular Health Metric, % (SE)
|
||||||
|---|---|---|---|---|---|---|---|
| Current Smoking | Body Mass Index | Physical Activity | Healthy Diet Score | Total Cholesterol | Blood Pressure | Hemoglobin A1C | |
| Current smoking | |||||||
| Yes | … | 33.3 (1.0) | 45.9 (1.7) | 12.9 (1.0) | 45.4 (0.8) | 40.0 (1.2) | 81.9 (1.0) |
| No | … | 40.8 (1.4) | 30.8 (1.2) | 4.7 (0.7) | 43.9 (1.6) | 42.0 (1.3) | 80.2 (1.5) |
| P | … | <0.001 | <0.001 | <0.001 | 0.308 | 0.155 | 0.221 |
| Body mass index | |||||||
| Yes | 68.3 (1.8) | … | 46.0 (2.4) | 13.7 (1.3) | 52.2 (1.3) | 48.7 (1.5) | 89.9 (0.9) |
| No | 74.6 (1.0) | … | 39.1 (1.2) | 9.6 (0.7) | 41.0 (1.0) | 35.9 (1.1) | 77.1 (1.0) |
| P | <0.001 | … | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Physical activity | |||||||
| Yes | 79.5 (1.5) | 39.5 (1.5) | … | 14.4 (1.2) | 45.0 (1.2) | 44.2 (1.3) | 86.1 (1.3) |
| No | 67.6 (1.0) | 32.4 (1.0) | … | 8.6 (0.8) | 45.3 (0.9) | 37.7 (1.1) | 78.2 (0.8) |
| P | <0.001 | <0.001 | … | <0.001 | 0.852 | <0.001 | <0.001 |
| Healthy diet score | |||||||
| Yes | 86.6 (1.6) | 41.0 (2.2) | 54.1 (3.3) | … | 48.8 (2.4) | 45.3 (2.5) | 87.0 (1.6) |
| No | 71.1 (1.2) | 34.0 (0.8) | 40.3 (1.3) | … | 44.8 (0.8) | 39.9 (1.1) | 80.5 (0.9) |
| P | <0.001 | 0.002 | <0.001 | … | 0.117 | 0.055 | <0.001 |
| Total cholesterol | |||||||
| Yes | 73.3 (1.1) | 40.5 (1.3) | 41.3 (1.5) | 11.7 (1.2) | … | 44.8 (1.4) | 82.6 (0.9) |
| No | 72.3 (1.4) | 29.2 (1.1) | 41.7 (1.7) | 10.4 (0.7) | … | 36.4 (1.3) | 80.1 (1.2) |
| P | 0.450 | <0.001 | 0.794 | 0.177 | … | <0.001 | 0.034 |
| Blood pressure | |||||||
| Yes | 70.8 (1.5) | 43.3 (1.8) | 45.9 (1.8) | 12.8 (1.4) | 50.6 (1.4) | … | 88.2 (1.1) |
| No | 72.9 (1.2) | 28.8 (1.0) | 38.4 (1.3) | 9.7 (0.8) | 41.1 (0.9) | … | 77.5 (1.1) |
| P | 0.152 | <0.001 | <0.001 | 0.037 | <0.001 | … | <0.001 |
| HbA1c | |||||||
| Yes | 73.2 (1.1) | 38.8 (0.9) | 44.3 (1.7) | 12.0 (0.9) | 45.9 (0.8) | 42.9 (1.0) | … |
| No | 70.4 (2.2) | 16.0 (1.8) | 29.4 (2.0) | 7.5 (0.9) | 39.6 (2.1) | 24.1 (2.1) | … |
| P | 0.203 | <0.001 | <0.001 | <0.001 | 0.008 | <0.001 | … |
P values represent the difference between percentages.
Table 3.
