Abstract
BACKGROUND
Most women with breast cancer who undergo breast-conserving surgery receive whole-breast irradiation. We examined whether the addition of regional nodal irradiation to whole-breast irradiation improved outcomes.
METHODS
We randomly assigned women with node-positive or high-risk node-negative breast cancer who were treated with breast-conserving surgery and adjuvant systemic therapy to undergo either whole-breast irradiation plus regional nodal irradiation (including internal mammary, supraclavicular, and axillary lymph nodes) (nodal-irradiation group) or whole-breast irradiation alone (control group). The primary outcome was overall survival. Secondary outcomes were disease-free survival, isolated locoregional disease-free survival, and distant disease-free survival.
RESULTS
Between March 2000 and February 2007, a total of 1832 women were assigned to the nodal-irradiation group or the control group (916 women in each group). The median follow-up was 9.5 years. At the 10-year follow-up, there was no significant between-group difference in survival, with a rate of 82.8% in the nodal-irradiation group and 81.8% in the control group (hazard ratio, 0.91; 95% confidence interval [CI], 0.72 to 1.13; P = 0.38). The rates of disease-free survival were 82.0% in the nodal-irradiation group and 77.0% in the control group (hazard ratio, 0.76; 95% CI, 0.61 to 0.94; P = 0.01). Patients in the nodal-irradiation group had higher rates of grade 2 or greater acute pneumonitis (1.2% vs. 0.2%, P = 0.01) and lymphedema (8.4% vs. 4.5%, P = 0.001).
CONCLUSIONS
Among women with node-positive or high-risk node-negative breast cancer, the addition of regional nodal irradiation to whole-breast irradiation did not improve overall survival but reduced the rate of breast-cancer recurrence.
Many women with early-stage breast cancer undergo breast-conserving surgery followed by whole-breast irradiation, which reduces the rate of local recurrence.1-3 Radiotherapy to the chest wall and regional lymph nodes, termed regional nodal irradiation, which is commonly used after mastectomy in women with node-positive breast cancer who are treated with adjuvant systemic therapy, reduces locoregional and distant recurrence and improves overall survival.4-6 An unanswered question is whether the addition of regional nodal irradiation to whole-breast irradiation after breast-conserving surgery has the same effect. Whole-breast irradiation may involve irradiation of the lower axillary and internal mammary lymph nodes.7 However, regional nodal irradiation increases the complexity of radiotherapy and may increase the risks of pneumonitis, lymphedema, cardiac disease, and late secondary neoplasms. In the NCIC Clinical Trials Group MA.20 trial, we compared whole-breast irradiation plus regional nodal irradiation with whole-breast irradiation alone in women with early-stage breast cancer who were treated with breast-conserving surgery and adjuvant systemic therapy.
METHODS
PATIENTS
Eligible patients were women with invasive carcinoma of the breast who were treated with breast-conserving surgery and sentinel-lymph-node biopsy or axillary-node dissection and who had positive axillary lymph nodes or negative axillary nodes with high-risk features. Such features were defined as a primary tumor measuring 5 cm or more or 2 cm or more with fewer than 10 axillary nodes removed and at least one of the following: grade 3 histologic categorization, estrogen-receptor (ER) negativity, or lymphovascular invasion. A level I or II axillary dissection was required for patients with positive results on sentinel-node biopsy. All patients received adjuvant systemic therapy with chemotherapy, endocrine therapy, or both.
Patients were excluded if they had T4 tumors (clinical evidence of direct extension to chest wall or skin) or N2–3 nodes (involvement of axillary nodes that are fixed or of internal mammary nodes), distant metastasis, or serious nonmalignant disease (e.g., cardiovascular or pulmonary) that would preclude definitive radiation therapy. Other exclusion criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Potentially eligible patients underwent chest radiography and liver-function testing. A bone scan and abdominal ultrasonography or computed tomography (CT) were performed if abnormal results were identified. Written informed consent was obtained from patients before randomization. The study protocol was approved by the institutional review board at each participating center and is available at NEJM.org.
