Abstract
Background
Many patients with benign serous cystadenoma (SCA) of the pancreas will undergo resection because of the inability to reliably discriminate between SCA and premalignant mucinous cysts (intraductal papillary mucinous neoplasm [IPMN], mucinous cystic neoplasm [MCN]).
Methods
Cyst fluid from patients with SCA (n = 15), non main-duct and noninvasive IPMN (n = 32), and noninvasive MCN (n = 12) was aspirated at the time of operative resection and analyzed. Commercially available and custom designed multiplex assays (Luminex) were performed using a biomarker panel developed for pancreatic cancer. Differential protein expression (fluorescence intensity, FI) was compared between the 3 groups for each protein (Wilcoxon rank sum test). Unsupervised sample clustering (hierarchical clustering) and supervised sample classification (prediction analysis for microarrays [PAM]) was then performed.
Results
Differential protein expression (P < 0.05) was identified between SCA and IPMN (34/51 proteins, 67%) and between SCA and MCN (13/51 proteins, 25%). The majority of proteins were down-regulated in IPMN and MCN compared with SCA. The only proteins significantly overexpressed in the cyst fluid of patients with mucinous cysts were CEA (median FI: IPMN 11.4, MCN 13.0, SCA 5.3; P < 0.001, IPMN vs. SCA) and CA72.4 (median FI: IPMN 10.4, MCN 10.5, SCA 9.9; P = 0.003, IPMN vs. SCA). Unsupervised cluster analysis demonstrated distinct clustering of SCA and IPMN with some cross-over between MCN. Supervised sample classification with 14 proteins had an overall accuracy rate of 92% between SCA and IPMN.
Conclusions
In this study differential cyst fluid protein expression was observed between SCA and IPMN for the majority of proteins assessed and multimarker sample classification accurately discriminated between SCA and IPMN in 92% of patients.
Over the past decade the increased availability of high quality cross-sectional imaging has resulted in more patients diagnosed with cystic lesions of the pancreas.1,2 At Massachusetts General Hospital, 16% of pancreatic resections performed in 1991 were for cystic lesions of the pancreas, and in 1998 the percentage of resections performed for pancreatic cysts had increased to 30%.1 This past year at our institution (Memorial Sloan-Kettering Cancer Center [MSKCC,] 2007), surgeons and gastroenterologists evaluated over 200 new patients with pancreatic cysts.
A “cystic lesion of the pancreas” is a radiographic finding with a broad histologic differential. This histologic differential includes non-neoplastic pseudocysts, as well as benign, premalignant, and malignant neoplasms. Improved imaging techniques, as well as an improved understanding of the nature of these lesions, has made possible the distinction between non-neoplastic pseudocysts and cystic neoplasms in most of cases.3 Because of this, current research efforts have focused on developing techniques to differentiate between the most common neoplastic cysts.
Currently, over 90% of patients undergoing resection for a cystic neoplasm of the pancreas will have the histologic diagnosis of a serous cystadenoma (SCA), intraductal papillary mucinous neoplasm (IPMN), or mucinous cystic neoplasm (MCN) with the majority of these being either SCA or IPMN.4 Preoperative determination of histologic subtype is critical, as SCAs are considered benign and generally do not require resection, whereas IPMNs and MCNs (mucinous cysts) are considered premalignant. Because current diagnostic tests are unable to reliably identify mucinous cysts, many institutions have recommended routine resection of pancreatic cysts.5,6 This approach exposes asymptomatic patients with benign lesions to the risks of pancreatectomy for no identifiable benefit.
The purpose of this study was to assess the diagnostic accuracy of proteomic profiling of pancreatic cyst fluid obtained from patients with noninvasive and radiographically equivocal cysts. Differential protein expression was assessed in the cyst fluid from resected SCA, IPMN, and MCN. Sample classification and unsupervised cluster analyses were performed to assess the accuracy of this proteomic approach.
METHODS
Patients undergoing resection for a cystic lesion of the pancreas at MSKCC were consented to the IRB approved tissue, serum, and cyst fluid protocol (MSKCC IRB 00–032). Resected specimens were immediately transported to the MSKCC tumor procurement facility where cyst aspiration was performed by a surgeon, pathologist, or technician. Cyst fluid samples were aspirated with an 18 to 21 gauge needle, divided into 500 μL aliquots, and stored at −80°C. The time between resection and freezing was recorded and only samples that had been frozen within 60 minutes of resection were used. None of the samples had undergone any freeze-thaw cycles before analysis.