Correlations Adjusted for Age and Sex Between the Ranks of Continuous Variables Used to Define Cardiovascular Health Metrics Among 7622 Participants ≥20 Years of Age, National Health and Nutrition Examination Survey 1999 to 2002
| Body Mass Index, kg/m2 | Physically Active, min/wk | Healthy Eating Index Score, % | Total Cholesterol, mg/dL | Systolic Blood Pressure, mm Hg | Diastolic Blood Pressure, mm Hg | Hemoglobin A1c, % | |
|---|---|---|---|---|---|---|---|
| Body mass index, kg/m2 | … | −0.10 | −0.09 | 0.11 | 0.19 | 0.15 | 0.29 |
| … | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Physically active, min/wk | … | 0.15 | −0.02 | −0.06 | −0.01 | −0.12 | |
| … | <0.001 | 0.236 | 0.001 | 0.531 | <0.001 | ||
| Healthy Eating Index score, % | … | −0.06 | −0.04 | −0.07 | −0.09 | ||
| … | 0.001 | 0.024 | 0.001 | <0.001 | |||
| Total cholesterol, mg/dL | … | 0.08 | 0.16 | 0.07 | |||
| … | <0.001 | <0.001 | <0.0001 | ||||
| Systolic blood pressure, mm Hg | … | 0.41 | 0.10 | ||||
| … | <0.001 | <0.001 | |||||
| Diastolic blood pressure, mm Hg | … | 0.06 | |||||
| … | 0.001 | ||||||
| Hemoglobin A1c, % | … |
During a median follow-up of 5.8 years, 532 deaths (186 deaths resulting from diseases of the circulatory system) were recorded among the 7622 participants. Smoking status, dietary score, HbA1c, physical activity, and blood pressure were significantly associated with all-cause mortality (Table 4). In the maximally adjusted model, only smoking status, physical activity, HEI score, blood pressure, and HbA1c retained their statistical significance. Five metrics (smoking status, physical activity, HEI score, blood pressure, and HbA1c) showed evidence of a graded response, indicating that the intermediate health categories for these metrics were associated with reduced all-cause mortality. With the exception of blood pressure, the hazard ratios for mortality from diseases of the circulatory system generally were aligned with those for all-cause mortality, although the confidence limits included unity.
Table 4.
Hazard Ratios (95% Confidence Interval) for Mortality From All Causes and Diseases of the Circulatory System Among 7622 Participants ≥20 Years of Age, National Health and Nutrition Examination Survey 1999 to 2002
| All-Cause Mortality
|
Diseases of the Circulatory System
|
|||||
|---|---|---|---|---|---|---|
| Poor Health | Intermediate Health | Ideal Health | Poor Health | Intermediate Health | Ideal Health | |
| Model 1: adjusted for age and gender | ||||||
| Smoking status | 1.00 | 0.63 (0.25–1.62) | 0.41 (0.27–0.64) | 1.00 | 0.02 (0.00–0.20) | 0.37 (0.20–0.68) |
| Body mass index | 1.00 | 0.74 (0.55–0.99) | 0.97 (0.70–1.35) | 1.00 | 1.08 (0.64–1.83) | 1.16 (0.70–1.94) |
| Physically active | 1.00 | 0.63 (0.44–0.90) | 0.47 (0.38–0.59) | 1.00 | 0.40 (0.21–0.77) | 0.42 (0.23–0.75) |
| Healthy Eating Index score | 1.00 | 0.64 (0.45–0.91) | 0.37 (0.23–0.59) | 1.00 | 1.01 (0.46–2.21) | 0.43 (0.21–0.90) |