RANDOMIZATION AND TREATMENT REGIMENS
Before randomization, patients were stratified according to the number of axillary nodes that were removed (<10 or ≥10), the number of positive axillary nodes (0, 1 to 3, or >3), the type of chemo-therapy (anthracycline-containing, other, or none), hormonal therapy (yes or no), and treatment center. With the use of a centralized minimization procedure, the NCIC Clinical Trials Group office in Kingston, Ontario, randomly assigned patients to undergo either whole-breast irradiation plus regional nodal irradiation (nodal-irradiation group) or whole-breast irradiation alone (control group).8
For patients in the control group who were assigned to undergo whole-breast irradiation alone, the breast was treated with a pair of opposed fields tangentially arranged across the chest. A dose of 50 Gy in 25 fractions was prescribed. Other radiotherapy planning details are provided in the Supplementary Appendix.
For patients in the nodal-irradiation group, the intention was to treat the breast at risk and the ipsilateral internal mammary lymph nodes in the upper three intercostal spaces, along with the supraclavicular and axillary lymph nodes. Two techniques for irradiation of the internal mam-mary lymph nodes were permitted: a modified wide-tangent technique and a technique involving a separate internal-mammary-node field plus tangents. For the modified wide-tangent technique, the upper tangent fields were widened to include the internal mammary nodes and narrowed inferiorly to reduce the dose to the underlying lung and heart. For the technique involving a separate internal-mammary-node field, a mixed electron and photon field was angled to match the tangent fields. CT planning was recommended, with the internal mammary node volume defined as 1 cm around internal mammary vessels in the first three intercostal spaces to be covered by at least the 80% isodose. Wedges, compensators, or intensity-modulated radiation therapy were permitted to ensure a uniform dose throughout the target volume. Dose correction for the different densities of tissues irradiated (e.g., lung) was permissible.
The supraclavicular and level III axillary nodes were treated with a nondivergent anterior field to include the head of the clavicle medially and the coracoid process laterally. The inferior border was matched to the superior field of the breast tangents, and the superior border included the supraclavicular fossa. For patients who had fewer than 10 axillary nodes removed or more than 3 positive axillary nodes, the lateral aspect of the field was extended laterally to include the level I and II axillary lymph nodes, and it was recommended that a nondivergent posterior field should match the anterior field or that a smaller patch field be used to cover the axilla. Patients were treated with 4 to 18 MV in the supine position with the arm abducted.
The dose to the breast and internal mammary node fields was 50 Gy in 25 fractions. The dose to the anterior supraclavicular and axillary field was 50 Gy in 25 fractions prescribed at a depth of 3 cm. For patients who were treated with anterior and posterior fields, a dose of 45 Gy in 25 fractions was prescribed at midseparation at the center of the fields. Treatment beam images were obtained to confirm adequate coverage. Boost radiation of 10 to 16 Gy in 5 to 8 fractions to the tumor bed with the use of external-beam radiation or brachytherapy was permitted in the two study groups according to the institution's policy (e.g., for close margins of excision or a young age).
We used a quality-assurance program that included a review of radiation plans before treatment for the first 25 patients who underwent randomization at each participating center and after treatment for all other patients. (Details are provided in the Supplementary Appendix.)
Adjuvant chemotherapy according to institutional practice was delivered before radiation. Endocrine therapy with tamoxifen or an aroma-tase inhibitor was administered either concurrently with or after radiotherapy. After June 2005, trastuzumab was recommended for patients with human epidermal growth factor receptor 2 (HER2)–positive disease.
FOLLOW-UP AND OUTCOMES
Patients were followed according to a prescribed schedule (see the Supplementary Appendix). Adverse events were assessed with the use of the National Cancer Institute Common Toxicity Criteria, version 2. Data with respect to quality of life, which was assessed with the use of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and cosmetic and arm-function modules that were developed for the trial, are not included in this report.
The primary outcome was overall survival, which was defined as the time from randomization to death from any cause. Prespecified secondary outcomes were disease-free survival, isolated locoregional disease-free survival, distant disease-free survival, and toxicity. Disease-free survival was defined as the time from randomization to the time of a first recurrence in the ipsilateral breast or in nodal or distant sites, a contralateral breast cancer, or death from breast cancer. Isolated locoregional disease-free survival was defined as the time from randomization to the time of a first recurrence in the ipsilateral breast or in axillary, supraclavicular, or internal mammary nodes without evidence of distant disease for 1 month. Distant disease-free survival was defined as the time from randomization to the time of a recurrence at a distant site (e.g., bone, liver, lung, or central nervous system) or death from breast cancer.