Histopathologic assessment of resected specimens was performed within the Department of Pathology (MSKCC). Results were reviewed at monthly multidisciplinary staging conferences and stored with the Department of Surgery pancreatic database. In this study only cyst fluid specimens from patients with SCA (n = 15), noninvasive IPMN (n = 32), or noninvasive MCN (n = 12) were used. Patients with invasive IPMN or isolated main duct IPMN were excluded as the radiographic findings in these patients are typically not equivocal and operative resection is routinely recommended.
Multianalyte analysis (Luminex) of the cyst fluid was performed at the University of Pittsburgh Medical Center using an antibody microsphere array panel that has been designed and developed for pancreatic cancer. Commercially available and custom designed multiplex assays were used (Biosource International, Linco Research). The 54 unique proteins measured in this analysis are listed in Table 1.
TABLE 1.
Biomarkers Used in the Multianalyte Analysis of 59 Patients Resected With SCA, IPMN, or MCN
| Growth factors/cell cycle | EGF/EGFR, IGFBP-1, ErbB2, GM-CSF, Fas, sFasL, DR5, IFNg |
| Immune modulator | MPO, MIF, CD4OL (TRAP) |
| Chemokines/cytokines | MIP1B, Eotaxin, IL-6, Fractalkine, TNF-a, IP-10, TNF-RI, TNF-RII, IL-2IL-2R, TGFA, IL-8, IL1B, IL-4, IL-5, IL-7, IL-10, IL-12P70, IL-13, IL-6R, IL-1Ra |
| Angiogenesis | MMP-9, sVCAM, TPAI-1 |
| Callular/tumor antigens | CEA, CA19-9, CA-125, CK-19, CA15-3, CA72-4, AFP, mesothelin, sICAM |
| Extracellular matrix | MMP-2, Kallikrein 10, MMP-3, adiponectin |
| Endocrine | FSH, LH, TSH, Prolactin, GH, ACTH |
Multianalyte analysis was performed according to previously described protocol (Biosource International). In brief, antibody-coated bead concentrates were initially vortexed and diluted with wash solution. All microtiter wells then received 25 μL of diluted antibody coated beads. Standard wells received 100 μL of serially diluted standards and sample wells received 50 μL of diluent and 50 μL of sample. Wells were then zeroed with assay diluent and placed on an orbital shaker in the dark for 2 hours. Biotinylated detection antibody was then added (100 μL), and the samples then incubated for an hour. The beads were then filtered, washed, mixed with 100 μL streptavidin-RPE, and incubated for 30 minutes. The solution was then filtered, washed, resuspended in wash solution and loaded into the Luminex platform. Standard curves were created and the sample concentrations then measured and recorded.
Data analysis was performed for all samples and/or proteins that had successful measurement of ≥85% of the data points. Cyst fluid samples were excluded from analysis if 15% or more of the proteins were not successfully measured (IPMN, n = 10 and MCN, n = 1). Individual proteins were excluded if 15% or more of the cyst fluid samples did not have successful measurement of the given protein (CA-125, Kallikrein 10, DR5). Serial dilution of the primary fluid was performed as indicated (mucinous fluid).
Protein expression levels were log2 transformed before analysis to improve the normality of the data. Supervised analyses utilizing the Wilcoxon rank sum test were performed to identify differentially expressed proteins between groups (SCA, IPMN, MCN). A P value cutoff of 0.05 was used to select significant proteins. With this cutoff, approximately 3 proteins would have such a P value by chance.
Sample classification was performed utilizing the prediction analysis for microarrays (PAM) method.7 The resulting sample classifier was evaluated utilizing 10-fold cross validation. Samples were divided into 10 folds; prediction rules were built on 9 folds of the samples and then evaluated on the left out samples–this process was repeated for each of the 10 folds and each sample was left out once. Both the protein selection step and the classifier building step were included in the cross-validation process to minimize potential bias in the estimated error rate. Unsupervised sample clustering was also performed to explore the sample grouping based on protein expression alone.