| Total cholesterol | 1.00 | 0.82 (0.59–1.14) | 0.91 (0.65–1.28) | 1.00 | 0.89 (0.52–1.51) | 0.88 (0.52–1.47) |
| Blood pressure | 1.00 | 0.80 (0.69–0.92) | 0.70 (0.51–0.97) | 1.00 | 0.78 (0.51–1.19) | 0.14 (0.04–0.43) |
| Hemoglobin A1c | 1.00 | 0.80 (0.57–1.13) | 0.48 (0.33–0.70) | 1.00 | 0.63 (0.36–1.10) | 0.41 (0.26–0.65) |
| Model 2: as model 1 plus race or ethnicity, education, health insurance, and alcohol use | ||||||
| Smoking status | 1.00 | 0.64 (0.24–1.67) | 0.46 (0.30–0.71) | 1.00 | 0.02 (0.00–0.21) | 0.40 (0.21–0.76) |
| Body mass index | 1.00 | 0.77 (0.57–1.03) | 1.05 (0.76–1.44) | 1.00 | 1.15 (0.69–1.92) | 1.27 (0.76–2.15) |
| Physically active | 1.00 | 0.71 (0.50–1.01) | 0.54 (0.43–0.68) | 1.00 | 0.45 (0.22–0.90) | 0.46 (0.24–0.88) |
| Healthy Eating Index score | 1.00 | 0.70 (0.49–0.99) | 0.44 (0.27–0.72) | 1.00 | 1.11 (0.49–2.52) | 0.54 (0.24–1.19) |
| Total cholesterol | 1.00 | 0.84 (0.61–1.17) | 0.90 (0.65–1.25) | 1.00 | 0.91 (0.53–1.57) | 0.83 (0.49–1.40) |
| Blood pressure | 1.00 | 0.82 (0.71–0.96) | 0.75 (0.54–1.05) | 1.00 | 0.79 (0.51–1.23) | 0.14 (0.05–0.43) |
| Hemoglobin A1c | 1.00 | 0.84 (0.58–1.20) | 0.52 (0.35–0.77) | 1.00 | 0.66 (0.38–1.16) | 0.45 (0.28–0.71) |
| Model 3: as model 2 plus self-reported health status, history of cardiovascular disease, and history of cancer | ||||||
| Smoking status | 1.00 | 0.65 (0.25–1.68) | 0.50 (0.32–0.77) | 1.00 | 0.03 (0.00–0.21) | 0.46 (0.24–0.89) |
| Body mass index | 1.00 | 0.83 (0.61–1.11) | 1.13 (0.81–1.56) | 1.00 | 1.26 (0.74–2.17) | 1.42 (0.82–2.46) |
| Physically active | 1.00 | 0.79 (0.54–1.14) | 0.67 (0.54–0.83) | 1.00 | 0.48 (0.23–0.99) | 0.59 (0.32–1.10) |
| Healthy Eating Index score | 1.00 | 0.70 (0.49–1.01) | 0.44 (0.27–0.72) | 1.00 | 1.11 (0.48–2.55) | 0.52 (0.24–1.17) |
| Total cholesterol | 1.00 | 0.78 (0.56–1.09) | 0.83 (0.62–1.13) | 1.00 | 0.84 (0.50–1.42) | 0.81 (0.48–1.36) |
| Blood pressure | 1.00 | 0.81 (0.69–0.96) | 0.74 (0.54–1.02) | 1.00 | 0.76 (0.47–1.21) | 0.14 (0.05–0.41) |
| Hemoglobin A1c | 1.00 | 0.94 (0.65–1.36) | 0.63 (0.42–0.94) | 1.00 | 0.82 (0.48–1.40) | 0.60 (0.37–1.00) |
| Model 4: as model 3 plus other cardiovascular health metrics | ||||||
| Smoking status | 1.00 | 0.71 (0.26–1.91) | 0.55 (0.35–0.87) | 1.00 | 0.03 (0.00–0.25) | 0.48 (0.23–1.02) |
| Body mass index | 1.00 | 0.86 (0.64–1.15) | 1.21 (0.85–1.73) | 1.00 | 1.34 (0.78–2.32) | 1.61 (0.88–2.93) |
| Physically active | 1.00 | 0.82 (0.56–1.21) | 0.75 (0.61–0.91) | 1.00 | 0.49 (0.25–0.98) | 0.65 (0.37–1.14) |
| Healthy Eating Index score | 1.00 | 0.76 (0.54–1.09) | 0.51 (0.30–0.85) | 1.00 | 1.25 (0.51–3.06) | 0.61 (0.25–1.49) |
| Total cholesterol | 1.00 | 0.83 (0.60–1.13) | 0.88 (0.65–1.19) | 1.00 | 0.86 (0.53–1.39) | 0.88 (0.53–1.45) |
| Blood pressure | 1.00 | 0.78 (0.65–0.93) | 0.75 (0.54–1.03) | 1.00 | 0.76 (0.48–1.21) | 0.14 (0.05–0.41) |
| Hemoglobin A1c | 1.00 | 0.87 (0.61–1.24) | 0.58 (0.39–0.88) | 1.00 | 0.74 (0.44–1.25) | 0.55 (0.33–0.93) |
The National Death Index was used to ascertain mortality through 2006.