STATISTICAL ANALYSIS
The study was designed to detect a hazard ratio of 0.73 for overall survival, which corresponded to an improvement of 5 percentage points (from 80% to 85%) in 5-year survival. We determined that 312 deaths among 1832 patients would provide a power of 80% to detect this hazard ratio, using a two-sided alpha level of 0.05 and assuming a 4-year accrual period with 3 years of follow-up. A planned interim analysis was performed after the occurrence of 170 deaths and was presented at the 2011 Annual Meeting of the American Society of Clinical Oncology.9
The final analysis was performed in the intention-to-treat population. We used the Kaplan–Meier method to describe the survival experiences of patients in the two study groups. Details with respect to data censoring are provided in the Supplementary Appendix. Stratified log-rank tests that were adjusted for the stratification factors excluding the study center were used to compare the study groups. Likelihood ratio tests of treatment according to covariate interactions were used to examine the heterogeneity of the treatment effect according to prespecified subgroups of the four stratification factors, status with respect to ER positivity and progesterone-receptor (PR) positivity, and tumor location. In a post hoc analysis, we estimated the rate of death from breast cancer by using the cumulative incidence function and Gray's test for competing risks for the between-group comparison. The corresponding hazard ratio was estimated with the use of the Fine–Gray model. In a similar manner, we conducted a sensitivity analysis for isolated locoregional and distant disease-free survival after adjustment for competing risks.
All patients who underwent irradiation were included in the safety analysis, according to the treatment they received. Fisher's exact test was used to compare toxic effects in the two groups. A P value of less than 0.05 was considered to indicate statistical significance, with no adjustment for multiple testing. All analyses were conducted with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
Between March 2000 and February 2007, a total of 1832 patients were enrolled, with 916 assigned to each study group. Among patients in the control group who were assigned to receive whole-breast irradiation, 5 received whole-breast and regional nodal irradiation and 3 received no radiation. Among patients in the nodal-irradiation group, 19 received whole-breast irradiation alone and 9 received no radiation. Thirty-five patients (1.9%) withdrew consent, and 37 (2.0%) were lost to follow-up (Fig. S1 in the Supplementary Appendix). The median follow-up at the time of this analysis was 9.5 years.
The characteristics of the patients at baseline were similar in the two study groups (Table 1). Most patients (99%) had tumors that were categorized as either T1 (measuring ≤2 cm) or T2 (measuring >2 cm but ≤5 cm), had one to three positive axillary nodes (85%), and had ER-positive disease (75%). Most of the patients received combination chemotherapy (91%) with an anthracycline (86%) or with a taxane (26%), along with endocrine therapy (76%). A modified wide-tangent approach was used in 80% of patients in the nodal-irradiation group.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | WBI (N = 916) | WBI+RNI (N = 916) |
|---|---|---|
| Median age (range) — yr | 53 (26-84) | 54 (29-84) |
| Patients who underwent initial sentinel-lymph-node biopsy — no. (%)† | 357 (39.0) | 360 (39.3) |
| No. of axillary nodes removed | ||
| Median (interquartile range) | 12 (8-16) | 12 (9-16) |
| 1-9 — no. (%) | 303 (33.1) | 294 (32.1) |
| ≥10 — no. (%) | 612 (66.8) | 622 (67.9) |
| No. of positive axillary nodes — no. (%) | ||
| 0 | 89 (9.7) | 88 (9.6) |
| 1 | 447 (48.8) | 460 (50.2) |
| 2 | 233 (25.4) | 209 (22.8) |
| 3 | 100 (10.9) | 109 (11.9) |
| >3 | 47 (5.1) | 50 (5.5) |
| Tumor size — no. (%) | ||
| ≤2 cm | 501 (54.7) | 459 (50.1) |
| 2.1-5 cm | 409 (44.7) | 443 (48.4) |
| >5 cm | 6 (0.7) | 13 (14) |
| Estrogen-receptor status — no. (%) | ||
| Positive | 682 (74.5) | 685 (74.8) |
| Negative | 234 (25.5) | 231 (25.2) |
| Progesterone-receptor status — no. (%) | ||
| Positive | 549 (59.9) | 553 (60.4) |
| Negative | 365 (39.8) | 360 (39.3) |
| Adjuvant chemotherapy — no. (%) | ||
| Anthracycline without taxane | 540 (59.0) | 554 (60.5) |
| Anthracycline with taxane | 244 (26.6) | 230 (25.1) |
| Other‡ | 45 (4.9) | 47 (5.1) |
| No chemotherapy | 87 (9.5) | 85 (9.3) |
| Adjuvant endocrine therapy — no. (%)§ | ||
| Aromatase inhibitor¶ | 529 (57.8) | 521 (56.9) |
| Tamoxifen | 167 (18.2) | 172 (18.8) |
| No endocrine therapy | 220 (24.0) | 223 (24.3) |
| Boost irradiation — no. (%)∥ | 317 (34.6) | 294 (32.1) |
There were no significant differences between the groups at baseline. Additional details regarding the baseline characteristics of the patients are provided in Table S1 in the Supplementary Appendix. WBI denotes whole-breast irradiation alone (control group), and WBI+RNI whole-breast irradiation plus regional nodal irradiation.
