Abstract
The differential diagnosis of parkinsonian syndromes can be challenging, particularly in early disease stages. However, prognosis and therapeutic regimes are not alike in Parkinson disease and atypical parkinsonism, and thus a correct diagnosis at the earliest possible stage is desirable. Over the past two decades,magnetic resonance imaging and radiotracer-based imaging techniques have proven to be helpful tools to enhance the accuracy of clinical diagnosis in these disorders. Here, we review recent advances in neuroimaging for the differential diagnosis of parkinsonian syndromes.
Keywords: Parkinson disease, atypical parkinsonism, differential diagnosis, neuroimaging
Parkinson disease (PD) is a progressive neurologic disease characterized by bradykinesia, rigidity, tremor, and postural instability. Its histopathological hallmark is the loss of dopaminergic cells in the substantia nigra (SN). The diagnosis of PD can be straightforward when patients present with the typical cardinal symptoms and respond well to dopaminergic treatment. However, particularly in early disease stages, symptoms often overlap with those observed in atypical parkinsonian syndromes (APS), such as multiple system atrophy (MSA), progressive supranuclear palsy (PSP), or corticobasal degeneration (CBD). Indeed, clinicopathological studies have found that up to 24% of patients clinically diagnosed with PD were classified as APS on autopsy.1 The sensitivity and specificity of clinical diagnosis even is lower for early-stage APS.2,3 Although the number of misclassifications can be reduced when strictly applying standardized criteria,1 the accuracy of early differential diagnosis remains suboptimal.
To date, there is no cure for PD. That said, the underlying pathology, prognosis, and symptomatic therapeutic regimes are not alike across the different forms of parkinsonism. Moreover, although the diagnosis of idiopathic PD relies on the presence of motor symptoms, it has become increasingly recognized that a variety of nonmotor symptoms can antecede motor symptoms in PD by many years.4
A potentially disease-modifying therapy should be initiated early on in the disease course, preferably in the “prodromal” phase before motor symptoms emerge. Therefore, it is crucial to establish reliable biomarkers to distinguish PD from APS at the earliest possible stage. Over recent years, neuroimaging has evolved as a particularly useful tool in this regard.
Here, we review recent advances in magnetic resonance imaging (MRI) and radiotracer-based imaging techniques in the differential diagnosis of parkinsonian syndromes.
Magnetic Resonance Imaging
The classic domain of conventional MRI is the exclusion of structural lesions that may cause secondary, symptomatic parkinsonism, such as normal pressure hydrocephalus, multiple sclerosis, or tumors. Cerebrovascular damage due to small vessel disease and infarctions, such as vascular parkinsonism, is perhaps the most common form of secondary parkinsonism, and abnormal structural MRI findings are present in up to 90 to 100% of these cases.5
In PD, structural MRI is usually normal, at least early in the disease course. In PSP, structural MRI may reveal the “hummingbird” sign on sagittal view (▶Fig. 1A) and the “Mickey Mouse” sign, also called “morning glory flower” sign, in the axial plane as a reflection of midbrain atrophy.6 Multiple system atrophy is characterized by putaminal and olivopontocerebellar atrophy.7 The putamen may appear hypointense on T2-weighted images with a concurrent hyperintense rim lateral to the putamen, most likely reflecting reactive micro- and astrogliosis (▶Fig. 1B).8 In addition, degeneration of pontocerebellar fibers can cause a hyperintense signal in the middle cerebellar peduncles (MCP) and pons that in some cases shows a cruciform configuration called the “hot cross bun” sign (▶Fig. 1C).6,7 Although these structural abnormalities are highly specific and comport well with postmortem macroscopic findings, their sensitivity is rather low.6,9
Fig. 1.
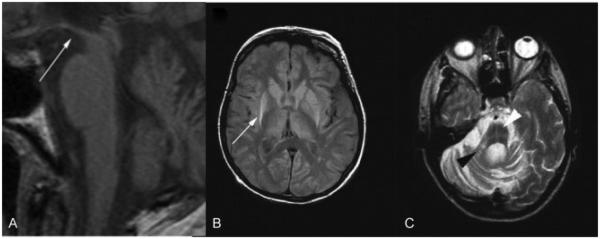
Structural magnetic resonance imaging in progressive supranuclear palsy (PSP) and multiple system atrophy (MSA). (A) T1-weighted image showing hummingbird sign (white arrow) in PSP 1.5 years after onset. (B) Proton density weighted image of pathologically confirmed MSA. Putaminal atrophy with hyperintense putaminal rim (white arrow) on the right and early hyperintense putaminal rim on the left 4.8 years after disease onset. (C) Right middle cerebellar peduncle sign (black arrowhead) and hot cross bun sign (white arrowhead) in pathologically confirmed MSA (T2-weighted image). (Adapted with permission from Massey et al. Conventional Magnetic Resonance Imaging in Confirmed Progressive Supranuclear Palsy and Multiple System Atrophy. Mov Disord 2012; 27:1754–1762.)