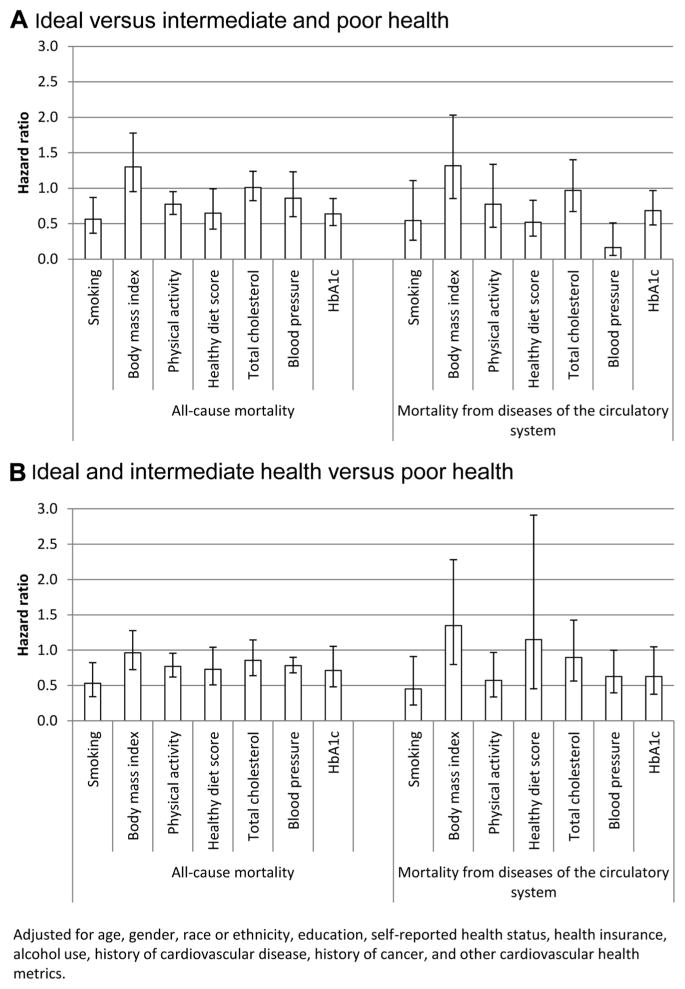
Four of the 7 dichotomized cardiovascular health metrics (ideal health versus intermediate or poor) were significantly associated with all-cause mortality (the Figure, A). Ranked according to the magnitude of the hazard ratio for all-cause mortality were ideal smoking status, HbA1c and dietary score, and physical activity. For mortality from diseases of the circulatory system, ideal health for blood pressure proved to be a particularly strong determinant. Furthermore, dietary score and HbA1c were also significantly associated with this outcome. We also redid the above models after revising the outcome variable by comparing ideal and intermediate health with poor health (the Figure, B). The resulting magnitude and rank order of the hazard ratios showed some differences from those of the models in part A of the Figure.
Figure.
Adjusted hazard ratios (95% confidence intervals) for mortality from all causes and diseases of the circulatory system among 7622 participants ≥20 years of age, National Health and Nutrition Examination Survey, 1999 to 2002. The National Death Index was used to ascertain mortality through 2006. A, Hazard ratios for ideal vs intermediate and poor health for each cardiovascular health metric. B, Hazard ratios for ideal and intermediate health vs poor health for each cardiovascular health metric. Hb indicates hemoglobin.
When the dichotomized cardiovascular health metrics were summed, a strong inverse relationship was present between the number of ideal health metrics and mortality from all causes or diseases of the circulatory system (Table 5). Because so few deaths occurred among participants with 6 or 7 ideal health metrics, we grouped participants with 5, 6, or 7 ideal health metrics into 1 category. Compared with participants with no ideal health metrics, those with ≥5 had reduced all-cause mortality (adjusted hazard ratio, 0.22; 95% confidence interval [CI], 0.10–0.50) and mortality from diseases of the circulatory system (adjusted hazard ratio, 0.12; 95% CI, 0.03–0.57). When participants who died during the first year of follow-up were excluded from the analyses (481 deaths, 7571 at risk), a strong reduction in risk with increasing number of ideal health metrics remained evident (adjusted hazard ratios for participants with 1, 2, 3, 4, and ≥5 ideal health metrics are 0.68 [95% CI, 0.32–1.45], 0.69 [95% CI, 0.31–1.52], 0.58 [95% CI, 0.24–1.38], 0.49 [95% CI, 0.19–1.27], and 0.22 [95% CI, 0.08–0.62]). Furthermore, after the exclusion of participants with self-reported cardiovascular disease from the analyses, the hazard ratios for participants with ≥5 ideal health metrics were similar to the hazard ratios for the entire analytic sample of 7622 participants.
Table 5.