A total of 35 patients in WBI group and 33 patients in the WBI+RNI group underwent only sentinel-lymph-node biopsy.
Other types of chemotherapy included cyclophosphamide, methotrexate, and fluorouracil (CMF).
Endocrine therapy was initiated after radiation therapy in some patients.
In these patients, an aromatase inhibitor was used alone or after previous receipt of tamoxifen.
Boost irradiation was delivered after WBI or WBI+RNI.
MORTALITY
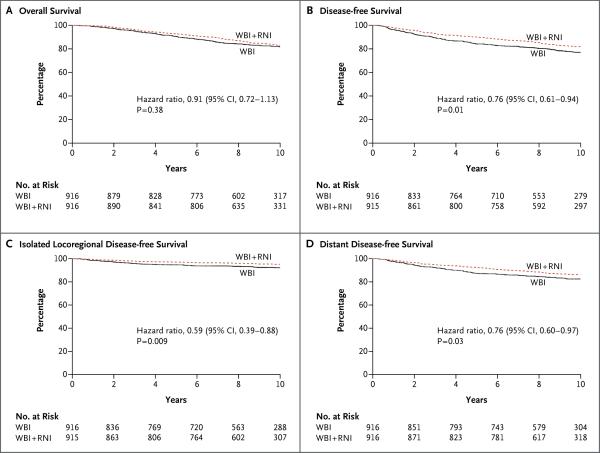
The data for recurrences and deaths are provided in Table 2. There was no significant between-group difference in overall survival, with 10-year rates of survival of 82.8% in the nodal-irradiation group and 81.8% in the control group (hazard ratio for death in the nodal-irradiation group as compared with the control group, 0.91; 95% confidence interval [CI], 0.72 to 1.13; P = 0.38) (Fig. 1A). In a prespecified subgroup analysis, patients with ER-negative disease in the nodal-irradiation group had a higher 10-year rate of overall survival than did patients in the control group (81.3% vs. 73.9%), a difference that approached statistical significance (hazard ratio, 0.69; 95% CI, 0.47 to 1.00; P = 0.05). The test for interaction was 0.08 (Fig. S2 in the Supplementary Appendix).
Table 2.
Disease Recurrence or Death.
| Event | WBI (N = 916) | WBI+RNI (N = 916) |
|---|---|---|
| no. of patients with event (%) | ||
| Isolated locoregional recurrence | 62 (6.8) | 39 (4.3) |
| Local (in breast) only | 38 (4.1) | 33 (3.6) |
| Regional only | 23 (2.5)* | 5 (0.5)† |
| Local and regional | 1 (0.1)* | 1 (0.1)† |
| Distant recurrence | 151 (16.5) | 118 (12.9) |
| First or concurrent with locoregional recurrence | 118 (12.9) | 100 (10.9) |
| After locoregional recurrence | 33 (3.6) | 18 (2.0) |
| Any recurrence or contralateral breast cancer | 195 (21.3) | 154 (16.8) |
| Any recurrence | 175 (19.1) | 134 (14.6) |
| Contralateral breast cancer | 20 (2.2) | 20 (2.2) |
| Death | 168 (18.3) | 155 (16.9) |
| Breast cancer | 113 (12.3) | 93 (10.2) |
| Other cancer | 26 (2.8) | 32 (3.5) |
| Cardiovascular cause | 11 (1.2) | 11 (12) |
| Other cause | 12 (1.3) | 8 (0.9) |
| Unknown | 6 (0.7) | 11 (12) |
Included among regional recurrences were 14 involving axillary nodes, 8 involving supraclavicular nodes, 1 involving infraclavicular nodes, and 1 involving multiple sites. The 10-year cumulative rate of regional recurrence was 2.7%.