Morphometric Magnetic Resonance Imaging
Magnetic resonance volumetry (MRV) allows for the quantitative assessment of brain atrophy. Magnetic resonance volumetry is performed in a semiautomated manner by defining a priori regions-of-interest (ROI). Employing this ROI-based approach, a variety of volume ratio indices have been proposed to assist in the differential diagnosis of parkinsonian syndromes. For example, Quattrone and colleagues10 measured volumes of the middle and superior cerebellar peduncles (SCP), pons, and midbrain in PSP, MSA, and PD patients. Subsequently, the pons to midbrain and MCP/SCP ratios were calculated. Both ratios were increased in PSP patients compared with the other two groups and normal controls. However, there was considerable overlap at the individual level. Of note, a combination of both ratios, termed the magnetic resonance parkinsonism index,10 provided excellent accuracy in identifying PSP patients. Similarly, others have reported reduced midbrain and SCP volumes in PSP patients, whereas MSA patients typically show pontocerebellar volume loss.11 In contrast, regional volumes usually are normal in PD.11,12 It has been suggested, however, that atrophy of the SN may be present even in early-stage PD.13,14 That said, SN atrophy does not reliably distinguish PD from APS.15 Findings from MRV studies in CBD are less consistent. Atrophy of the corpus callosum and parietal cortices has been reported.16 However, others have found that these regional changes are not specific to CBD, but can also be observed in other APS.17
Brain atrophy can also be assessed at the whole-brain level. Voxel-based morphometry (VBM) is a fully automated and rater-independent approach that does not necessitate the a priori delineation of ROIs, but relies on whole-brain voxel-wise comparisons.18 Using VBM, several groups have reported cortical atrophy mainly in temporal associative, limbic, paralimbic, frontal, and parietal regions in PD.19–22 Two recent meta-analyses23,24 investigated atrophy patterns of the cerebral white and gray matter in PSP patients. Subjects showed consistent white matter reductions in the pons, midbrain, and adjacent to the basal ganglia.23 Grey matter loss was observed in the thalamus, midbrain, basal ganglia, and insular and frontal cortices.24 In MSA, putaminal volume loss is common.25–27 Atrophy may also be revealed in the cerebellum, pons, sensorimotor, premotor, frontal, and insular cortices in these patients.26,28,29 In CBD, asymmetric cortical atrophy may be discerned, whereas subcortical atrophy is often less severe compared with other APS.30,31
Morphometric changes on VBM have been found to generally correlate well with clinical phenotype in both PD and APS.19,32,33 Voxel-based morphometry findings can aid in the differential diagnosis of idiopathic PD and APS, providing satisfactory specificity and sensitivity.34,35 Recent studies suggest that VBM also might be effective in distinguishing APS subtypes, for example, the cerebellar and parkinsonian variants of MSA.26
However, there remains substantial overlap at the individual level, and VBM is not yet established in routine clinical practice. Moreover, it remains to be proven whether disease-specific patterns of cortical and subcortical atrophy can be reliably detected in very early disease stages when the need for a correct diagnosis is most urgent.
Diffusion Weighted Imaging
Diffusion weighted imaging (DWI) is a novel MRI technique that allows for the assessment of brain microstructural integrity. It measures the movement of water molecules, which is mainly directed along the white matter fibers. Axonal damage and cell loss, as commonly observed in neurodegenerative diseases, lead to an increase in molecule movement, and consequently, the apparent diffusion coefficient. Diffusion tensor imaging (DTI) provides additional quantitative information about the average diffusivity within one voxel (mean diffusivity) and directionality of diffusion (fractional anisotropy). Compared with conventional MRI techniques, DWI/DTI provides a major advantage to detect early, subtle structural changes that might not be apparent on conventional MRI.36
Increased mean diffusivity and reduced fractional anisotropy in the SN of PD patients indicate nigral cell loss, but do not differentiate PD from APS.37 The topography of apparent diffusion coefficient, mean diffusivity, and fractional anisotropy changes in APS is in good agreement with histopathological findings. For example, increased regional apparent diffusion coefficient values in the MCP separate MSA from PSP and PD patients with a sensitivity and specificity ranging between 84 to 100%.38,39 Patients with MSA regularly show increased mean diffusivity in the putamen compared with PSP and PD patients.40 In PSP, regional apparent diffusion coefficient has been found to be elevated in the midbrain and globus pallidus as compared with PD and MSA patients, and increased apparent diffusion coefficient values in the SCP and caudate nucleus separated PSP from MSA patients.41 Studies exploring DWI/DTI abnormalities in CBD are scarce. One study42 reported increased mean diffusivity in the anterior/medial thalamus and SCP in PSP compared with CBD patients. In contrast, the latter group exhibited increased mean diffusivity in the pre- and postcentral gyri. In addition, fractional anisotropy values were lower in the anterior cingulum in PSP compared with CBD patients. However, these findings are restricted to the group level, and their utility to differentiate PD from APS in single cases remains to be elucidated.
In summary, DWI/DTI may overcome the rather low sensitivity of conventional MRI measures, particularly in early-stage patients. However, longitudinal studies involving larger samples are warranted to confirm the promising findings from recent studies.
Imaging of Basal Ganglia Iron Deposition
Patients with PD show increased nigral iron content.43 T2*-weighted MRI allows for the in vivo assessment of cerebral iron deposition, and more recently, susceptibility weighted imaging (SWI) further improved the visualization of cerebral iron accumulation in neurodegenerative disorders.44,45 Indeed, numerous studies have reported increased iron content of the SN in PD.46 Moreover, patterns of basal ganglia iron deposition can be utilized to differentiate PD from APS. Iron accumulation has been reported to be increased in the globus pallidus and caudate nucleus in PSP compared with PD patients, and iron levels in the thalamus, globus pallidus, red nucleus, and substantia nigra have been found to be higher in PSP compared with MSA patients.45,47 A substantial increase in iron accumulation in the putamen is a characteristic feature of MSA, and it facilitates the separation between MSA and both PD and PSP patients.45,47,48
Magnetic Resonance Spectroscopy Imaging
Proton magnetic resonance spectroscopy imaging (H1-MRSI) allows for the assessment of marker molecules in brain energy metabolism and neuronal integrity. Particularly, the ratio of N-acetylaspartate (NAA) as a surrogate marker of neuronal structural integrity, and creatine (Cr), an indicator of energy metabolism, has been in the focus of several recent studies in parkinsonian syndromes.