Sample Sizes, Rates, and Hazard Ratios for Mortality From All Causes and Diseases of the Circulatory System Among Participants ≥20 Years of Age, National Health and Nutrition Examination Survey 1999 to 2002
| No. of Ideal Cardiovascular Health Metrics
|
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ≥5 | |
| All participants | ||||||
| All causes | ||||||
| Deaths/at risk, n | 16/134 | 99/829 | 170/1711 | 156/2112 | 71/1587 | 20/1249 |
| Age- and sex-adjusted rate/1000 PY (95% CI) | 17.3 (7.0–27.6) | 10.4 (7.6–13.2) | 10.9 (8.4–13.4) | 8.1 (5.9–10.4) | 6.3 (4.5–8.2) | 3.5 (1.1–5.9) |
| Age- and sex-adjusted hazard ratios (95% CI) | 1.00 | 0.53 (0.28–1.01) | 0.48 (0.25–0.94) | 0.36 (0.17–0.76) | 0.29 (0.13–0.64) | 0.13 (0.06–0.30) |
| Adjusted hazard ratios (95% CI)* | 1.00 | 0.55 (0.29–1.06) | 0.52 (0.26–1.01) | 0.41 (0.19–0.87) | 0.34 (0.15–0.77) | 0.17 (0.07–0.39) |
| Adjusted hazard ratios (95% CI)† | 1.00 | 0.59 (0.31–1.11) | 0.63 (0.33–1.21) | 0.50 (0.24–1.04) | 0.44 (0.20–0.96) | 0.22 (0.10–0.50) |
| Diseases of the circulatory system | ||||||
| Deaths/at risk, n | 8/134 | 35/829 | 66/1711 | 49/2112 | 24/1587 | 4/1249 |
| Age- and sex-adjusted rate/1000 PY (95% CI) | 6.1 (1.2–11.0) | 3.6 (2.1–5.1) | 3.8 (2.7–4.9) | 2.1 (1.4–2.8) | 1.8 (1.0–2.6) | 0.6 (0.0–1.2) |
| Age- and sex-adjusted hazard ratios (95% CI) | 1.00 | 0.41 (0.16–1.02) | 0.34 (0.13–0.90) | 0.19 (0.07–0.49) | 0.17 (0.07–0.44) | 0.08 (0.02–0.32) |
| Adjusted hazard ratios (95% CI)* | 1.00 | 0.41 (0.15–1.09) | 0.36 (0.13–1.02) | 0.20 (0.07–0.58) | 0.19 (0.07–0.54) | 0.09 (0.02–0.40) |
| Adjusted hazard ratios (95% CI)† | 1.00 | 0.40 (0.15–1.09) | 0.46 (0.17–1.22) | 0.25 (0.09–0.67) | 0.27 (0.10–0.72) | 0.12 (0.03–0.57) |
| Limited to participants without CVD | ||||||
| All causes | ||||||
| Deaths/at risk, n | 10/111 | 57/675 | 104/1468 | 104/1900 | 49/1497 | 15/1204 |
| Age- and sex-adjusted rate/1000 PY (95% CI) | 13.6 (2.4–24.7) | 7.8 (4.7–11.0) | 8.5 (6.0–11.0) | 6.9 (4.6–9.1) | 5.1 (3.3–6.9) | 2.5 (0.8–4.1) |
| Age- and sex-adjusted hazard ratios (95% CI) | 1.00 | 0.50 (0.20, 1.21) | 0.47 (0.20, 1.15) | 0.39 (0.15, 1.01) | 0.27 (0.10, 0.71) | 0.13 (0.05, 0.35) |
| Adjusted hazard ratios (95% CI)* | 1.00 | 0.52 (0.20–1.33) | 0.52 (0.21–1.30) | 0.45 (0.16–1.21) | 0.32 (0.12–0.89) | 0.17 (0.06–0.48) |
| Adjusted hazard ratios (95% CI)† | 1.00 | 0.59 (0.23–1.51) | 0.61 (0.24–1.55) | 0.54 (0.21–1.44) | 0.39 (0.14–1.09) | 0.21 (0.07–0.59) |
| Diseases of the circulatory system | ||||||
| Deaths/at risk, n | 7/111 | 13/675 | 26/1468 | 27/1900 | 13/1497 | 4/1204 |
| Age- and sex-adjusted rate/1000 PY (95% CI) | 7.0 (1.5–12.6) | 1.4 (0.5–2.2) | 1.5 (0.7–2.2) | 1.5 (0.8–2.3) | 0.9 (0.4–1.5) | 0.7 (0.0–1.4) |
| Age- and sex-adjusted hazard ratios (95% CI) | 1.00 | 0.13 (0.04–0.36) | 0.09 (0.03–0.26) | 0.09 (0.03–0.26) | 0.06 (0.02–0.16) | 0.07 (0.02–0.30) |
| Adjusted hazard ratios (95% CI)* | 1.00 | 0.12 (0.04–0.41) | 0.10 (0.03–0.32) | 0.10 (0.03–0.35) | 0.07 (0.02–0.22) | 0.09 (0.02–0.41) |
| Adjusted hazard ratios (95% CI)† | 1.00 | 0.13 (0.04–0.43) | 0.12 (0.04–0.35) | 0.12 (0.04–0.36) | 0.09 (0.03–0.25) | 0.10 (0.02–0.47) |
PY indicates person-years; CI, confidence interval. The National Death Index was used to ascertain mortality through 2006.
Adjusted for age, sex, race or ethnicity, education, health insurance, and alcohol use.
Adjusted for age, sex, race or ethnicity, education, self-reported health status, health insurance, alcohol use, history of cardiovascular disease (for models involving all participants only), and history of cancer.