Included among regional recurrences were 5 involving axillary nodes and 1 involving multiple sites. The 10-year cumulative rate of regional recurrence was 0.7%.
Figure 1. 10-Year Kaplan–Meier Estimates of Survival.
Shown are rates of overall survival (Panel A), disease-free survival (Panel B), isolated locoregional disease-free survival (Panel C), and distant disease-free survival (Panel D) among patients who underwent whole-breast irradiation plus regional nodal irradiation (WBI + RNI) and those who underwent whole-breast irradiation alone (WBI, control group).
No significant difference was detected in breast-cancer mortality, with 10-year rates of 10.3% in the nodal-irradiation group and 12.3% in the control group (hazard ratio, 0.80; 95% CI, 0.61 to 1.05; P = 0.11) (Fig. S3 in the Supplementary Appendix). The causes of non–breast-cancer deaths were similar in the two groups (Table 2).
RECURRENCE
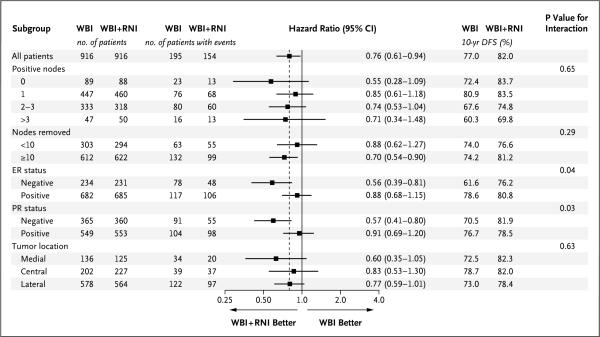
The rate of disease-free survival was higher in the nodal-irradiation group than in the control group, with 10-year rates of 82.0% and 77.0%, respectively (hazard ratio, 0.76; 95% CI, 0.61 to 0.94; P = 0.01) (Fig. 1B). In prespecified subgroup analyses, the treatment effects were greater for patients with ER-negative or PR-negative tumors than for those with hormone receptor–positive tumors (Fig. 2). There were no major treatment differences according to other factors.
Figure 2. Disease-free Survival at 10 Years, According to Subgroup.
Shown are hazard ratios and rates of disease-free survival among patients who underwent whole-breast irradiation plus regional nodal irradiation (WBI+RNI) and those who underwent whole-breast irradiation alone (WBI, control group). The dashed vertical line at 0.76 in dicates the overall hazard ratio estimate. Hazard ratios are shown on a logarithmic scale. DFS denotes disease-free survival, ER estrogen receptor, and PR progesterone receptor.
The 10-year rates of isolated locoregional disease-free survival were 95.2% in the nodal-irradiation group and 92.2% in the control group (hazard ratio, 0.59; 95% CI, 0.39 to 0.88; P = 0.009) (Fig. 1C). The treatment effect was associated mainly with a reduction in the rate of regional recurrence (Table 2). The majority of regional treatment failures included the axillary nodes (in 63% of patients) or the supraclavicular nodes (in 27%). The rates of distant disease-free survival at 10 years were 86.3% in the nodal-irradiation group and 82.4% in the control group (hazard ratio, 0.76; 95% CI, 0.60 to 0.97; P = 0.03) (Fig. 1D). The improvements in isolated locoregional and distant disease-free survival in the nodal-irradiation group were similar when the study groups were compared with the use of competing-risks analyses (Fig. S4 and S5 in the Supplementary Appendix).