Regionally decreased NAA/Cr ratios have been reported in PD patients, particularly in those showing cognitive impairment.49,50 However, findings are equivocal, and particularly in early disease stages NAA levels can be normal.51 In one study,52 PD patients exhibited a higher NAA/Cr ratio in the caudal SN compared with the rostral portion. Reversed ratios were observed in APS patients and normal controls. However, due to the limited sample size, APS subtypes (MSA, PSP, CBD) could not be differentiated, and NAA/Cr ratios did not differ between APS patients and healthy volunteers. Guevara et al53 observed decreased NAA levels in the putamen and globus pallidus in MSA and PSP patients compared with PD patients, and putaminal NAA levels also distinguished MSA from PSP patients.
Although findings from these and other54,55 studies are promising and suggest a potential role of MRSI in the differential diagnosis of parkinsonian syndromes, more research is required to test the utility of H1-MRSI at the individual level.
Magnetization Transfer Imaging
Magnetization transfer imaging (MTI) assesses the energy transfer between protons that are highly bound to structures, such as myelin, and very mobile protons in free water. Thus, the magnitude of the magnetization transfer ratio (MTR) between these protons is highly dependent on axonal myelination. Consequently, demyelination and neurodegeneration lead to changes in MTR that have been used to visualize microstructural damage in the parkinsonian brain.
In keeping with DWI/DTI studies, microstructural damage of the SN has been reported to be present even in early-stage PD patients.56,57 Reduced MTR might also be observed in the caudate nucleus, putamen, thalamus, and in the periventricular, frontal, and parietal white matter.57 Limited data are available on the utility of MTR to differentiate among parkinsonian syndromes. Eckert et al identified several regions exhibiting distinct MTR changes in PD and APS.58 For example, MTR in the globus pallidus was reduced in MSA-compared PD patients, and even lower values separated PSP from MSA patients. In the putamen, the lowest MTR was observed in MSA patients, distinguishing this group from PD subjects and healthy controls. However, putaminal MTR did not differ between MSA and PSP patients. Magnetization transfer ratio in the SN was lowest in PSP patients, and separated this group from both MSA and PD patients, and also MSA from PSP patients.
Radiotracer Imaging
Both positron emission tomography (PET) and single photon emission computed tomography (SPECT) using a variety of radioactive tracers have been used to investigate brain function in parkinsonian syndromes. Positron emission tomography has a higher resolution and sensitivity compared with SPECT, whereas the latter technique is more widely available and less expensive. Additionally, more tracers are commercially available for SPECT, mitigating the need for an onsite cyclotron.
Dopaminergic Imaging
Loss of presynaptic striatal dopaminergic function is the hallmark of PD, and a large body of studies employing different tracers (e.g., 18F-fluorodopa [FDOPA], 123I-β-CIT, and 123I-FP-CIT [FPCIT]) have investigated patterns of dopaminergic decline in parkinsonian syndromes. Dopaminergic decline typically is pronounced in the dorsal striatum, whereas dopaminergic function is preserved in the anterior putamen and caudate nucleus in early-stage disease.59
Dopaminergic imaging readily distinguishes PD from essential tremor with very high accuracy.60 Presynaptic dopaminergic imaging is less useful in distinguishing PD from APS. It has been suggested that the anterior caudate to anterior striatum uptake ratio distinguishes PD from PSP patients, and that the posterior to anterior putamen ratio might be lower in PD compared with MSA patients.61 However, there was substantial overlap between MSA and PD patients at the individual level in this study, and others have found that patterns of presynaptic dopaminergic decline are similar in PD and APS.62,63 The low accuracy of dopaminergic imaging to separate APS from PD is also reflected by the notion that in the USA and Europe, FPCIT SPECT is only approved for the differential diagnosis of PD/APS versus essential tremor, and to distinguish dementia with Lewy bodies from Alzheimer disease.64
It has been suggested that the additional use of postsynaptic dopaminergic tracers (e.g., 11C-raclopride [RAC] and 123I-IBZM) might supplement the differential diagnosis in parkinsonism. Indeed, postsynaptic dopaminergic function is usually unaltered in PD,63 whereas reduced tracer uptake is commonly observed in PSP, MSA, and CBD patients.65–67 However, normal postsynaptic function does not rule out APS. For example, Plotkin et al65 reported reduced RAC uptake in six of eight PSP patients, whereas sensitivity in MSA patients (seven of 13 patients), and particularly CBD (two of nine patients), was rather disappointing. Similarly, Vlaar and colleagues68 reported a sensitivity of 80% and specificity of only 62% to separate PD from APS based on IBZM SPECT in a cohort of 154 parkinsonian patients. Of note, a pooled analysis of pre- and postsynaptic imaging in the same cohort improved diagnostic accuracy only marginally.68
In summary, dopaminergic imaging is a powerful tool to augment clinical diagnosis in cases in which there is doubt about the diagnosis of PD/APS versus essential tremor. In addition, decreasing tracer uptake correlates with disease progression and severity in PD patients.69,70 That said, imaging of presynaptic dopaminergic function is not useful to differentiate PD from APS. Postsynaptic dopaminergic imaging might have some merit in this regard, but sensitivity is still low and normal postsynaptic tracer uptake does not rule out APS.