RESULTS
There were 59 patients (SCA, n = 15; noninvasive IPMN, n = 32; noninvasive MCN, n = 12) who underwent resection, had pancreatic cyst fluid banked according to protocol, and met eligibility criteria as noted above (Table 2). All patients with the exception of 2 patients with large SCA (>5 cm) were recommended to resection because of concern for a mucinous lesion. Before resection all patients underwent CT imaging, 24 patients (41%) underwent MR/MRCP, and 32 patients (54%) underwent endoscopy with endoscopic ultrasound (EUS)/FNA. An EUS with or without FNA was performed in 6 of the 13 patients with SCA who were presumed to have a mucinous lesion based on imaging. In all 6 cases the EUS suggested a mucinous lesion. In 2 cases the cytology revealed mucinous cells, in 3 cases the cyst was macrocystic and presumed mucinous based on morphology, and in 1 case the pancreatic duct distal to the lesion was dilated.
TABLE 2.
Demographic and Pathologic Data for the 59 Patients Who Underwent Resection for SCA, IPMN, or MCN of the Pancreas
| Factor | SCA (n = 15) | IPMN (n = 32) | MCN (n = 12) |
|---|---|---|---|
| Age (yr) | |||
| Median (range) | 64 (44–79) | 71 (39–90) | 55 (43–68) |
| Male gender | |||
| n (%) | 1 (7%) | 16 (50%) | 0 |
| Cyst diameter (cm) | |||
| Median (range) | 3.2 (2.5–9.0) | 2.8 (1.0–10.0) | 3.7 (1.5–15.0) |
| Main pancreatic duct dilation | |||
| Yes | 0 | 11 (34%) | 0 |
| Dysplasia | |||
| Low-grade | |||
| N (%) | N/A | 3 (9%) | 11 (92%) |
| Moderate | 23 (72%) | ||
| High-grade | 6 (19%) | 1 (8%) | |
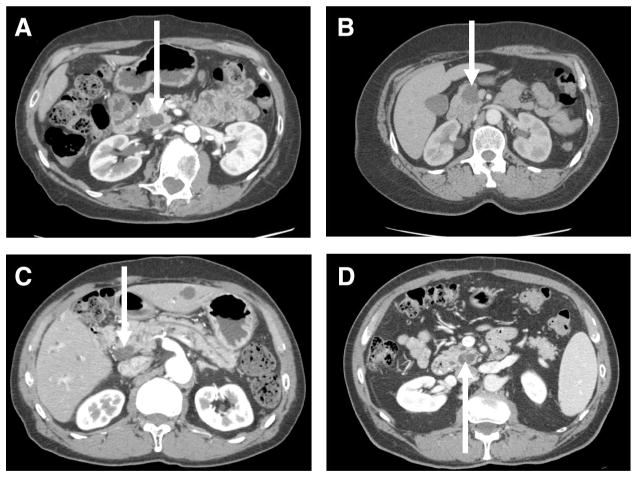
The median diameter of the cystic lesions was not significantly different between SCA (median: 3.2 cm, range, 2.5–9.0), IPMN (median: 2.8 cm, range, 1.0–10.0 cm), and MCN (median: 3.7 cm, range, 1.5–15.0 cm). Within the group of patients resected for IPMN the final pathology revealed low-grade dysplasia in 3 patients (3/32, 9%), moderate dysplasia in 23 patients (72%), and high-grade dysplasia in 6 patients (19%). Isolated branch duct disease was present in 21 of these patients (69%) and the remaining had some component of main duct dilation in addition to the primary cyst. Within the group of 21 patients with isolated branch duct disease there were 3 patients with low-grade dysplasia, 16 patients with moderate dysplasia, and 2 patients with high-grade dysplasia. A single patient resected for MCN had high-grade dysplasia (1/12, 8%). Because of the noninvasive nature of the mucinous lesions, and the similar size distribution, most of these cysts had similar radiographic appearance (Fig. 1).
FIGURE 1.
Representative CT images of selected patients resected for SCA (A and B) and IPMN (C and D).
Despite serial dilution, 10 patients with IPMN and a single patient with MCN had ≥15% of overall protein data missing. These patients were excluded from analysis. Within this group of patients there was a similar distribution of dysplasia (1/10 low-grade, 7/10 moderate-grade, 2/10 high-grade) as compared with the larger group of 32 patients. In addition, only 5 of the 51 measured proteins were differentially expressed (IL-2, P = 0.002; IL-1b, P = 0.006; IFN gamma, P = 0.02; CA72–4, P = 0.03; and MPO, P = 0.03) between excluded and included IPMN patients. None of the cyst fluid samples from patients with SCA experienced a ≥15% dropout in protein measurement. This finding likely represents the overall difference in fluid viscosity and bead agglomeration in the fluid from some patients with mucinous cysts.