ADVERSE EVENTS
Table 3 summarizes selected potential radiation-related adverse events of grade 2 or higher. Grade 4 toxic effects were rare, and no grade 5 events were reported. For acute events (those occurring ≤3 months after the completion of radiation), significant increases in the rates of radiation dermatitis and pneumonitis were reported in the nodal-irradiation group. For delayed events (those occurring >3 months after the completion of radiation), there were significant increases in the rates of lymphedema, telangiectasia of the skin, and subcutaneous fibrosis in the nodal-irradiation group. No increases in rates of brachial neuropathy, cardiac disease, or secondary cancers were observed in the nodal-irradiation group, but the period of follow-up was not sufficiently long to rule out a difference in the rate of secondary cancers.
Table 3.
Adverse Events of Grade 2 or Higher.*
| Adverse Event | WBI (N = 927) | WBI+RNI (N = 893) | P Value† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Total | Grade 2 | Grade 3 | Grade 4 | Total | ||
| no. of patients with event (%) | |||||||||
| Acute | |||||||||
| Fatigue | 156 | 13 | 0 | 169 (18.2) | 154 | 16 | 0 | 170 (19.0) | 0.67 |
| Pain‡ | 35 | 5 | 0 | 40 (4.3) | 46 | 7 | 0 | 53 (5.9) | 0.14 |
| Pneumonitis§ | 2 | 0 | 0 | 2 (0.2) | 11 | 0 | 0 | 11 (1.2) | 0.01 |
| Radiation dermatitis | 349 | 23 | 0 | 372 (40.1) | 397 | 45 | 0 | 442 (49.5) | <0.001 |
| Delayed | |||||||||
| Cardiac¶ | 2 | 2 | 0 | 4 (0.4) | 0 | 6 | 2 | 8 (0.9) | 0.26 |
| Lymphedema∥ | 38 | 4 | 0 | 42 (4.5) | 65 | 10 | 0 | 75 (8.4) | 0.001 |
| Neuropathy** | 16 | 1 | 0 | 17 (1.8) | 16 | 5 | 1†† | 22 (2.5) | 0.42 |
| Pneumonitis or fibrosis§‡‡ | 2 | 1 | 0 | 3 (0.3) | 4 | 0 | 0 | 4 (0.4) | 0.72 |
| Joint | 12 | 2 | 0 | 14 (1.5) | 21 | 0 | 0 | 21 (2.4) | 0.23 |
| Skin§§ | 38 | 2 | 0 | 40 (4.3) | 51 | 11 | 0 | 62 (6.9) | 0.02 |
| Subcutaneous tissue | 19 | 0 | 0 | 19 (2.0) | 34 | 3 | 0 | 37 (4.1) | 0.01 |
| Second cancer¶¶ | NA | NA | NA | 93 (10.0) | NA | NA | NA | 98 (11.0) | 0.54 |
Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0. Events were considered to be acute if they occurred within 3 months after the completion of radiation and delayed if they occurred more than 3 months after completion. NA denotes not applicable.
P values were calculated by means of Fisher's exact test for the between-group comparison of the proportion of patients with an adverse event of grade 2 or higher.
Listed are events that were attributed to radiation.
Grade 2 events were radiographic changes requiring the use of glucocorticoids or diuretics; grade 3 events were radiographic changes and those requiring the use of oxygen.
Cardiac events included left ventricular failure, cardiac ischemia or infarction, supraventricular arrhythmia, and pericardial effusion.
Grade 2 events were moderate lymphedema requiring compression; grade 3 events were severe lymphedema limiting function.
Neuropathy includes both sensory and motor events.
One patient was reported to have transient grade 4 motor neuropathy in the ipsilateral arm.
Included in this category are two patients with pulmonary fibrosis in the WBI+RNI group.
Included in this category are late radiation changes such as atrophy or telangiectasia.
Excluded from this category are skin and contralateral breast cancers.
DISCUSSION
In this study, we evaluated the addition of regional nodal irradiation to whole-breast irradiation in patients treated with breast-conserving surgery and adjuvant systemic therapy. No improvement was detected with respect to the primary outcome of overall survival. Most of the study patients had no more than three positive lymph nodes. It is likely that patients with more than three nodes were routinely treated off trial with regional nodal irradiation, which would potentially decrease the probability of detecting a significant effect on overall survival in this trial.10
In addition, since the majority of patients were treated with contemporary multiagent chemotherapy containing anthracyclines or taxanes and endocrine therapy, the baseline risk of death and the power to detect a between-group improvement in overall survival were probably further reduced.
