Abstract
Background
Early life psychobiological and psychosocial factors play a key role in influencing child health outcomes. Longitudinal studies may help elucidate the relevant risk and resilience profiles, and the underlying mechanisms that impact on child health, but there is a paucity of birth cohort data from low and middle-income countries (LMIC). We describe the rationale for and present baseline findings from the psychosocial component of the Drakenstein Child Health Study (DCHS).
Methods
We review the psychosocial measures used in the DCHS, a multidisciplinary birth cohort study in a peri-urban area in South Africa, and provide initial data on psychological distress, depression, substance use, and exposure to traumatic stressors and intimate partner violence (IPV). These and other measures will be assessed longitudinally in mothers in order to investigate associations with child neurodevelopmental and health outcomes.
Results
Baseline psychosocial data is presented for mothers (n = 634) and fathers (n = 75) who have completed antenatal assessments to date. The sample of pregnant mothers is characterized by multiple psychosocial risk factors, including a high prevalence of psychological distress and depression, high levels of substance use, and high exposure to traumatic stressors and IPV.
Discussion
These data are consistent with prior South African studies which have documented a high prevalence of a multitude of risk factors during pregnancy. Further longitudinal assessment of mothers and children may clarify the underlying psychobiological and psychosocial mechanisms which impact on child health, and so inform clinical and public health interventions appropriate to the South African and other LMIC contexts.
Keywords: birth cohort, child health, depression, substance use, intimate partner violence, South Africa
Background
There is growing evidence that early life events have a profound impact on later health outcomes (Gluckman et al., 2008 and Heim et al., 2010). The first few years of life constitute a critical period, during which psychobiological and psychosocial factors may influence not only developmental outcomes, but also lifelong health trajectories (Anda et al., 2006 and Maggi et al., 2010). Examples of psychosocial risk factors include low socioeconomic status (SES) during childhood; psychological distress and exposure to stressors during pregnancy and the postnatal period; and an adverse early family environment (Taylor, 2010 and Kingston et al., 2012). The underlying mechanisms which account for these associations are also increasingly understood (Gluckman et al., 2008 and Heim et al., 2010).
Early psychobiological and psychosocial risk factors may impact a range of organs, including the nervous and respiratory systems, and parental psychological factors may influence both biological and psychological infant outcomes. Maternal prenatal anxiety, for example, has been associated with infant respiratory illness (Beijers et al., 2010), while perinatal depression has been associated with infant lower respiratory tract infections (LRTI) (Ban et al., 2010). Use of nicotine (Hollams et al., 2014 and Stocks et al., 2013) and alcohol (Stocks et al., 2013) during pregnancy have been associated with both reduced lung function and detrimental neurodevelopmental outcomes, with the underlying mechanisms being partially elucidated. Associations have also been found between intimate partner violence (IPV) and child or adolescent behavioral problems (Carter et al., 2010 and Flach et al., 2011).
The majority of such work has, however, taken place in high-income countries, where only a relatively small percentage of the world's population lives. Psychobiological and psychosocial risk factor profiles in low and middle-income countries (LMICs) differ from those of developed countries. In LMICs, maternal depression and exposure to violence may be more prevalent, and there is a higher prevalence of low birthweight, childhood under-nutrition, and infectious disease, and such differences may be associated with lifetime health trajectories (Walker et al., 2007 and Walker et al., 2011). Still, there is a paucity of data from LMICs, and much remains unknown about risk and protective factors within these contexts – particularly about how multiple risk factors intersect to impact on developmental and health outcomes (Lund, 2014).
Given the paucity of longitudinal birth cohort data in LMICs, we have undertaken the Drakenstein Child Health Study (DCHS), a multidisciplinary early life study investigating the determinants of child health, including childhood respiratory function and infant neurodevelopmental outcomes, in two peri-urban communities in the Western Cape Province of South Africa (Zar et al., 2014). The study investigates the role and interaction of risk factors in the environmental, infectious, nutritional, genetic, psychosocial, maternal and immunological spheres, and is one of the first birth cohort studies globally to investigate multiple risk factors for child health. Recruitment from two communities characterized by substantially different psychosocial risk factors will enable a comparison of the impact of such factors on child health and development.
The methods of the larger DCHS study have been described elsewhere (Zar et al., 2014). This paper describes the design and methods of the psychosocial component of the study specifically, and provides baseline psychosocial findings from pregnant women and fathers enrolled in the cohort to date. Given the prevalence and potential impact of parental psychosocial risk factors in these communities and in other LMIC populations, this paper provides data which will potentially be useful in understanding the effects of a range of risk factors on infant health and developmental outcomes both locally and worldwide.
Methods
Design
The birth cohort design recruits pregnant women attending one of two primary health care clinics in the Drakenstein sub-district of the Cape Winelands, Western Cape, South Africa – Mbekweni (serving a black African population) and TC Newman (serving a mixed race population). Consenting mothers are enrolled during pregnancy, and mother–infant dyads are followed longitudinally until children reach 5 years of age. Mothers are asked to request that the father of the index pregnancy attend a single antenatal study visit. Follow-up visits for mother–infant dyads take place at the two primary health care clinics and at Paarl Hospital.
Setting
The Drakenstein sub-district is a peri-urban area which includes the town of Paarl, and is located 60 km outside of Cape Town, South Africa. This is a stable, accessible, low socioeconomic community that is characterized by a high prevalence of a range of risk factors such as alcohol abuse (Watt et al., 2014), tobacco smoke exposure, HIV, single-parent households and poverty; as well as a high burden of childhood diseases, including infant pneumonia. In this respect, it is representative of many peri-urban regions in South Africa, as well as in other LMICs. The majority of the population accesses health care in the public sector.
Participants
Pregnant women are eligible to participate if they are 18 years or older, are accessing one of the two primary health care clinics for antenatal care, state no intention to move out of the district within the following year, and sign written informed consent (the study was approved by the Faculty of Health Sciences, Human Research Ethics Committee, University of Cape Town (401/2009), by Stellenbosch University (N12/02/0002), and by the Western Cape Provincial Health Research committee (2011RP45)). Participants are enrolled between 20 and 28 weeks’ gestation, upon presenting for an initial antenatal care visit. In addition, consenting fathers of the index pregnancy are enrolled in the study and attend a single antenatal study visit.