We also created a score out of the 7 cardiovascular health metrics ranging from 0 to 14 by assigning a value of 0 for poor health, 1 for intermediate health, and 2 for ideal health for each metric. The maximally adjusted hazard ratio per unit score was 0.86 (95% CI, 0.81–0.91) for all-cause mortality and 0.83 (95% CI, 0.76–0.91) for mortality resulting from diseases of the circulatory system.
Discussion
Using a recent national sample of US adults, we show that the number of ideal cardiovascular health metrics was strongly and inversely related to mortality from all causes and diseases of the circulatory system. These data, which are consistent with the results from a recent prospective study,11 suggest that attainment of the AHA 2020 goals could result in substantial reductions in mortality.
Since the 1960s, mortality from coronary heart disease has enjoyed a more or less sustained decrease.17 The early period of this drop was likely governed primarily by reductions in the prevalence of smoking,18 mean concentration of total cholesterol,19 and blood pressure.20 The introduction of the coronary care unit during the 1960s and its subsequent expansion also saved lives. The development of ever more sophisticated medical and surgical treatments in ensuing decades contributed further to reducing the mortality rate from coronary heart disease. Between 1980 and 2000, treatments accounted for ≈47% and reductions in risk factors for ≈44% of the deaths from coronary heart disease that were prevented or postponed in the United States.21 Models consistently show that enormous reductions in the mortality from coronary heart disease are achievable with meaningful reductions in risk factors.22 Thus, the development of the AHA 2020 goals that emphasize cardiovascular health represents an important evolution from disease management to health promotion.
Controlling for confounding constitutes a major challenge in observational studies. To examine the impact of various possible confounders on our risk estimates, we presented several proportional hazards models that contained different sets of covariates. We included self-reported health and prevalent chronic disease as possible confounders because these factors are related to mortality and to the AHA cardiovascular health metrics. Conceivably, these factors could also form part of the causal pathways between the AHA metrics and mortality, in which case the model was overadjusted and the benefits attributable to the metrics were underestimated. However, a comparison of models with and without these factors shows that self-reported health and prevalent chronic disease did not materially affect the hazard ratios. Similar considerations apply to the model that adds all the AHA metrics.
Our analyses using the NHANES 1999 to 2002 data show that only a small percentage of US adults met ideal criteria for all 7 cardiovascular health metrics, a result that is disappointing but perhaps not surprising. One consequence of the very small percentage of participants in the extremes of the distribution is that large cohorts with large numbers of deaths are needed to form stable and accurate estimates of the risk for mortality among those with 6 or 7 ideal health metrics. Most adults met 2, 3, or 4 ideal health metrics, all of which are associated with a substantial reduction in mortality compared with participants with no ideal health metric. The challenge for clinical and public health professionals is to keep moving the distribution of the number of health metrics in the desired direction. In this regard, a number of major efforts are underway. An example of a clinical program is the AHA’s The Guideline Advantage, jointly managed by the American Cancer Society, American Diabetes Association, and AHA.23 An example of a recent major public health initiative launched by the US Department of Health and Human Services is the Communities Putting Prevention to Work initiative, which funded 50 communities with the goal of reducing tobacco use, increasing physical activity, improving nutrition, and reducing obesity.24 In addition to continued efforts to promote healthy lifestyles, public health programs have put a greater focus on systems and environmental changes to effect changes and options for healthy lifestyle choices.25–28 Examples of such policy and systems level efforts include smoking bans and restrictions in public places29,30 and recent efforts to address the amount of sodium in foods.31,32
In the AHA statement, the prevalence of an ideal healthy dietary score was <0.5%. In contrast, our use of the HEI score and the cut points we chose yielded a prevalence of an ideal healthy dietary score of ≈11%. How well the AHA’s healthy dietary score predicts mortality remains unknown. However, 3 of its 5 primary components are shared with the HEI score.
Five of the 7 metrics (current smoking, physical activity, healthy diet score, blood pressure, and HbA1c) showed a gradient in risk, suggesting that health gains are feasible when adults move from poor to intermediate health and when they move from intermediate to ideal health for most health metrics. Future studies comparing the gain in health benefits associated with a change from poor to intermediate health with the gain associated with a change from intermediate to ideal health for each metric may yield helpful insights that will be of value to clinicians in optimizing the cardiovascular health of their patients.