On the other hand, the addition of regional nodal irradiation to whole-breast irradiation significantly increased relative disease-free survival by 24%, which was an absolute improvement of 5 percentage points at 10 years. The benefit reflected reductions in both isolated locoregional and distant recurrences. The relative reduction of 40% in the rate of locoregional recurrence that was associated with regional nodal irradiation was anticipated on the basis of reported trials of postmastectomy radiotherapy. The relative reduction of 24% in the rate of distant recurrences with regional nodal irradiation was probably due to the reduction in regional nodal recurrence and possibly to the reduction of subclinical regional nodal disease.11,12
In patients with breast cancer, distant metastasis typically leads to death. However, we did not observe an improvement in overall survival at 10 years. In the recent meta-analysis by the Early Breast Cancer Trialists’ Collaborative Group of postmastectomy radiotherapy in node-positive patients, for every 1.5 recurrences (either locoregional or distant) that were prevented during the first 10 years after radiation, one breast-cancer death was prevented at 20 years.13
The observed effects on overall and distant recurrence are consistent with the results of the EORTC 22922-10925 trial, in which patients with node-positive breast cancer or node-negative disease with central or medial tumors were randomly assigned to undergo regional nodal irradiation that included the medial supraclavicular and internal mammary nodes or no regional nodal irradiation after breast-conserving surgery or mastectomy.14 At a median follow-up of 10.9 years, regional nodal irradiation was associated with improvements in disease-free and distant disease-free survival. In our study, it is not clear which sites of nodal irradiation (internal mammary, supraclavicular, level III axillary, or all three) led to improvements in disease-free survival. All areas are at risk for residual disease after surgery, but the EORTC trial suggests that irradiation of the internal mammary nodes is important.
Although subgroup analyses were prespecified, they were generally not adequately powered to assess the benefit of treatment in different subgroups. Furthermore, the P values of the subgroup analyses were not adjusted for multiple testing.15 Patients with ER-negative or PR-negative tumors appeared to benefit more from regional nodal irradiation than those with ER-positive or PR-positive tumors. Although this effect was not observed in previous trials of postmastectomy radiotherapy,16 it supports the hypothesis that further research on the molecular characterization of the primary tumor may identify patients who are more likely to benefit from regional nodal irradiation.17,18 Since the number of node-negative patients in our trial was relatively small, the application of our results to node-negative patients is unclear. In addition, at the time of our study, the size of nodal metastasis was not routinely measured, so it is difficult to generalize our findings to patients with micrometastases.
Our study was an international effort involving more than 1800 patients from Canada, the United States, and Australia. Adherence to treatment was high, and relatively few patients were lost to follow-up. The trial was conducted with contemporary CT radiotherapy planning to optimize the quality and safety of treatment and with a rigorous radiotherapy quality-assurance process, including pretreatment reviews of radiation plans, to confirm technical protocol compliance. These approaches potentially contributed to the limited radiation-related toxicity that was observed. At a median follow-up of nearly 10 years, there were no increases in cardiac morbidity and mortality or in rates of death from other causes associated with regional nodal irradiation. We observed a near doubling (absolute increase, 4 percentage points) in the rate of lymphedema among patients who were treated with regional nodal irradiation, a finding that was consistent with that of previous trials of locoregional therapy.4,19 The rate of acute radiation pneumonitis was also significantly higher in the nodal-irradiation group (absolute increase, 1 percentage point), although the condition was uncommon in the two groups. It remains too early to assess the influence of the additional radiotherapy on heart disease and second cancers.
When we designed our trial, there was no plan to adjust the P value for multiple comparisons of secondary outcomes in the event that the primary outcome was not significant. Inferences on the observed treatment effects for secondary outcomes in our analysis may be less robust because of the issue of multiple testing.20
Axillary dissection occurred in 96% of patients in our trial. Increasingly, sentinel-lymph-node biopsy without axillary dissection is being performed in patients with sentinel-node–positive disease on the basis of the results of the American College of Surgeons Oncology Group Z0011 trial.21 In the absence of a randomized trial evaluating the addition of regional nodal irradiation to whole-breast irradiation in patients with positive results on sentinel-lymph-node biopsy without axillary dissection, we feel that it is reasonable to speculate that the results of our study may be applicable to such patients, since many of the cancer-containing axillary nodes are removed. In addition, in the Z0011 trial, the recurrence rates were similar regardless of whether an axillary dissection was performed.