Sample size
The projected sample size for the cohort is 1000 mother–infant dyads. This sample size is designed to provide at least 550 pneumonia episodes, as a primary aim of the DCHS is to investigate risk factors for childhood pneumonia. This sample will provide adequate statistical power to detect relative associations of at least 1.5-fold for prevalent risk factors in the cohort, taking into account an estimated cumulative attrition of 20% over 5 years (including losses due to child mortality). Given potential loss to follow-up between enrolment and birth, approximately 1150 women will be enrolled in the cohort in order to achieve this target sample. At the time of writing, enrolment is ongoing.
Measures
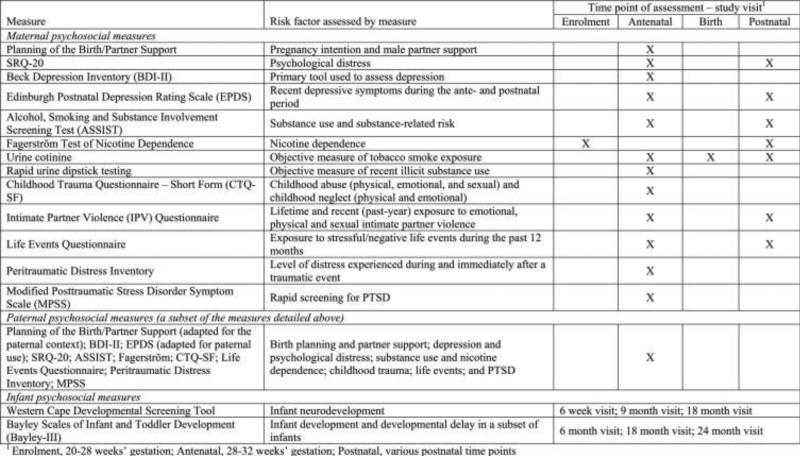
After providing consent, participants are asked to complete a battery of self-report and clinician-administered measures at a number of antenatal and postnatal study visits (see Fig. 1), as described below. These measures aim to assess maternal psychosocial risk factors over time, paternal psychosocial risk factors antenatally, and infant development. This combination of methods will allow the exploration of a range of risk factors and their underlying mechanisms, and will enable a comparison of their impact on child health and developmental outcomes. As such, the DCHS is one of the first birth cohort studies globally to investigate multiple risk factors for child neurodevelopment. Although the majority of measures were originally designed to be self-administered, questionnaires are administered by trained fieldworkers in the participants’ preferred language of English, Afrikaans, or isiXhosa, as literacy levels are low in this context. Translation of measures from English to Afrikaans and isiXhosa was done using a standard forward and back-translation process (Smit et al., 2006).
Figure 1.
Table of antenatal and perinatal psychosocial measures
Sociodemographic characteristics
Following an initial enrolment assessment, sociodemographic characteristics are assessed longitudinally. The interviewer-administered sociodemographic questionnaire designed for the purposes of this study was adapted from items used in the South African Stress and Health Study (SASH) (Myer et al., 2008), and includes an assessment of employment status, highest level of education completed, and participants’ population group. Race classification was historically required under the apartheid system in South Africa. While we do not intend to reify these categories, information on population group is collected in this study, as persistent health disparities exist across groups in South Africa, and disparities in child health and development related to ethnicity and SES are well-documented worldwide (Cheng and Goodman, 2015).
Planning of pregnancy and partner support
The Planning of the Birth/Partner Support Questionnaire was developed for the purposes of this. The questionnaire assesses pregnancy intention and support received from a male partner. Partner support and ability to rely on a male partner for help are assessed on a frequency scale ranging from 1 (“not at all”) to 5 (“extremely”), with higher scores indicating greater support and partner reliability.
Psychological distress, depression severity, and psychiatric disorders
The SRQ-20 (Beusenberg and Orley, 1994) is a WHO-endorsed measure of psychological distress. This tool has been widely used internationally and in South African settings, and has shown good reliability and face validity (Harpham et al., 2003 and Rumble et al., 1996). The SRQ-20 consists of 20 items which assess non-psychotic symptoms, including symptoms of depressive and anxiety disorders. Each item is scored according to whether the participant responds in the affirmative (scored as 1) or negative (scored as 0) to the presence of a symptom. Individual items are summed to generate a total score. A cut-off score of ≥8 can be used to dichotomize participants into “high risk” versus “low risk”, as has been widely used elsewhere (Harpham et al., 2003 and Ventevogel et al., 2007).
The Beck Depression Inventory (BDI-II) is a widely-used and reliable measure of depressive symptoms (Beck et al., 1961, Beck et al., 1988 and Beck et al., 1996). The BDI-II has shown good validity and internal consistency when used in both psychiatric and non-psychiatric populations (Beck et al., 1988 and Beck et al., 1996) and has been used in numerous studies conducted in South Africa (Kagee et al., 2014, Kagee and Martin, 2010 and Nel and Kagee, 2013). The BDI-II comprises 21 items, each of which assesses the severity of a symptom of major depression. Each item is assessed on a severity scale ranging from 0 (absence of symptoms) to 3 (severe, often with functional impairment). A total score is then obtained by summing individual item responses, with a higher score indicative of more severe depressive symptoms. A cut-off score of ≥20 has been used to dichotomize participants into “probable moderate/severe clinical cases” versus “probable sub-threshold participants” (Lasa et al., 2000).
The Edinburgh Postnatal Depression Rating Scale (EPDS) (Cox et al., 1987) is a 10-item self-report measure of recent depressive symptoms. The tool was originally developed for use in postnatal women, and assesses the presence of mood changes characteristic of postnatal depression, under the assumption that normal symptoms experienced during the perinatal period (for example, changes in sleep and appetite) could be misattributed to a depressive disorder on many standard screening tools for depression (Cox et al., 1987). This tool has been validated for use in pregnancy (Murray and Cox, 1990), and is used alongside the BDI-II to measure depression in this study. Each item is scored on a frequency scale ranging from 0 to 3. A total score is then obtained by summing individual item responses, with a higher score indicative of more severe depressive symptoms. A cut-off score of ≥13 has been used to indicate probable depression, as described in the original development of the scale (Cox et al., 1987) and in South Africa (Hartley et al., 2011).