Metabolic Imaging
18F-fluorodeoxyglucose (FDG) PET directly assesses synaptic activity, a main contributor to brain metabolism. The use of FDG PET has revealed regional metabolic alterations in PD and APS. In PD, metabolism in the putamen and globus pallidus internus is typically increased, whereas parietal associative cortices show reduced glucose utilization. In contrast, in MSA patients, bilateral putaminal hypometabolism and reduced pontocerebellar activity are commonly observed. Brain metabolism in PSP patients is characterized by reduced activity in the premotor and motor cortices, as well as the midbrain. Asymmetric cortical and basal ganglia hypometabolism is a typical feature of patients with CBD.71,72 Of note, the accuracy of metabolic changes to distinguish among parkinsonian syndromes appears to be higher compared with dopaminergic imaging techniques, particularly in differentiating among the various APS.71
Alongside these regional analyses, PET has also been used to characterize functional brain networks at the whole-brain level. Principal component analysis (PCA), as a multivariate and purely data-driven analytic approach, renders possible the identification of such networks, and has been used extensively by our group and others to identify disease-specific abnormal functional brain networks in parkinsonian syndromes.73,74 Indeed, distinct brain networks specific to PD, MSA, and PSP have been identified using PCA (▶Fig. 2).73,75–78 Tang et al have shown recently that these networks are very useful in the differential diagnosis of parkinsonian syndromes.79 In this study, 167 patients with parkinsonian symptoms, but uncertain clinical diagnoses underwent FDG PET. Final clinical diagnosis was made by movement disorder specialists after an average follow-up time of 2.6 years. Image-based classification for idiopathic Parkinson disease had 84% sensitivity and 97% specificity. The PCA algorithm yielded similar accuracy in the identification of MSA (85% sensitivity, 96% specificity) and PSP patients (88% sensitivity, 94% specificity).
Cardiac Imaging
Autonomic dysfunction including cardiovascular dysautonomia is common in PD, and cardiac sympathetic denervation is present in the vast majority of patients. Loss of visceral sympathetic nerve function can be attributed to Lewy body inclusions in the cardiac plexus, and may antecede the manifestation of motor symptoms by several years.80
A variety of radiotracers, such as 123I-metaiodobenzylguanidine (MIBG), have been developed to visualize sympathetic cardiac denervation. A large body of studies has shown decreased cardiac MIBG uptake in patients with PD.81 Although autonomic dysfunction is also common in PSP and even more so in MSA, sympathetic innervation has been reported to be largely normal in these patients.82,83 That said, the utility of cardiac imaging in the differential diagnosis of parkinsonian syndromes has been discussed controversially. Some authors have reported excellent accuracy of MIBG PET to separate APS from PD,84 whereas others have found that MIBG uptake can be reduced in some APS patients, and a normal MIBG SPECT does not rule out PD.81 Indeed, a recent meta-analysis including 391 parkinsonian patients revealed that abnormal MIBG myocardial SPECT had a high sensitivity (87.7%), but unsatisfactory specificity (37.4%) to detect PD.85
Functional Imaging in Prodromal Parkinson Disease
As pointed out earlier, it is of great importance to initiate potentially disease-modifying therapies at the earliest possible stage of PD when evaluating these treatments. In recent years, it has become increasingly recognized that nonmotor symptoms can emerge many years before the onset of motor symptoms in PD. Rapid-eye-movement (REM) sleep behavior disorder (RBD) is a case in point. About 28 to 45% of patients with RBD go on to develop a parkinsonian syndrome within 5 years of diagnosis. Even higher conversion rates have been reported after 10 years.4
Consequently, several studies have explored dopaminergic integrity in individuals with idiopathic RBD, consistently reporting reduced striatal dopamine levels in these individuals.86,87 Moreover, reduced striatal tracer uptake in RBD patients is a risk factor for the subsequent development of a parkinsonian syndrome.88 Recently, we have found that the PD-related metabolic network (▶Fig. 2A) is abnormally expressed in individuals with RBD.89 In addition, high network expression was predictive of the subsequent development of PD and dementia with Lewy bodies in these subjects. Of note, network expression also separated RBD patients that were eventually diagnosed with MSA from converters to PD, indicating that metabolic network imaging might be useful in the very early differential diagnosis of parkinsonian syndromes before motor symptoms emerge.
Conclusions
The diagnosis of PD and APS relies on the clinical exam. Although imaging techniques hitherto available have to be regarded as adjunct to clinical diagnosis, promising findings from recent studies support a significant role of neuroimaging in the differential diagnosis of parkinsonism.
The role of conventional structural MRI is largely limited to the exclusion of structural lesions causing secondary parkinsonism; DWI/DTI has higher sensitivity to discern subtle microstructural changes than conventional MRI, and has shown some potential in the separation of APS from PD. Studies employing novel MRI techniques such as H1-MRSI and MTI have provided promising results, but larger prospective studies are required to determine the merits of these techniques in the differential diagnosis of parkinsonian syndromes. Dopaminergic imaging is useful in differentiating PD and APS from essential tremor, but its utility in distinguishing PD from APS is limited.
Both structural and functional imaging, and particularly network-based metabolic imaging, has enhanced our understanding of parkinsonian disorders as systemic diseases causing widespread alterations of whole-brain circuitry. Functional alterations occur early on in the disease course, and highly specific networks have been identified to separate PD from APS. Given the need to correctly diagnose PD as early as possible, future research focusing on the premotor stage of PD is encouraged, and might open new avenues for the use of neuroimaging when clinical motor exam is yet unrevealing.
Fig. 2.