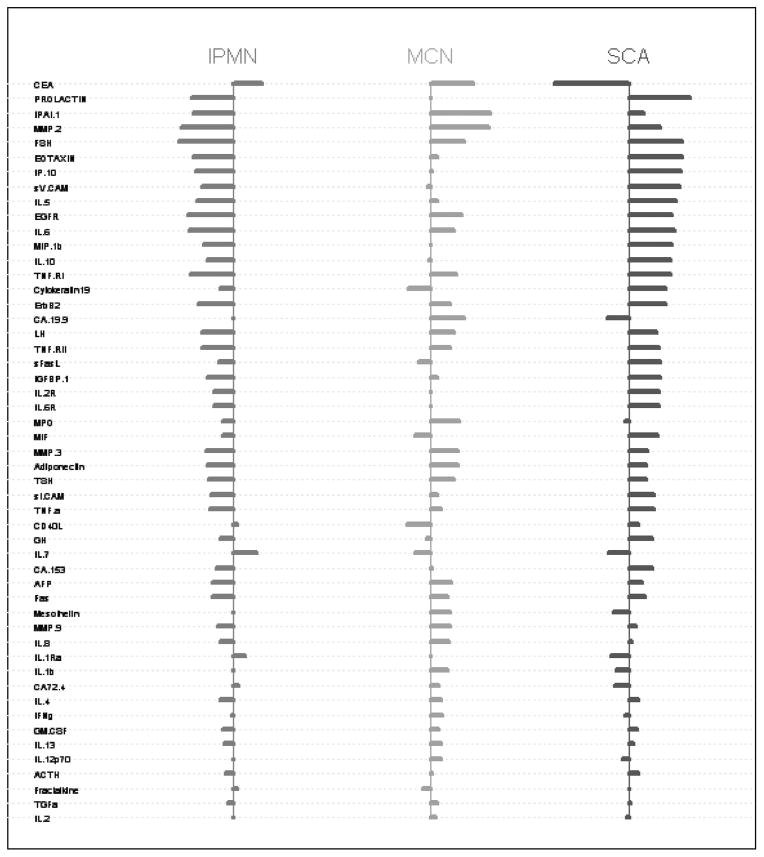
Pairwise comparisons of individual protein expression revealed significant differences between all 3 groups. The 2 groups with largest number of individual differences were SCA and IPMN. Differential protein expression (P < 0.05) was identified between SCA and IPMN in 34 of the 51 proteins evaluated (66%). Differential protein expression (P < 0.05) was identified between SCA and MCN in 13 of 51 proteins evaluated (25%). These differences in expression were almost exclusively manifested as overexpression within the SCA group (Fig. 2). The only proteins significantly overexpressed in the cyst fluid of patients with IPMN and MCN were CEA (median FI: IPMN 11.4, MCN 13.0, SCA 5.3; P < 0.001 IPMN vs. SCA) and CA72.4 (median FI: IPMN 10.4, MCN 10.5, SCA 9.9; P = 0.003 IPMN vs. SCA).
FIGURE 2.
Relative differential protein expression for the 51 proteins analyzed from patients with SCA, IPMN, and MCN.
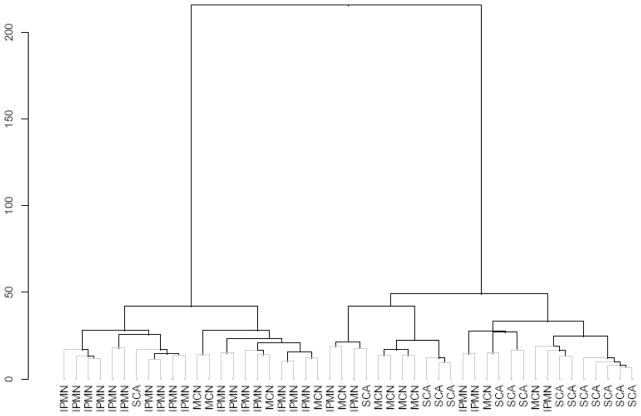
Unsupervised hierarchical cluster analysis revealed distinct clustering between the SCA and IPMN groups (Fig. 3). The dendrogram presented in Figure 3 also illustrates some of the cross-over between patients with IPMN and SCA. The length of each vertical limb in the dendrogram is proportional to the strength of the association between individual proteins and the initial vertical branch point is CEA which was the most powerful single marker. Patients with MCN, did experience some cross-over with IPMN and SCA. As noted above, differential expression was not as prevalent as it was between IPMN and SCA (Fig. 1).