The Mini International Neuropsychiatric Interview (MINI) is an abridged version of the Structured Clinical Interview for DSM-IV (Lecrubier et al., 1997, Sheehan et al., 1997 and Sheehan et al., 1998). This clinician-administered interview provides diagnoses of a range of common mental disorders, and shows good psychometric properties (Lecrubier et al., 1997, Sheehan et al., 1997 and Sheehan et al., 1998). The MINI is administered to a sample of participants, partly in order to obtain data on the clinical validity of measures such as the SRQ-20 and BDI-II in our sample, and partly to study women with PTSD in more detail. As nested sub-studies, these protocols and analyses will be reported elsewhere.
Substance use
The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) (WHO ASSIST Working Group, 2002) is a tool that was developed by the WHO to detect and manage substance use among people attending primary health care services. It has shown good reliability and validity in international, multi-site studies (Humeniuk et al., 2008 and WHO ASSIST Working Group, 2002). The tool assesses substance use and substance-related risk across 10 categories (tobacco, alcohol, cannabis, cocaine, amphetamine-type stimulants, inhalants, sedatives/sleeping pills, hallucinogens, opioids, and other substances), as well as enquiring about a history of intravenous drug use. Total scores are obtained for each substance by summing individual item responses, with a higher score indicative of greater risk for substance-related health problems. Scores of 0–10 for alcohol and 0–3 for illicit drugs have been used to indicate that a participant is at low risk for substance-related health problems from their current pattern of use; scores of 11–26 for alcohol and 4–26 for illicit drugs to indicate moderate risk; and scores >26 to indicate that a participant is at high risk of experiencing severe problems as a result of their current pattern of use and is likely to be dependent (World Health Organization, 2010).
The Fagerström Test for Nicotine Dependence is a 6-item tool that has been widely-used and provides a rapid assessment of nicotine dependence (Fagerström et al., 1990 and Heatherton et al., 1991). It is used in this study to supplement the findings of the tobacco category of the ASSIST. Individual items are scored based on the original development of the scale (Heatherton et al., 1991) and are summed to generate a total score, with a higher score indicative of greater nicotine dependence. Participants are then categorized according to this level of dependence, with scores of 1–2 indicating low dependence; scores of 3–4 indicating low-moderate dependence; scores of 5–7 indicating moderate dependence; and scores of 8 or above indicating high dependence.
Objective measures of substance use are also included in the DCHS. Maternal urine cotinine is measured antenatally and at the time of birth; and for infants at birth, 6–10 weeks, annually, and at the time of pneumonia episodes, as an objective measure of tobacco smoke exposure. In addition, maternal urine samples are tested antenatally via rapid urine dipstick testing for recent use of common illicit substances, including methamphetamines, cocaine, cannabis, methaqualone (locally referred to as mandrax), opiates, and MDMA.
Exposure to trauma and posttraumatic stress disorder
The Childhood Trauma Questionnaire–Short Form (CTQ-SF) (Bernstein et al., 1994) is a 28-item inventory assessing childhood abuse (physical, emotional, and sexual) and childhood neglect (physical and emotional) occurring up until the age of 12 years. Each item is scored on a frequency scale from 1 (“never true”) to 5 (“very often true”), such that each subscale (domain of abuse or neglect) is scored on a spectrum from 5 (no history of abuse or neglect) to 25 (very extreme history of abuse or neglect). Thus, higher scores in each domain signify a greater severity of abuse or neglect. Participants can also be categorized according to the cut-off scores for each domain, as defined in the CTQ manual (Bernstein and Fink, 1998). Respondents may then be dichotomized into those with a history of childhood trauma (those scoring within the “low to moderate”, “moderate to severe”, or “severe to extreme” category) versus those without (those scoring within the “none or minimal” category). Three items are also included as a Minimization/Denial scale to detect potential under-reporting of abuse by participants. These items are dichotomized (with a response of “never” scored 0, and all other responses scored 1) and added, with a sum total ≥ 1 indicating possible under-reporting (Bernstein and Fink, 1998 and Villano et al., 2004).
The Intimate Partner Violence (IPV) Questionnaire used in this study was adapted from the WHO multi-country study (Jewkes, 2002) and the Women's Health Study in Zimbabwe (Shamu et al., 2011) in order to assess lifetime and recent (past-year) exposure to emotional, physical and sexual IPV. Each category of violence is assessed across multiple items measuring the frequency of a number of specified violent acts. Each item is scored using a frequency scale from 1 (“never”) to 4 (“many times”). Scoring guidelines were devised for the purposes of this study, and were based on prior work in South Africa (Dunkle et al., 2004). Participants are categorized as having experienced no IPV if all responses are “never”; an isolated incident of IPV if one response is “once”; a low frequency of violence if the response is “once” to more than one item; a mid frequency if they respond “a few times” to at least one item, but do not respond “many times” to any item; and a high frequency if there are any responses of “many times”. In addition, those who respond that the violence has occurred during the past 12 months are categorized as having experienced recent (past-year) violence.
The World Mental Health Life Events Questionnaire is a 17-item tool which assesses exposure to stressful/negative life events during the past 12 months. The questionnaire used in this study is based on the items used in the SASH study in South Africa (Myer et al., 2008). A total score is obtained by summing the total number of life events that participants report experiencing during the past 12 months, with higher scores indicating greater exposure to stressful life events.
The Peritraumatic Distress Inventory is a 13-item tool which assesses the level of distress experienced during and immediately after a traumatic event (Brunet et al., 2001). Each item assesses the extent to which a participant experienced a symptom of distress related to the event. Each item is scored using a frequency scale from 0 (“not at all”) to 4 (“extremely”). A total score is obtained by calculating the mean response across all 13 items, with higher scores indicating greater distress related to the traumatic event.