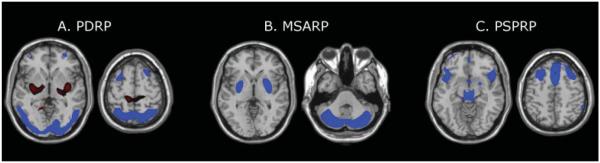
Disease-related spatial covariance patterns. (A) Parkinson disease-related pattern (PDRP) identified by spatial covariance analysis of 18F-fluorodeoxyglucose positron emission tomography (FDG PET) scans from 33 patients with Parkinson disease and 33 age-matched normal volunteer subjects. This pattern was characterized by relative metabolic increases (red) in the pallidum and thalamus, in the pons and cerebellum, and in the sensorimotor cortex. These changes covaried with metabolic decreases (blue) in the lateral premotor cortex and in parieto-occipital association regions. (Adapted from Ma et al. Abnormal metabolic network activity in Parkinson’s disease: test-rest reproducibility. J Cereb Blood Flow Metab 2007;27:597–605.) (B) Multiple system atrophy-related pattern (MSARP) identified by spatial covariance analysis of FDG PET scans from 10 patients with multiple system atrophy and 10 healthy controls. This pattern was characterized by covarying metabolic decreases (blue) in the putamen and the cerebellum. (Adapted with permission from Eckert et al. Abnormal Metabolic Networks in Atypical Parkinsonism. Mov Disord 2008;23:727–733.) (C) Progressive supranuclear palsy-related pattern (PSPRP) identified by spatial covariance analysis of FDG PET scans from 10 patients with progressive supranuclear palsy and 10 healthy controls. This pattern was characterized by covarying metabolic decreases (blue) in the medial prefrontal cortex, the frontal eye fields, the ventrolateral prefrontal cortex, the caudate nuclei, the medial thalamus, and the upper brainstem. (Adapted with permission from Eckert et al. Abnormal Metabolic Networks in Atypical Parkinsonism. Mov Disord 2008;23:727–733.) (The covariance maps were overlaid on T1-weighted magnetic resonance-template images. Voxels with positive region weights [metabolic increases] are color-coded red and those with negative region weights [metabolic decreases] are color-coded blue.)
Acknowledgments
This work was supported in part by the National Institute of Neurological Disorders and Stroke Morris K. Udall Center of Excellence for Parkinson’s Disease Research at The Feinstein Institute for Medical Research (P50 NS071675 to D.E.). Dr. Eidelberg has received research support from the NIH (NINDS, NIDCD, NIAID), High Q Foundation, the Dana Foundation, and the Bachmann-Strauss Dystonia and Parkinson Foundation; and has served as scientific advisor for the Thomas Hartman Foundation for Parkinson’s Research, Inc., and as a consultant for Pfizer. The authors wish to thank Ms. Yoon Young Choi for assistance in manuscript preparation.
Footnotes
Issue Theme Atypical Parkinsonian Disorders; Guest Editors, Yvette Bordelon, MD, PhD, and Carlos Portera-Cailliau, MD, PhD
References
- 1.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litvan I, Booth V, Wenning GK, et al. Retrospective application of a set of clinical diagnostic criteria for the diagnosis of multiple system atrophy. J Neural Transm. 1998;105(2-3):217–227. doi: 10.1007/s007020050050. [DOI] [PubMed] [Google Scholar]
- 3.Respondek G, Roeber S, Kretzschmar H, et al. Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov Disord. 2013;28(4):504–509. doi: 10.1002/mds.25327. [DOI] [PubMed] [Google Scholar]
- 4.Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27(5):617–626. doi: 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- 5.Kalra S, Grosset DG, Benamer HT. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: a systematic review. Mov Disord. 2010;25(2):149–156. doi: 10.1002/mds.22937. [DOI] [PubMed] [Google Scholar]
- 6.Massey LA, Micallef C, Paviour DC, et al. Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy. Mov Disord. 2012;27(14):1754–1762. doi: 10.1002/mds.24968. [DOI] [PubMed] [Google Scholar]
- 7.Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3(2):93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz J, Weis S, Kraft E, et al. Signal changes on MRI and increases in reactive microgliosis, astrogliosis, and iron in the putamen of two patients with multiple system atrophy. J Neurol Neurosurg Psychiatry. 1996;60(1):98–101. doi: 10.1136/jnnp.60.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5(1):75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 10.Quattrone A, Nicoletti G, Messina D, et al. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246(1):214–221. doi: 10.1148/radiol.2453061703. [DOI] [PubMed] [Google Scholar]
- 11.Paviour DC, Price SL, Jahanshahi M, Lees AJ, Fox NC. Regional brain volumes distinguish PSP, MSA-P, and PD: MRI-based clinico-radiological correlations. Mov Disord. 2006;21(7):989–996. doi: 10.1002/mds.20877. [DOI] [PubMed] [Google Scholar]
- 12.Messina D, Cerasa A, Condino F, et al. Patterns of brain atrophy in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Parkinsonism Relat Disord. 2011;17(3):172–176. doi: 10.1016/j.parkreldis.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Kashihara K, Shinya T, Higaki F. Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson’s disease. J Clin Neurosc. 2011;18:1093–1096. doi: 10.1016/j.jocn.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler DA, Wonderlick JS, Ashourian P, et al. Substantia nigra volume loss before basal forebrain degeneration in early Parkinson disease. JAMA Neurol. 2013;70(2):241–247. doi: 10.1001/jamaneurol.2013.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesse S, Kassubek J, Müller HP, Ludolph AC, Unrath A. Signal alterations of the basal ganglia in the differential diagnosis of Parkinson’s disease: a retrospective case-controlled MRI data bank analysis. BMC Neurol. 