FIGURE 3.
Dendrogram demonstrating distinct clustering of IPMN and SCA with some cross-over of MCN.
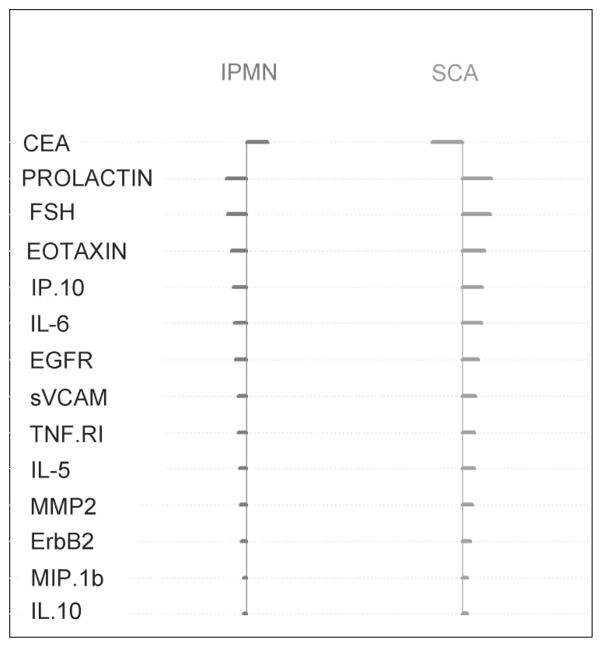
These findings were further substantiated in the sample classification. Sample classification with 3 classes (SCA, IPMN, and MCN) resulted in a classification error rate of 27% with a model utilizing 51 proteins. When this classification model was limited to 2 classes (SCA and IPMN) the error rate decreased to 8% and was built on a model of 14 proteins. These individual proteins, and their relative expression levels are presented in Figure 4. The error rate of CEA alone in discriminating between SCA and IPMN was 14%.
FIGURE 4.
Specific markers used in 2-class sample classification (SCA vs. IPMN) with overall error rate of 8%.
DISCUSSION
Over 90% of patients undergoing resection for a cystic neoplasm of the pancreas will have the histologic diagnosis of SCA, IPMN, or MCN.4 Preoperative determination of histologic subtype is critical, as asymptomatic SCAs are considered benign and do not require resection, whereas IPMNs and MCNs (mucinous cysts) are considered premalignant. The development of a diagnostic test that could discriminate between these entities with a high degree of certainty would be extremely beneficial to clinical decision making.
The ability of current laboratory, radiographic, and cytologic tests to differentiate between serous and mucinous cysts of the pancreas is limited. CT is considered by many to be one of the best tests available for differentiating between serous and mucinous cysts. The CT characteristics of serous and mucinous lesions have been described, however, studies have shown that CT is discriminatory in only about 40% of cases.8,9 The imaging presented in Figure 1 demonstrates the limitations of cross-sectional radiography in distinguishing between certain serous and mucinous lesions. The 4 patients presented in Figure 1 were presumed to have mucinous cysts based on preoperative CT, however, only 2 of the 4 were found to have mucinous lesions (IPMN) on pathologic analysis, and none had malignancy. Some have reported that MRI and MRCP can provide improved visualization of cysts and the associated ductal system, however, the reported accuracy of MRI/MRCP in distinguishing between serous and mucinous cysts appears to be similar to CT.10
When radiographic studies are not characteristic of histologic subtype, EUS and cyst fluid aspiration have been recommended as useful diagnostic techniques. Brugge et al reported on the accuracy of EUS morphology in a group of 341 patients with cystic lesions of the pancreas. In this study EUS morphology was able to discriminate mucinous from nonmucinous in 57 of 112 patients (51%) who underwent this test. Cytology performed on the fluid obtained at the time of EUS is also of limited diagnostic value. These specimens are often small in quantity, are often acellular, and reports evaluating the sensitivity of cytology for determining histologic subtype have reported sensitivities of 28% to 60%.12 Much of the inaccuracy of cyst fluid cytology results from the presumed contamination of duodenal or gastric epithelial cells at the time of cyst puncture which are misread on cytology as mucinous cyst lining cells. This was observed in 2 of the 15 patients in the current study who were resected for SCA.