The Modified Posttraumatic Stress Disorder Symptom Scale (MPSS) (Foa et al., 1993) is a 17-item interview that mirrors the DSM-IV criteria for posttraumatic stress disorder (PTSD). The tool is used as a rapid screening for PTSD in this study, and was selected for use due to its reasonably good diagnostic validity for PTSD. The MPSS includes items assessing three symptom clusters for PTSD, namely re-experiencing, avoidance/emotional numbing, and increased arousal. Each item is scored on a frequency scale ranging from 0 (absence of symptom) to 3 (symptom occurs five or more times per week/very much/almost always). A final item was added to assess the duration of symptoms, with response options of <1 month; 1–3 months; 3 months–1 year; and >1 year. A proxy variable for PTSD diagnosis can be generated when re-experiencing symptoms total ≥ 1, avoidance/emotional numbing symptoms ≥ 3, increased arousal symptoms ≥ 2, and symptom duration is of at least 1 month.
The Clinician Administered PTSD Scale (CAPS) is an interviewer-administered diagnostic instrument which measures PTSD (Blake et al., 1990, Blake et al., 1995 and Weathers et al., 2001). The CAPS has been shown to have excellent psychometric properties (Blake et al., 1990, Blake et al., 1995 and Weathers et al., 2001), and is administered to those who score above threshold for PTSD on the MINI in this study, in order to assess PTSD symptom severity .
Paternal psychosocial measures
A subset of the measures described above is additionally completed by fathers enrolled in the study at one antenatal visit. Specifically, fathers complete the measures related to birth planning and partner support; psychological distress and depression; childhood trauma; life events; self-reported substance use and nicotine dependence; and PTSD, using the MPSS and Peritraumatic Distress Inventory.
Infant development
The Western Cape Developmental Screening Questionnaire is administered to caregivers of the infants at 6 weeks, 9 months and 18 months of age. The screening tools were developed as part of a Western Cape Government Department of Health provincial primary health care screening program that aims to identify children with moderate and severe neurodevelopmental disabilities for referral to health services for further formal evaluation and management. Each age-specific tool consists of yes/no questions pertaining to the acquisition of developmental milestones in the domains of hearing, vision, gross motor, fine motor, language and communication and psychosocial development. The tools were designed to be administered to children's caregivers by primary health care workers in a clinical setting during routine immunization visits. Prior to the screening program implementation during 2001, the tools were piloted in provincial primary health care settings, including the Drakenstein sub-district. Domains within the 9-month and 18-month screening tools have been validated. In the present study, infants who are identified by the screening tools as being at-risk for neurodevelopmental problems are referred for further assessment via the accepted child health service pathways for the sub-district.
The Bayley Scales of Infant and Toddler Development (Bayley-III) is a comprehensive assessment tool for measuring infant development and assessing developmental delay in children aged 0–2 years (Bayley, 2006). This tool is a gold-standard measure of infant development globally, and has been widely used in LMIC settings, including South Africa (Ballot et al., 2012). In the DCHS, the Bayley-III is administered by a trained professional on a subset of infants at ages 6 months and 2 years in order to assess childhood development over time. The tool consists of five scales of infant development, namely cognitive, language, motor, socio-emotional, and adaptive behavior, and assesses development using direct observation of the infant as well as caregiver report. Each scale is scored according to the Bayley-III manual (Bayley, 2006) using specialized software (Bayley-III Scoring Assistant Update Version 2.0.2 with Bayley-III PDA conduit). Scaled scores for each subscale are calculated using age-specific reference norms, and a cut-off score of 1 or more standard deviations below the mean in any of the subscales has been used to identify infants with poor developmental outcomes.
Nested studies of mother–infant interactions and of infant brain imaging are being conducted. However, as these only include a sub-sample of participants, they are not detailed or reported here.
Results
Enrolment into the DCHS cohort began in March 2012, with approximately 950 women enrolled to date. As fewer than 40% of participants report that they are currently in a stable relationship, the number of fathers enrolled in the study is far lower (less than 10% of the total number of mothers enrolled). Complete baseline psychosocial data is available and presented below for 634 mothers, and 75 fathers.
Sociodemographic characteristics
Baseline maternal sociodemographic characteristics are presented in Table 1. The median age of mothers at enrolment was 25.6 years (inter-quartile range: 21.8–30.6). Education and employment levels are low at both clinics, with less than half of participants having completed secondary education, and only a quarter reporting that they are currently employed. A minority of participants (39%) reported that they are currently married or cohabiting.
Table 1.
Maternal baseline sociodemographic characteristics, pregnancy planning and partner
| Variable | Mbekweni n (%) | TC Newman n (%) | Total n (%) | P-valuea |
|---|---|---|---|---|
| Number of mothers | 317 (50) | 317 (50) | 634 (100) | |
| Median age at enrollment (IQR) | 6.7 (22.0-31.7) | 24.7 (21.4-29.0) | 25.6 (21.8-30.6) | <0.001 |
| Race | ||||
| Black/African | 317 (100) | 7 (2) | 324 (51) | |
| White | 0 (0) | 3 (1) | 3 (0.5) | |
| Mixed race | 0 (0) | 305 (96) | 305 (48) | |
| Other | 0 (0) | 2 (0.6) | 2 (0.3) | |
| Married/Cohabitating | 113 (36) | 133 (42) | 246 (39) | 0.103 |
| Educational attainment | ||||
| Primary | 34 (11) | 22 (7) | 56 (9) | 0.111 |
| Some secondary | 168 (53) | 158 (50) | 326 (51) | |
| Completed secondary | 97 (31) | 122 (38) | 219 (35) | |
| Any tertiary | 18 (6) | 15 (5) | 33 (5) | |
| Currently employed | 61 (19) | 97 (31) | 158 (25) | 0.001 |
| Primigravida | 103 (32) | 126 (40) | 229 (36) | 0.057 |
| Unplanned pregnancy | 219 (69) | 196 (62) | 415 (65)0.055 | 0.055 |
| Partner support | ||||
| Not at all/slightly supportive | 31 (10) | 30 (9) | 61 (10) | <0.001 |
| Moderately supportive | 52 (16) | 13 (4) | 65 (10) | |
| Extremely supportive | 234 (74) | 274 (86) | 508 (80) | |
| Reliance on partner for help | ||||
| Not at all/not very often | 39 (12) | 31 (10) | 70 (11) | <0.001 |
| Some of the time | 102 (32) | 17 (5) | 119 (19) | |
| Most of the time/all of the time | 176 (56) | 269 (85) | 445 (70) |
Variables compared across site using χ2 tests for categorical variables and Wilcoxon rank sum tests (Mann-Whitney tests) for continuous variables
Pregnancy planning and partner support
Self-report information regarding pregnancy planning and support received from a male partner is presented in Table 1. The majority of participants (64%) had previously been pregnant, and a high proportion (65%) reported that the current pregnancy was unplanned. Most participants reported high levels of male partner support and reliance on their partners for help.