2012;12:163. doi: 10.1186/1471-2377-12-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gröschel K, Hauser TK, Luft A, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21(2):714–724. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Tang-Wai DF, Edland SD, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004;61(12):1881–1884. doi: 10.1001/archneur.61.12.1881. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 19.Pereira JB, Ibarretxe-Bilbao N, Marti MJ, et al. Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp. 2012;33(11):2521–2534. doi: 10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78(3):254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biundo R, Formento-Dojot P, Facchini S, et al. Brain volume changes in Parkinson’s disease and their relationship with cognitive and behavioural abnormalities. J Neurol Sci. 2011;310(1-2):64–69. doi: 10.1016/j.jns.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Ramírez-Ruiz B, Martí MJ, Tolosa E, et al. Longitudinal evaluation of cerebral morphological changes in Parkinson’s disease with and without dementia. J Neurol. 2005;252(11):1345–1352. doi: 10.1007/s00415-005-0864-2. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Shao N, Li J, Shang H. Voxelwise meta-analysis of white matter abnormalities in progressive supranuclear palsy. Neurol Sci. 2014;35(1):7–14. doi: 10.1007/s10072-013-1512-8. [DOI] [PubMed] [Google Scholar]
- 24.Shi HC, Zhong JG, Pan PL, et al. Gray matter atrophy in progressive supranuclear palsy: meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34(7):1049–1055. doi: 10.1007/s10072-013-1406-9. [DOI] [PubMed] [Google Scholar]
- 25.Hauser TK, Luft A, Skalej M, et al. Visualization and quantification of disease progression in multiple system atrophy. Mov Disord. 2006;21(10):1674–1681. doi: 10.1002/mds.21032. [DOI] [PubMed] [Google Scholar]
- 26.Minnerop M, Specht K, Ruhlmann J, et al. Voxel-based morphometry and voxel-based relaxometry in multiple system atrophy-a comparison between clinical subtypes and correlations with clinical parameters. Neuroimage. 2007;36(4):1086–1095. doi: 10.1016/j.neuroimage.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Shigemoto Y, Matsuda H, Kamiya K, et al. In vivo evaluation of gray and white matter volume loss in the parkinsonian variant of multiple system atrophy using SPM8 plus DARTEL for VBM. Neuroimage Clin. 2013;2:491–496. doi: 10.1016/j.nicl.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenneis C, Boesch SM, Egger KE, et al. Cortical atrophy in the cerebellar variant of multiple system atrophy: a voxel-based morphometry study. Mov Disord. 2006;21(2):159–165. doi: 10.1002/mds.20656. [DOI] [PubMed] [Google Scholar]
- 29.Brenneis C, Seppi K, Schocke MF, et al. Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord. 2003;18(10):1132–1138. doi: 10.1002/mds.10502. [DOI] [PubMed] [Google Scholar]
- 30.Boxer AL, Geschwind MD, Belfor N, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63(1):81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- 31.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29(2):280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CC, Chang YY, Chang WN, et al. Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol. 2009;16(10):1144–1150. doi: 10.1111/j.1468-1331.2009.02661.x. [DOI] [PubMed] [Google Scholar]
- 33.Mak E, Zhou J, Tan LC, Au WL, Sitoh YY, Kandiah N. Cognitive deficits in mild Parkinson’s disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2013-305805. [DOI] [PubMed] [Google Scholar]
- 34.Focke NK, Helms G, Scheewe S, et al. Individual voxel-based subtype prediction can differentiate progressive supranuclear palsy from idiopathic Parkinson syndrome and healthy controls. Hum Brain Mapp. 2011;32(11):1905–1915. doi: 10.1002/hbm.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price S, Paviour D, Scahill R, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson’s disease. Neuroimage. 2004;23(2):663–669. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Vaillancourt DE, Spraker MB, Prodoehl J, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80(9):857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicoletti G, Lodi R, Condino F, et al. Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain. 2006;129(Pt 10):2679–2687. doi: 10.1093/brain/awl166. [DOI] [PubMed] [Google Scholar]
- 39.Paviour DC, Thornton JS, Lees AJ, Jäger HR. Diffusion-weighted magnetic resonance imaging differentiates Parkinsonian variant of multiple-system atrophy from progressive supranuclear palsy. Mov Disord. 2007;22(1):68–74. doi: 10.1002/mds.21204. [DOI] [PubMed] [Google Scholar]
- 40.Baudrexel S, Seifried C, Penndorf B, et al. The value of putaminal diffusion imaging versus 18-fluorodeoxyglucose positron emission tomography for the differential diagnosis of the Parkinson variant of multiple system atrophy. Mov Disord. 2014;29(Suppl 3):380–387. doi: 10.1002/mds.25749. [DOI] [PubMed] [Google Scholar]
- 41.Tsukamoto K, Matsusue E, Kanasaki Y, et al. Significance of apparent diffusion coefficient measurement for the differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and Parkinson’s disease: evaluation by 3.0-T MR imaging. Neuroradiology. 2012;54(9):947–955. doi: 10.1007/s00234-012-1009-9. [DOI] [PubMed] [Google Scholar]
- 42.Erbetta A, Mandelli ML, Savoiardo M, et al. Diffusion tensor imaging shows different topographic involvement of the thalamus in progressive supranuclear palsy and corticobasal degeneration. AJNR Am J Neuroradiol. 2009;30(8):1482–1487. doi: 10.3174/ajnr.A1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griffiths PD, Crossman AR. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson’s disease and Alzheimer’s disease. Dementia. 1993;4(2):61–65. doi: 10.1159/000107298. [DOI] [PubMed] [Google Scholar]