Not all of the individual metrics contributed to the reduction in risk; body mass index and total cholesterol status were not significantly related to mortality from all causes. The reasons for these findings are not clear. Of note, a previous analysis of the mortality experience by NHANES III participants found a weak relationship between body mass index and all-cause mortality.33 The authors of that study postulated that treatment for some of downstream effects of obesity such as dyslipidemia and hypertension may have blunted some of the risk associated with being obese. In addition, follow-up time may not have been long enough to discern the effects of body mass index. An earlier study among men observed no associations for body mass index or other measures of adiposity with coronary heart disease mortality during up to 14 years of follow-up but found positive associations after 14 years of follow-up,34 suggesting that the effects of adiposity may not become apparent for some time. A recent meta-analysis found that anthropometric measures such as body mass index did not improve risk prediction for cardiovascular disease once traditional cardiovascular risk factors were considered.35 From a public health perspective, the prevention and control of excess weight represents a valuable public health goal because, in addition to other considerations, excess weight causes dyslipidemia, high blood pressure, and hyperglycemia.
Hypercholesterolemia is considered 1 of the 3 cardinal risk factors for coronary heart disease and has been shown to be a risk factor for all-cause mortality.36 Consequently, the poor prediction by this risk factor in the present study was unexpected. The use of cholesterol-lowering medications, particularly in the form of statins, has been increasing rapidly in the United States,37 and some of the non–cholesterol-lowering effects of statin use may have played a role in our findings. Furthermore, the use of cholesterol-lowering medications among the participants with untreated hypercholesterolemia at baseline likely increased, which may have negated some of the adverse effects suggested by elevated baseline concentrations. Alternatively, the lack of a significant association may have represented a chance occurrence. It is also possible that a significant association may emerge with longer follow-up of the cohort.
Our results should be considered in light of several limitations. First, the follow-up of our cohort was of limited duration. Consequently, the number of deaths, even at >500, posed a major challenge in estimating the risks in the extremes of the distribution of the number of ideal health metrics. Thus, collapsing some categories for some analyses was necessary. Furthermore, the limited number of deaths also led us not to undertake stratified analyses by various demographic or other factors. Second, we had only a single measurement for all the health metrics; thus, were unable to account for changes in these metrics that may have occurred during the course of the follow-up period. Finally, as noted before, we did not adhere perfectly to all of the AHA 2020 health metrics for practical reasons.
Other studies are needed to examine the relationships between the AHA 2020 goals and the incidence and mortality of cardiovascular disease. Examining these relationships in historical and in more current cohorts will provide important feedback about the choice of metrics and the selected definitions for poor, intermediate, and ideal health.
CLINICAL PERSPECTIVE.
In 2010, the American Heart Association presented a set of 7 metrics that were designed to promote cardiovascular health: smoking status, body mass index, physical activity, healthy dietary score, total cholesterol, blood pressure, and fasting plasma glucose. For each metric, 3 levels of health were defined (poor, intermediate, and ideal health). The state of ideal health for each metric emphasizes the concept of primordial prevention. Using data from a nationally representative cohort of US adults, we found that about half of adults had 3 or 4 ideal health metrics (only ≈1% met the ideal health criteria for all 7 metrics). Furthermore, we observed a strong dose-response relationship between the number of ideal health metrics and mortality from all causes and diseases of the circulatory system. Clinicians can reduce cardiovascular morbidity and mortality in their patient populations not only by helping their patients maximize the number of attained ideal cardiovascular health metrics but also by helping patients with poor health for some metrics transition to intermediate health. Optimizing cardiovascular health involves addressing key lifestyle behaviors of smoking, diet, and physical activity. Besides the resources in their practices, clinicians can opt to draw on available institutional, clinical, and community resources. An implication of the AHA goals and our findings is that the cardiovascular health of patients should be monitored from an early age. Clinicians should strive to maximize the numbers of patients with hypercholesterolemia, hypertension, and hyperglycemia who require pharmacological management who are on treatment and under control.
Acknowledgments
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Disclosures
None.
References
- 1.Murphy SL, Xu J, Kochanek KD. [Accessed February 7, 2012];Deaths: Preliminary Data for 2010. http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf.
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van HL, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Strasser T. Reflections on cardiovascular diseases. Interdisciplinary Sci Rev. 1978;3:225–230. [Google Scholar]
- 4.Stamler J, Stamler R, Neaton JD, Wentworth D, Daviglus ML, Garside D, Dyer AR, Liu K, Greenland P. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282:2012–2018. doi: 10.1001/jama.282.21.2012. [DOI] [PubMed] [Google Scholar]
- 5.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Liu K, Pirzada A, Yan LL, Garside DB, Feinglass J, Guralnik JM, Greenland P, Stamler J. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Arch Intern Med. 2003;163:2460–2468. doi: 10.1001/archinte.163.20.2460. [DOI] [PubMed] [Google Scholar]
- 7.Akesson A, Weismayer C, Newby PK, Wolk A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med. 2007;167:2122–2127. doi: 10.1001/archinte.167.19.2122. [DOI] [PubMed] [Google Scholar]
- 8.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects: Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–579. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 9.King DE, Mainous AG, 3rd, Geesey ME. Turning back the clock: adopting a healthy lifestyle in middle age. Am J Med. 2007;120:598–603. doi: 10.1016/j.amjmed.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Li C, Zhao G, Pearson WS, Capewell S. Trends in the prevalence of low risk factor burden for cardiovascular disease among United States adults. Circulation. 2009;120:1181–1188. doi: 10.1161/CIRCULATIONAHA.108.835728. [DOI] [PubMed] [Google Scholar]
- 11.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. [Accessed January 7, 2008];National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm.