Psychological distress and depression
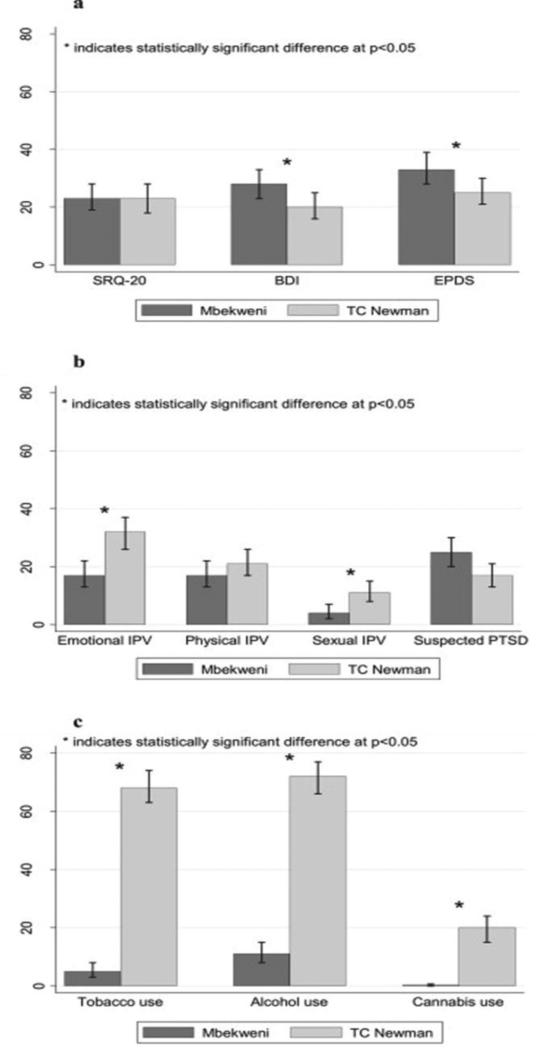
Reported rates of psychological distress and depression are high in this population (Table 2; Fig. 2a). Many participants at both clinics (n = 74, 23% and n = 72, 23%) scored above threshold scores for psychological distress. Overall, 24% of women scored above threshold scores for depression on the BDI-II, with the prevalence of depression significantly higher at Mbekweni (28%) than at TC Newman (20%).
Table 2.
Maternal psychological distress and depression; exposure to trauma and PTSD
| Variable | Mbekweni n (%) | TC Newman n (%) | Total n (%) | P-valuea |
|---|---|---|---|---|
| Psychological distress and depression | ||||
| SQR-20 – median score (IQR) | 4 (1-7) | 4 (2-7) | 4 (2-7) | 0.042 |
| Beck Depression Inventory – median score (IQR) | 12 (6–21) | 10 (5–18) | 11 (5–19) | 0.018 |
| Edinburgh Postnatal Depression Rating Scale – median score (IQR) | 11 (8–13) | 8 (4–13) | 10 (6–13) | <0.001 |
|
Exposure to trauma and PTSD | ||||
| Childhood trauma | ||||
| Emotional neglect – above threshold | 82 (26) | 123 (39) | 205 (32) | <0.001 |
| Physical neglect – above threshold | 111 (35) | 112 (35) | 223 (35) | 0.934 |
| Emotional abuse – above threshold | 71 (22) | 130 (41) | 201 (32) | <0.001 |
| Physical abuse – above threshold | 82 (26) | 57 (18) | 139 (22) | 0.016 |
| Sexual abuse – above threshold | 56 (18) | 57 (18) | 113 (18) | 0.917 |
| Lifetime Intimate partner violence (IPV) | ||||
| Emotional IPV – above threshold | 83 (26) | 145 (46) | 228 (36) | <0.001 |
| Physical IPV – above threshold | 87 (27) | 121 (38) | 208 (33) | 0.004 |
| Sexual IPV – above threshold | 24 (8) | 45 (14) | 69 (11) | 0.007 |
| Life events – median (IQR) | 1 (0 – 3) | 2 (1 – 4) | 2 (0 – 3) | 0.0001 |
| Peritraumatic Distress Inventory – median (IQR) | 1.2 (0.5 – 1.8) | 1.2 (0.5 – 1.8) | 1.2 (0.5 – 1.8) | 0.668 |
Variables compared across site using χ2 tests for categorical variables and Wilcoxon rank sum tests (Mann-Whitney tests) for continuous variables
Fig. 2.
(a) Prevalence of maternal psychological distress and depression across recruitment site. (b) Prevalence of recent intimate partner violence (IPV) and suspected PTSD across recruitment site. (c) Prevalence of self-reported lifetime substance use across
Exposure to traumatic stressors and PTSD
High levels of exposure to traumatic stressors were reported (Table 2), with many mothers having experienced neglect and abuse during childhood. In addition, reported levels of lifetime and recent IPV are high in this population, particularly among women from TC Newman (Table 2 and Fig. 2b). Almost half of the mothers from this clinic (46%) reported experiencing lifetime emotional IPV, with 38% and 14% reportedly experiencing lifetime physical and sexual IPV respectively. More than half (52%) of the women who reported experiencing a recent negative life event fit the MPSS-criteria for suspected PTSD related to this event.
Substance use
Self-reported lifetime use of tobacco (37%), alcohol (41%), and cannabis (10%) was high among mothers, particularly those at TC Newman (Fig. 2c). Lifetime use of other substances was negligible (lower than 4% of participants) in this sample. Among those who reported lifetime substance use, many scored above the threshold for current moderate-high substance-related problems (Table 3). Substance-related problems (moderate and high) over the past 3 months were most frequently reported for tobacco, with 189 mothers (30%) reporting at-risk tobacco use, followed by alcohol use (n = 68, 11%) and cannabis use (n = 14, 2%). The Fagerström test indicated moderate levels of nicotine dependence, with 35 mothers (6%) reporting moderate-high dependence. Objective measures of tobacco smoke exposure among those for whom these measures were available (n = 580) indicated a high prevalence of current exposure to tobacco smoke (n = 266, 46%) and current active smoking (n = 172, 30%). Objectives measures of illicit substance use confirmed a low prevalence of recent substance use. However, many participants who screened positive for recent substance use showed evidence of recent use of multiple substances.