- 44.Lehéricy S, Sharman MA, Dos Santos CL, Paquin R, Gallea C. Magnetic resonance imaging of the substantia nigra in Parkinson’s disease. Mov Disord. 2012;27(7):822–830. doi: 10.1002/mds.25015. [DOI] [PubMed] [Google Scholar]
- 45.Han YH, Lee JH, Kang BM, et al. Topographical differences of brain iron deposition between progressive supranuclear palsy and parkinsonian variant multiple system atrophy. J Neurol Sci. 2013;325(1-2):29–35. doi: 10.1016/j.jns.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Péran P, Cherubini A, Assogna F, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133(11):3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Han YH, Kang BM, Mun CW, Lee SJ, Baik SK. Quantitative assessment of subcortical atrophy and iron content in progressive supranuclear palsy and parkinsonian variant of multiple system atrophy. J Neurol. 2013;260(8):2094–2101. doi: 10.1007/s00415-013-6951-x. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Butros SR, Shuai X, et al. Different iron-deposition patterns of multiple system atrophy with predominant parkinsonism and idiopathetic Parkinson diseases demonstrated by phase-corrected susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2012;33(2):266–273. doi: 10.3174/ajnr.A2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis SJ, Shine JM, Duffy S, Halliday G, Naismith SL. Anterior cingulate integrity: executive and neuropsychiatric features in Parkinson’s disease. Mov Disord. 2012;27(10):1262–1267. doi: 10.1002/mds.25104. [DOI] [PubMed] [Google Scholar]
- 50.Nie K, Zhang Y, Huang B, et al. Marked N-acetylaspartate and choline metabolite changes in Parkinson’s disease patients with mild cognitive impairment. Parkinsonism Relat Disord. 2013;19(3):329–334. doi: 10.1016/j.parkreldis.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Weiduschat N, Mao X, Beal MF, Nirenberg MJ, Shungu DC, Henchcliffe C. Usefulness of proton and phosphorus MR spectroscopic imaging for early diagnosis of Parkinson’s disease. J Neuroimaging. 2013 doi: 10.1111/jon.12074. [DOI] [PubMed] [Google Scholar]
- 52.Gröger A, Bender B, Wurster I, Chadzynski GL, Klose U, Berg D. Differentiation between idiopathic and atypical parkinsonian syndromes using three-dimensional magnetic resonance spectroscopic imaging. J Neurol Neurosurg Psychiatry. 2013;84(6):644–649. doi: 10.1136/jnnp-2012-302699. [DOI] [PubMed] [Google Scholar]
- 53.Guevara CA, Blain CR, Stahl D, Lythgoe DJ, Leigh PN, Barker GJ. Quantitative magnetic resonance spectroscopic imaging in Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. Eur J Neurol. 2010;17(9):1193–1202. doi: 10.1111/j.1468-1331.2010.03010.x. [DOI] [PubMed] [Google Scholar]
- 54.Tedeschi G, Litvan I, Bonavita S, et al. Proton magnetic resonance spectroscopic imaging in progressive supranuclear palsy, Parkinson’s disease and corticobasal degeneration. Brain. 1997;120(Pt 9):1541–1552. doi: 10.1093/brain/120.9.1541. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe H, Fukatsu H, Katsuno M, et al. Multiple regional 1H-MR spectroscopy in multiple system atrophy: NAA/Cr reduction in pontine base as a valuable diagnostic marker. J Neurol Neurosurg Psychiatry. 2004;75(1):103–109. [PMC free article] [PubMed] [Google Scholar]
- 56.Morgen K, Sammer G, Weber L, et al. Structural brain abnormalities in patients with Parkinson disease: a comparative voxel-based analysis using T1-weighted MR imaging and magnetization transfer imaging. AJNR Am J Neuroradiol. 2011;32(11):2080–2086. doi: 10.3174/ajnr.A2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tambasco N, Belcastro V, Sarchielli P, et al. A magnetization transfer study of mild and advanced Parkinson’s disease. Eur J Neurol. 2011;18(3):471–477. doi: 10.1111/j.1468-1331.2010.03184.x. [DOI] [PubMed] [Google Scholar]
- 58.Eckert T, Sailer M, Kaufmann J, et al. Differentiation of idiopathic Parkinson’s disease, multiple system atrophy, progressive supranuclear palsy, and healthy controls using magnetization transfer imaging. Neuroimage. 2004;21(1):229–235. doi: 10.1016/j.neuroimage.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 59.Jokinen P, Helenius H, Rauhala E, Brück A, Eskola O, Rinne JO. Simple ratio analysis of 18F-fluorodopa uptake in striatal subregions separates patients with early Parkinson disease from healthy controls. J Nucl Med. 2009;50(6):893–899. doi: 10.2967/jnumed.108.061572. [DOI] [PubMed] [Google Scholar]
- 60.Antonini A, Moresco RM, Gobbo C, et al. The status of dopamine nerve terminals in Parkinson’s disease and essential tremor: a PET study with the tracer [11-C]FE-CIT. Neurol Sci. 2001;22(1):47–48. doi: 10.1007/s100720170040. [DOI] [PubMed] [Google Scholar]
- 61.Oh M, Kim JS, Kim JY, et al. Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med. 2012;53(3):399–406. doi: 10.2967/jnumed.111.095224. [DOI] [PubMed] [Google Scholar]
- 62.Burn DJ, Sawle GV, Brooks DJ. Differential diagnosis of Parkinson’s disease, multiple system atrophy, and Steele-Richardson-Olszewski syndrome: discriminant analysis of striatal 18F-dopa PET data. J Neurol Neurosurg Psychiatry. 1994;57(3):278–284. doi: 10.1136/jnnp.57.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghaemi M, Hilker R, Rudolf J, Sobesky J, Heiss WD. Differentiating multiple system atrophy from Parkinson’s disease: contribution of striatal and midbrain MRI volumetry and multi-tracer PET imaging. J Neurol Neurosurg Psychiatry. 2002;73(5):517–523. doi: 10.1136/jnnp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2013;84(11):1288–1295. doi: 10.1136/jnnp-2012-304436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plotkin M, Amthauer H, Klaffke S, et al. Combined 123I-FP-CIT and 123I-IBZM SPECT for the diagnosis of parkinsonian syndromes: study on 72 patients. J Neural Transm. 2005;112(5):677–692. doi: 10.1007/s00702-004-0208-x. [DOI] [PubMed] [Google Scholar]