In conclusion, the addition of regional nodal irradiation to whole-breast irradiation after breast-conserving surgery in women with node-positive or high-risk node-negative breast cancer did not improve overall survival but did reduce breast-cancer recurrence. Our findings indicate the importance of basing treatment decisions on a careful discussion of the potential benefits and risks with each patient.
Supplementary Material
Acknowledgments
Supported by grants from the Canadian Cancer Society Research Institute to the NCIC Clinical Trials Group (021039 and 015469), the Canadian Breast Cancer Research Initiative (010415), and the U.S. National Cancer Institute (CA077202, CA32102, and CA27057) and the Cancer Council of Victoria, New South Wales, Queensland, and South Australia (288720). Dr. Whelan is a recipient of a Canada Research Chair.
Dr. Whelan reports receiving fees for serving on an advisory board from Genomic Health and testing reagents for another study from NanoString Technologies; and Dr. Pritchard, receiving fees for serving on advisory boards from AstraZeneca, Pfizer, Roche, Amgen, Novartis, GlaxoSmithKline, and Eisai, consulting fees from Pfizer and Novartis, lecture fees from Novartis, and grant support from AstraZeneca, Pfizer, Roche, Novartis, and Eisai. No other potential conflict of interest relevant to this article was reported.
We thank the women with breast cancer who participated in this trial; and acknowledge Dr. Veronique Benk, a coinvestigator in the trial, who died during the conduct of the study.
Footnotes
(Funded by the Canadian Cancer Society Research Institute and others; MA.20 ClinicalTrials.gov number, NCT00005957.)
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–73. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328:1587–91. doi: 10.1056/NEJM199306033282202. [DOI] [PubMed] [Google Scholar]
- 3.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: an update. J Natl Cancer Inst. 1996;88:1659–64. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 4.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–62. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–55. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 6.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–8. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 7.Recht A, Siddon RL, Kaplan WD, Andersen JW, Harris JR. Three-dimensional internal mammary lymphoscintigraphy: implications for radiation therapy treat ment planning for breast carcinoma. Int J Radiat Oncol Biol Phys. 1988;14:477–81. doi: 10.1016/0360-3016(88)90263-5. [DOI] [PubMed] [Google Scholar]
- 8.Pocock SJ. Clinical trials: a practical approach. John Wiley and Sons; New York: 1983. [Google Scholar]
- 9.Whelan TJ, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29(Suppl):LBA1003. [Google Scholar]
- 10.Ceilley E, Jagsi R, Goldberg S, et al. Radiotherapy for invasive breast cancer in North America and Europe: results of a survey. Int J Radiat Oncol Biol Phys. 2005;61:365–73. doi: 10.1016/j.ijrobp.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 11.Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107:379–87. doi: 10.1007/s10549-007-9561-4. [DOI] [PubMed] [Google Scholar]
- 12.Veronesi U, Arnone P, Veronesi P, et al. The value of radiotherapy on metastatic internal mammary nodes in breast cancer: results on a large series. Ann Oncol. 2008;19:1553–60. doi: 10.1093/annonc/mdn183. [DOI] [PubMed] [Google Scholar]
- 13.McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poortmans P, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–27. doi: 10.1056/NEJMoa1415369. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine — reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 16.Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–26. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 17.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28:1677–83. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voduc KD, Cheang MCU, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 19.Højris I, Andersen J, Overgaard M, Overgaard J. Late treatment-related morbidity in breast cancer patients randomized to postmastectomy radiotherapy and systemic treatment versus systemic treatment alone. Acta Oncol. 2000;39:355–72. doi: 10.1080/028418600750013131. [DOI] [PubMed] [Google Scholar]
- 20.Cook R, Farewell VT. Multiplicity considerations in the design and analysis of clinical trials. J R Stat Soc Ser A Stat Soc. 1996;159:93–110. [Google Scholar]
- 21.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.