Table 3.
Maternal Substance use
| Variable | Mbekweni n (%) | TC Newman n (%) | Total n (%) |
|---|---|---|---|
| Tobacco use: substance-related risk | |||
| Moderate risk | 13 (4) | 140 (44) | 153 (24) |
| High risk | 3 (1) | 33 (10) | 36 (6) |
| Tobacco dependence (self-reported) | |||
| Low dependence | 3 (1) | 60 (19) | 63 (10) |
| Low-moderate dependence | 6 (2) | 45 (14) | 51 (8) |
| Moderate dependence | 2 (0.6) | 31 (10) | 33 (5) |
| High dependence | 0 (0) | 2 (0.6) | 2 (0.3) |
| Alcohol use: Substance-related risk | |||
| Moderate risk | 9 (3) | 40 (13) | 49 (8) |
| High risk | 14 (4) | 5 (2) | 19 (3) |
| Cannabis use: Substance-related risk | |||
| Moderate risk | 1 (0.3) | 9 (3) | 10 (2) |
| High risk | 0 (0) | 4 (1) | 4 (0.6) |
| Tobacco smoke exposure (objective; n = 580) | |||
| Non-smoker | 106 (36) | 36 (13) | 142 (24) |
| Passive smoker/exposed | 157 (53) | 109 (38) | 266 (46) |
| Active smoker | 31 (11) | 141 (49) | 172 (30) |
| Illicit substance use (objective; n = 569) | |||
| Methamphetamines | 0 (0) | 14 (5) | 14 (2) |
| Cocaine | 0 (0) | 0 (0) | 0 (0) |
| Cannabis | 1 (0.3) | 20 (7) | 21 (4) |
| Methaquolone (mandrax) | 0 (0) | 6 (2) | 6 (1) |
| Opiates | 1 (0.3) | 1 (0.4) | 2 (0.4) |
| MDMA | 0 (0) | 3 (1) | 3 (0.5) |
Paternal measures
Paternal measures are presented in Table 4. Given the low number of fathers, however, data are not presented separately for each clinic. Among fathers, there is a moderate prevalence of depression (15% according to the BDI-II), and a high lifetime prevalence of substance use, with 65% reporting tobacco use; 65% reporting alcohol use; and 17% reporting cannabis use. Substance-related risk (moderate and high) is particularly prevalent, with 44 fathers (59%) reporting at-risk tobacco use, followed by alcohol use (n = 37, 50%) and cannabis use (n = 9, 12%). The Fagerström test indicates fairly high levels of nicotine dependence, with 19 fathers (25%) reporting moderate-high dependence. High levels of childhood neglect and abuse were also reported, particularly in the domain of physical neglect (reported by 41% of fathers)
Table 4.
Paternal psychosocial risk factors.
| Variable | n (%) |
|---|---|
| Number of fathers | 75 |
| Beck Depression Inventory – median score (IQR) | 5 (1–15) |
| Beck Depression Inventory – above threshold ≥20 | 11 (15) |
| Edinburg Postnatal Depression Rating Scale – median score (IQR) | 7 (4–10) |
| Edinburg Postnatal Depression Rating Scale – above threshold ≥13 | 12 (16) |
| SRQ-20 – median score (IQR) | 2 (0–4) |
| SRQ-20 – above threshold ≥8 | 9 (12) |
| Tobacco use | |
| Self-reported lifetime use | 49 (65) |
| Substance-related risk | |
| Moderate risk | 29 (39) |
| High risk | 15 (20) |
| Tobacco dependence (self-reported) | |
| Low dependence | 13 (17) |
| Low-moderate dependence | 13 (17) |
| Moderate dependence | 16 (21) |
| High dependence | 3 (4) |
| Alcohol use | |
| Self-reported lifetime use | 49 (65) |
| Substance-related risk | |
| Moderate risk | 11 (15) |
| High risk | 26 (35) |
| Cannabis use | |
| Self-reported lifetime use | 13 (17) |
| Substance-related risk | |
| Moderate risk | 7 (9) |
| High risk | 2 (3) |
| Childhood trauma | |
| Emotional neglect – above threshold | 20 (27) |
| Physical neglect – above threshold | 31 (41) |
| Emotional abuse – above threshold | 19 (25) |
| Physical abuse – above threshold | 21 (28) |
| Sexual abuse – above threshold | 13 (17) |
| Life events – median (IQR) | 2 (0–4) |
| Suspected PTSD | 14 (19) |
| Peritraumatic Distress Inventory – median (IQR) | 0.9 (0.2–1.5) |
Discussion
Several aspects of the methods outlined here deserve further consideration, and several findings from these baseline data warrant further discussion.
First, these data reinforce the point that in many parts of the globe, pregnant mothers and their infants face considerable health challenges. This cohort has low levels of educational attainment and employment, and unplanned pregnancy is common. There is a high prevalence of antenatal psychological distress and depression, consistent with previous work in the Western Cape (Dewing et al., 2013, Hartley et al., 2011, Roos et al., 2013 and Tomlinson et al., 2014). Substance use, particularly tobacco and alcohol use, is common, again consistent with prior local work (Dewing et al., 2013, Petersen Williams et al., 2014 and Vythilingum et al., 2012). In addition, there is a high reported prevalence of IPV within this cohort, as has been previously found during pregnancy in South Africa (Groves et al., 2014).
Second, there is considerable variance within the sample. The legacy of apartheid has affected different communities disparately, and it is notable that the two clinics studied here exhibit differing baseline characteristics. For example, participants at Mbekweni are shown to have higher levels of depression; with those at TC Newman reporting a higher prevalence of substance use and IPV. The higher levels of both substance use and reported IPV observed in TC Newman participants is consistent with previous literature which demonstrates a clear link between substance use and both perpetration of and victimization from IPV (World Health Organization, 2012). Furthermore, despite the adverse circumstances that many pregnant women must endure, there is some evidence of social capital in this cohort (e.g. many consider their partners a support), and a considerable number do not show evidence of psychological distress or depression. While clinical and public mental health interventions should be made available to those in need, it is also important to emphasize the resilience of the communities and their members.