- 66.Antonini A, Leenders KL, Vontobel P, et al. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson’s disease. Brain. 1997;120(Pt 12):2187–2195. doi: 10.1093/brain/120.12.2187. [DOI] [PubMed] [Google Scholar]
- 67.Van Laere K, Clerinx K, D’Hondt E, de Groot T, Vandenberghe W. Combined striatal binding and cerebral influx analysis of dynamic 11C-raclopride PET improves early differentiation between multiple-system atrophy and Parkinson disease. J Nucl Med. 2010;51(4):588–595. doi: 10.2967/jnumed.109.070144. [DOI] [PubMed] [Google Scholar]
- 68.Vlaar AM, de Nijs T, Kessels AG, et al. Diagnostic value of 123I-ioflupane and 123I-iodobenzamide SPECT scans in 248 patients with parkinsonian syndromes. Eur Neurol. 2008;59(5):258–266. doi: 10.1159/000115640. [DOI] [PubMed] [Google Scholar]
- 69.Broussolle E, Dentresangle C, Landais P, et al. The relation of putamen and caudate nucleus 18F-Dopa uptake to motor and cognitive performances in Parkinson’s disease. J Neurol Sci. 1999;166(2):141–151. doi: 10.1016/s0022-510x(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 70.Brück A, Aalto S, Rauhala E, Bergman J, Marttila R, Rinne JO. A follow-up study on 6-[18F]fluoro-L-dopa uptake in early Parkinson’s disease shows nonlinear progression in the putamen. Mov Disord. 2009;24(7):1009–1015. doi: 10.1002/mds.22484. [DOI] [PubMed] [Google Scholar]
- 71.Hellwig S, Amtage F, Kreft A, et al. [18F]FDG-PET is superior to [123I] IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology. 2012;79(13):1314–1322. doi: 10.1212/WNL.0b013e31826c1b0a. [DOI] [PubMed] [Google Scholar]
- 72.Zhao P, Zhang B, Gao S. 18F-FDG PET study on the idiopathic Parkinson’s disease from several parkinsonian-plus syndromes. Parkinsonism Relat Disord. 2012;18(Suppl 1):S60–S62. doi: 10.1016/S1353-8020(11)70020-7. [DOI] [PubMed] [Google Scholar]
- 73.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32(10):548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holtbernd F, Eidelberg D. Functional brain networks in movement disorders: recent advances. Curr Opin Neurol. 2012;25(4):392–401. doi: 10.1097/WCO.0b013e328355aa94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eckert T, Tang C, Ma Y, et al. Abnormal metabolic networks in atypical parkinsonism. Mov Disord. 2008;23(5):727–733. doi: 10.1002/mds.21933. [DOI] [PubMed] [Google Scholar]
- 76.Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14(5):783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 77.Poston KL, Tang CC, Eckert T, et al. Network correlates of disease severity in multiple system atrophy. Neurology. 2012;78(16):1237–1244. doi: 10.1212/WNL.0b013e318250d7fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson’s disease: test-retest reproducibility. J Cereb Blood Flow Metab. 2007;27(3):597–605. doi: 10.1038/sj.jcbfm.9600358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang CC, Poston KL, Eckert T, et al. Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol. 2010;9(2):149–158. doi: 10.1016/S1474-4422(10)70002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis. 2012;46(3):572–580. doi: 10.1016/j.nbd.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rascol O, Schelosky L. 123I-metaiodobenzylguanidine scintigraphy in Parkinson’s disease and related disorders. Mov Disord. 2009;24(Suppl 2):S732–S741. doi: 10.1002/mds.22499. [DOI] [PubMed] [Google Scholar]
- 82.Yoshita M. Differentiation of idiopathic Parkinson’s disease from striatonigral degeneration and progressive supranuclear palsy using iodine-123 meta-iodobenzylguanidine myocardial scintigraphy. J Neurol Sci. 1998;155(1):60–67. doi: 10.1016/s0022-510x(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 83.Braune S, Reinhardt M, Schnitzer R, Riedel A, Lücking CH. Cardiac uptake of [123I]MIBG separates Parkinson’s disease from multiple system atrophy. Neurology. 1999;53(5):1020–1025. doi: 10.1212/wnl.53.5.1020. [DOI] [PubMed] [Google Scholar]
- 84.Courbon F, Brefel-Courbon C, Thalamas C, et al. Cardiac MIBG scintigraphy is a sensitive tool for detecting cardiac sympathetic denervation in Parkinson’s disease. Mov Disord. 2003;18(8):890–897. doi: 10.1002/mds.10461. [DOI] [PubMed] [Google Scholar]
- 85.Nagayama H, Hamamoto M, Ueda M, Nagashima J, Katayama Y. Reliability of MIBG myocardial scintigraphy in the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2005;76(2):249–251. doi: 10.1136/jnnp.2004.037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisensehr I, Linke R, Noachtar S, Schwarz J, Gildehaus FJ, Tatsch K. Reduced striatal dopamine transporters in idiopathic rapid eye movement sleep behaviour disorder. Comparison with Parkinson’s disease and controls. Brain. 2000;123(Pt 6):1155–1160. doi: 10.1093/brain/123.6.1155. [DOI] [PubMed] [Google Scholar]
- 87.Iranzo A, Valldeoriola F, Lomeña F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol. 2011;10(9):797–805. doi: 10.1016/S1474-4422(11)70152-1. [DOI] [PubMed] [Google Scholar]
- 88.Iranzo A, Lomeña F, Stockner H, et al. Sleep Innsbruck Barcelona (SINBAR) group Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected] Lancet Neurol. 2010;9(11):1070–1077. doi: 10.1016/S1474-4422(10)70216-7. [DOI] [PubMed] [Google Scholar]
- 89.Holtbernd F, Gagnon JF, Postuma RB, et al. Abnormal metabolic network activity in REM sleep behavior disorder. Neurology. 2014;82(7):620–627. doi: 10.1212/WNL.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]