- 13.Centers for Disease Control and Prevention. [Accessed January 7, 2011];NHEFS linked mortality file. http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhefs_linkage.htm.
- 14.US Department of Health and Human Services. [Accessed January 3, 2012];Physical activity guidelines for Americans. 2008 http://www.health.gov/paguidelines/guidelines/default.aspx.
- 15.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 16.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–1901. doi: 10.1016/j.jada.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health, National Heart Lung, and Blood Institute. [Accessed April 27, 2011];Morbidity & Mortality: 2009 Chartbook on Cardiovascular, Lung, and Blood Diseases. http://www.nhlbi.nih.gov/resources/docs/2009_Chart-Book.pdf.
- 18.Centers for Disease Control and Prevention. [Accessed January 3, 2012];Trends in current cigarette smoking among high school students and adults, United States, 1965–2007. http://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.
- 19.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA. 2005;294:1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 20.Burt VL, Cutler JA, Higgins M, Horan MJ, Labarthe D, Whelton P, Brown C, Roccella EJ. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population: data from the Health Examination Surveys, 1960 to 1991. Hypertension. 1995;26:60–69. doi: 10.1161/01.hyp.26.1.60. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Capewell S. Proportion of the decline in cardiovascular mortality disease due to prevention versus treatment: public health versus clinical care. Annu Rev Public Health. 2011;32:5–22. doi: 10.1146/annurev-publhealth-031210-101211. [DOI] [PubMed] [Google Scholar]
- 23.American Heart Association. [Accessed April 27, 2011];The guideline advantage. http://www.guidelineadvantage.org/TGA/
- 24.Centers for Disease Control and Prevention. [Accessed May 27, 2011];Communities putting prevention to work. http://www.cdc.gov/CommunitiesPuttingPreventionto-Work/
- 25.Kumanyika SK, Obarzanek E, Stettler N, Bell R, Field AE, Fortmann SP, Franklin BA, Gillman MW, Lewis CE, Poston WC, Stevens J, Hong Y. Population-based prevention of obesity: the need for comprehensive promotion of healthful eating, physical activity, and energy balance: a scientific statement from American Heart Association Council on Epidemiology and Prevention, Interdisciplinary Committee for Prevention (formerly the Expert Panel on Population and Prevention Science) Circulation. 2008;118:428–464. doi: 10.1161/CIRCULATIONAHA.108.189702. [DOI] [PubMed] [Google Scholar]
- 26.Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm Rep. 2009;58:1–26. [PubMed] [Google Scholar]
- 27.Institute of Medicine. A Population-Based Policy and Systems Change Approach to Prevent and Control Hypertension. Washington DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. [Accessed May 3, 2011];The Guide to Community Preventive Services. http://www.thecommunityguide.org/index.html.
- 29.Khuder SA, Milz S, Jordan T, Price J, Silvestri K, Butler P. The impact of a smoking ban on hospital admissions for coronary heart disease. Prev Med. 2007;45:3–8. doi: 10.1016/j.ypmed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Lightwood JM, Glantz SA. Declines in acute myocardial infarction after smoke-free laws and individual risk attributable to secondhand smoke. Circulation. 2009;120:1373–1379. doi: 10.1161/CIRCULATIONAHA.109.870691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Institute of Medicine. Strategies to Reduce Sodium Intake in the United States. Washington DC: National Academies Press; 2010. [Google Scholar]
- 32.Gase LN, Kuo T, Dunet DO, Simon PA. Facilitators and barriers to implementing a local policy to reduce sodium consumption in the County of Los Angeles government, California, 2009. Prev Chronic Dis. 2011;8:A33. [PMC free article] [PubMed] [Google Scholar]
- 33.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 34.Spataro JA, Dyer AR, Stamler J, Shekelle RB, Greenlund K, Garside D. Measures of adiposity and coronary heart disease mortality in the Chicago Western Electric Company Study. J Clin Epidemiol. 1996;49:849–857. doi: 10.1016/0895-4356(96)00067-4. [DOI] [PubMed] [Google Scholar]
- 35.Wormser D, Kaptoge S, Di AE, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 37.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140:226–235. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]