Third, within a complex multidisciplinary study such as this, there are inevitably many claims on participants’ time. Thus, only a limited number of psychosocial measures can be administered, and a balance needs to be struck between efficiently assessing a limited range of key issues (e.g. psychological distress, substance use, IPV), and addressing a broader range of possibly relevant factors. One strategy employed is the use of nested sub-studies, which allow exploration of particular questions in greater depth. A particular advantage of our site in Cape Town, for example, is that such sub-studies can include advanced methods such as infant brain imaging. While our focus here has been on presenting the baseline findings on a small number of core measures, we hope to present sub-study results in the future.
Fourth, it is worth mentioning the range of methodological issues that arise when assessing participants from diverse cultural and language groups. For example, translation of questionnaires needs to be done with great care in order to ensure semantic equivalence (Smit et al., 2006). Furthermore, given the low levels of literacy among our respondents, fieldworkers provide help in administering questionnaires that are ordinarily self-administered. Important limitations may of course ensue, for example respondents may be reluctant to disclose personal or embarrassing information. However, in our setting the benefits of interviewer-administration outweigh the potential for bias, as understanding and correct completion of the questionnaires is ensured. In our view, the longitudinal design of this study fosters a close and open relationship between participants and members of the research team.
Finally, it is worth considering some of the ethical aspects surrounding research such as this. Given the high levels of psychopathology and of exposure to traumatic stressors in this cohort, there was a need to develop standardized clinical protocols for referral of at-risk participants. Fortunately, a basic public health system is in place in the Drakenstein sub-district, and is able to provide care for women with severe psychological distress or depression. However, there remains an insufficiency of public health resources to assist all women in the community. It is our hope that by enrolling in the study, women are guaranteed a minimal level of care; and that the data emerging from this study will be useful in understanding health outcomes in this and other LMIC populations, thus contributing to the advancement of relevant interventions.
In summary, the DCHS cohort is representative of many populations of pregnant mothers and their infants – both locally and worldwide – who face considerable health challenges. While much has been discovered about the effects of early adversity on subsequent health outcomes, relatively little work has been done in LMICs, where psychobiological and psychosocial risk factor profiles differ from those in high-income countries. Further longitudinal assessment of mothers and children may therefore address a critical gap by clarifying the ways in which risk factors intersect and interact, and delineating underlying mechanisms which impact on child health. Such work may ultimately inform clinical and public health interventions appropriate to the South African and other LMIC contexts.
Acknowledgments
We thank the study staff and the staff at Paarl Hospital, Mbekweni and TC Newman clinics for their support of the study. We thank the families and children who participated in this study. Support for this study was provided by the Bill and Melinda Gates Foundation [OPP 1017641] and by the National Institute of Mental Health (NIMH) Brain Disorders in the Developing World: Research Across the Lifespan program (grant number 1R21MH098662-01). Professor Stein is supported by the Medical Research Council of South Africa.
References
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, et al. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballot DE, Potterton J, Chirwa T, Hilburn N, Cooper PA. Developmental outcome of very low birth weight infants in a developing country. BMC Pediatrics. 2012;12:11. doi: 10.1186/1471-2431-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban L, Gibson JE, West J, Tata LJ. Association between perinatal depression in mothers and the risk of childhood infections in offspring: a population-based cohort study. BMC Public Health. 2010;10:799. doi: 10.1186/1471-2458-10-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley scales of infant and toddler development. third edition Harcourt Assessment, Inc; San Antonio, TX: 2006. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio: 1996. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Beusenberg M, Orley J. Division of Mental Health. World Health Organization; Geneva: 1994. A user's guide to the self reporting questionnaire (SRQ). [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, et al. A clinician rating scale for assessing current lifetime PTSD: the CAPS-1. Behav Ther. 1990;13:187–8. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Traum Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, et al. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–5. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- Carter AS, Wagmiller RJ, Gray SAO, McCarthy KJ, Horwitz SM, Briggs-Gowan MJ. Prevalence of DSM-IV disorder in a representative, healthy birth cohort at school entry: sociodemographic risks and social adaptation. J Am Acad Child Adolesc Psychiatry. 2010;49(7):686–98. doi: 10.1016/j.jaac.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TL, Goodman E. The Committee on Pediatric Research, Race, ethnicity, and socioeconomic status in research on child health. Pediatrics. 2015;135(1):e225–e237. doi: 10.1542/peds.2014-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychotherapy. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Dewing S, Tomlinson M, le Roux IM, Chopra M, Tsai AC. Food insecurity and its association with co-occurring postnatal depression, hazardous drinking, and suicidality among women in peri-urban South Africa. J Affect Disord. 2013;150:460–5. doi: 10.1016/j.jad.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle KL, Jewkes RK, Brown HC, Gray GE, McIntryre JA, Harlow SD. Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. Lancet. 2004;363(9419):1415–21. doi: 10.1016/S0140-6736(04)16098-4. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69:763–768. [PubMed] [Google Scholar]
- Flach C, Leese M, Heron J, Evans J, Feder G, Sharp D, et al. Antenatal domestic violence, maternal mental health and subsequent child behaviour: a cohort study. BJOG. 2011;118:1383–1391. doi: 10.1111/j.1471-0528.2011.03040.x. [DOI] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;6(4):459–473. [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves AK, Moodley D, McNaughton-Reyes L, Martin SL, Foshee V, Maman S. Prevalence, rates and correlates of intimate partner violence among South African women during pregnancy and the postpartum period. Matern Child Health J. 2014 doi: 10.1007/s10995-014-1528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpham T, Reichenheim M, Oser R, Thomas E, Hamid N, Jaswal S, et al. Measuring mental health in a cost-effective manner. Health Policy Plan. 2003;18(3):344–9. doi: 10.1093/heapol/czg041. [DOI] [PubMed] [Google Scholar]
- Hartley M, Tomlinson M, Greco E, Comulada WS, Stewart J, le Roux I, et al. Depressed mood in pregnancy: prevalence and correlates in two Cape Town peri-urban settlements. Reprod Health. 2011;8:9. doi: 10.1186/1742-4755-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189(4):401–407. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- Humeniuk RE, Ali RA, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Jewkes R. Intimate partner violence: causes and prevention. Lancet. 2002;359(9315):1423–9. doi: 10.1016/S0140-6736(02)08357-5. [DOI] [PubMed] [Google Scholar]
- Kagee A, Martin L. Symptoms of depression and anxiety among a sample of South African patients living with HIV. AIDS Care. 2010;22(3):381–8. doi: 10.1080/09540120903111445. [DOI] [PubMed] [Google Scholar]
- Kagee A, Nel A, Saal W. Factor structure of the Beck Depression Inventory-II among South Africans receiving antiretroviral therapy. AIDS Care. 2014;26(2):257–62. doi: 10.1080/09540121.2013.802278. [DOI] [PubMed] [Google Scholar]
- Kingston D, Tough S, Whitfield H. Prenatal and postpartum maternal psychological distress and infant development: a systematic review. Child Psychiatry Hum Dev. 2012;43:683–714. doi: 10.1007/s10578-012-0291-4. [DOI] [PubMed] [Google Scholar]
- Lasa L, Ayuso-Mateos JL, Vázquez-Barquero JL, Díez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57(1-3):261–5. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan K, et al. The MINI International Neuropsychiatric Interview (M.I.N.I.) a short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12:224. [Google Scholar]
- Lund C. Poverty and mental health: towards a research agenda for low and middle-income countries, Commentary on Tampubolon and Hanandita. Soc Sci Med. 2014;111:134–136. doi: 10.1016/j.socscimed.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Maggi S, Irwin LJ, Siddiqi A, Hertzman C. The social determinants of early child development: an overview. J Paediatr Child Health. 2010;46:627–35. doi: 10.1111/j.1440-1754.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- Maggi S, Irwin LJ, Siddiqi A, Hertzman C. The social determinants of early child development: an overview. J Paediatr Child Health. 2010;46:627–635. doi: 10.1111/j.1440-1754.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EPDS). J Reprod Infant Psychol. 1990;8:99–107. [Google Scholar]
- Myer L, Stein DJ, Grimsrud A, Seedat S, Williams DR. Social determinants of psychological distress in a nationally-representative sample of South African adults. Soc Sci Med. 2008;66(8):1828–40. doi: 10.1016/j.socscimed.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Kagee A. The relationship between depression, anxiety and medication adherence among patients receiving antiretroviral treatment in South Africa. AIDS Care. 2013;25(8):948–55. doi: 10.1080/09540121.2012.748867. [DOI] [PubMed] [Google Scholar]
- Petersen Williams P, Jordaan E, Mathews C, Lombard C, Parry CDH. Alcohol and other drug use during pregnancy among women attending midwife obstetric units in the Cape Metropole, South Africa. Adv Prev Med. 2014 doi: 10.1155/2014/871427. Article ID 871427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A, Faure S, Lochner C, Vythilingum B, Stein DJ. Predictors of distress and anxiety during pregnancy. Afr J Psychiatry. 2013;16:118–22. doi: 10.4314/ajpsy.v16i2.15. [DOI] [PubMed] [Google Scholar]
- Rumble S, Swartz L, Parry C, Zwarenstein M. Prevalence of psychiatric morbidity in the adult population of a rural South African village. Psychol Med. 1996;26:997–1007. doi: 10.1017/s0033291700035327. [DOI] [PubMed] [Google Scholar]
- Shamu S, Abrahams N, Temmerman M, Musekiwa A. Zarowsky C A systematic review of African studies on intimate partner violence against pregnant women: prevalence and risk factors. PLoS One. 2011;6(3):e17591. doi: 10.1371/journal.pone.0017591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSMIV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonora LI, et al. Reliability and validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): according to the SCID-P. Eur Psychiatry. 1997;12:232–41. [Google Scholar]
- Smit J, van den Berg CE, Bekker L-G, Seedat S, Stein DJ. Translation and cross-cultural adaptation of a mental health battery in an African setting. Afr Health Sci. 2006;6(4):215–222. doi: 10.5555/afhs.2006.6.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med. 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107(19):8507–12. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, O'Connor MJ, le Roux IM, Stewart J, Mbewu N, Harwood J, et al. Multiple risk factors during pregnancy in South Africa: the need for a horizontal approach to perinatal care. Prev Sci. 2014;15:277–82. doi: 10.1007/s11121-013-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventevogel P, De Vries G, Scholte WF, Shinwari NR, Faiz H, Nassery R, et al. Properties of the Hopkins Symptom Checklist-25 (HSCL-25) and the Self-Reporting Questionnaire (SRQ-20) as screening instruments used in primary care in Afghanistan. Soc Psychiatry Psychiatr Epidemiol. 2007;42(4):328–35. doi: 10.1007/s00127-007-0161-8. [DOI] [PubMed] [Google Scholar]
- Villano CL, Cleland C, Rosenblum A, Fong C, Nuttbrock L, Marthol M, et al. Psychometric utility of the childhood trauma questionnaire with female street-based sex workers. J Trauma Dissociation. 2004;5(3):33–41. doi: 10.1300/J229v05n03_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingum B, Roos A, Faure SC, Geerts L, Stein DJ. Risk factors for substance use in pregnant women in South Africa. S Afr Med J. 2012;102(11) doi: 10.7196/samj.5019. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Grantham-McGregor S, Black MM, Nelson CA, Huffman SL, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378:1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Meeks Gardner J, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–57. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- Watt MH, Eaton LA, Choi KW, Velloza J, Kalichman SC, Skinner D, et al. It's better for me to drink, at least the stress is going away: perspectives on alcohol use during pregnancy among South African women attending drinking establishments. Soc Sci Med. 2014;116:119–125. doi: 10.1016/j.socscimed.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JRT. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- WHO ASSIST Working Group The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): manual for use in primary care. World Health Organization; Geneva: 2010. [Google Scholar]
- World Health Organization . Intimate Partner Violence. World Health Organization; Geneva: 2012. [Google Scholar]
- World Health Organization . The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Manual for Use in Primary Care. World Health Organization; 2010. [Google Scholar]
- Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax. 2015;70:592–594. doi: 10.1136/thoraxjnl-2014-206242. [DOI] [PMC free article] [PubMed] [Google Scholar]