Multiple studies have investigated the diagnostic value of individual cyst fluid protein measurements. Proteins that have been evaluated in cyst fluid include CEA, CA19.9, CA 125, CA72–4, CA15–3, amylase, mucin-like carcinoma-associated antigen, and tissue polypeptide antigen.11 Currently, the most useful marker in distinguishing between mucinous and nonmucinous cysts is cyst fluid CEA. Most studies have reported that a CEA level ≥200 ng/mL is associated with the presence of a mucinous cyst.13–15 Elevated CEA levels (>200 ng/mL), and the presence of extracellular mucin, have been shown to have a positive predictive value for mucinous lesions of approximately 75%.
In general, the power of individual protein markers for diagnosis or staging of malignancy has been limited. Serum CA19–9, the most commonly used serum biomarker for the diagnosis of pancreatic adenocarcinoma has a diagnostic sensitivity and specificity of approximately 80% and 42%, respectively. The technique used in the current study, multianalyte antibody array analysis, allows for the quantitative measurement of multiple predetermined protein markers from a single sample. This approach is currently being applied to a variety of malignant and premalignant conditions and has resulted in a serum assay that can reliably diagnose over 97% of women with early stage ovarian cancer.16
The current study evaluated this multimarker approach for diagnosis in patients with serous and mucinous cysts of the pancreas. This prevalidation study identified differential cyst fluid protein expression between SCA and IPMN in 34 of the 51 proteins evaluated (66%). Differential protein expression was identified between SCA and MCN in 13 of 51 proteins evaluated (25%). These differences allowed for a classification model to be developed that with cross-validation was accurate in discriminating between SCA and IPMN 92% of the time. This technique has many theoretical advantages for the diagnosis of pancreatic cysts. This assay can be easily modified and can be uniquely customized. Commercial antibody bead sets are readily available and additional antibody-bead sets can be developed when novel markers are identified. In our current array, the commercial antibody bead sets targeting known tumor associated antigens have been used (CEA, CA19–9, CA 125, CA 72–4) as well as individual antibodies for proteins recently identified to be differentially expressed in pancreatic adenocarcinoma (mesothelin, heat shock protein 27, macrophage inflammatory protein-1 alpha). Once the antibody-bead panel is established the process is high-throughput and compared with other molecular tests the costs are low. Current material costs for running a panel of 50 proteins is approximately $400/sample. Furthermore, this method can quantitatively assess up to 100 different biomarkers from a single sample as small as 50 μL. This can be extremely important in this group of patients as the amount of fluid endoscopically or percutaneously aspirated from these lesions is typically 300 μL to 500 μL.
In patients who present with radiographically equivocal lesions our diagnostic assessment generally includes triple-phase contrast enhanced CT imaging of the pancreas and endoscopic ultrasound with cyst fluid aspiration. As noted above, elevated cyst fluid CEA (>200 ng/mL) is currently the single most accurate test in identifying mucinous cysts with a positive predictive value of between 75% and 80%. In the current study the error rate for CEA alone in discriminating between SCA and IPMN was 14% compared with 8% for the multimarker panel. The importance of increasing this diagnostic accuracy cannot be overstated as this test may be the primary factor in the decision to perform pancreaticoduodenectomy which in high-volume centers has a reported major morbidity rate of 20% to 30% and a mortality rate of 2% to 4%.
The results presented in this study are preliminary and the potential difficulties in reproducing these results from endoscopically obtained samples is acknowledged. This platform needs further testing in the prevalidation setting with additional samples, and will eventually require validation on endoscopically acquired samples in patients who undergo resection. The reproducibility of these results between samples obtained at the time of resection and those obtained by a transduodenal or transgastric method is unknown. Methods need to be developed to allow more consistent measurement of mucinous fluid through decreasing the tendency for bead agglomeration. Additionally, new markers are currently being incorporated that may be more specific for mucinous lesions (MUC-1, MUC-2).
The results however are encouraging, and the strengths of this study are several. First, the accuracy of this panel was determined from the pathologic diagnosis of resected specimens (gold standard). Some reports evaluating the accuracy of endoscopic and even molecular tests for cysts of the pancreas have used cytologic (nonresectional) and imaging findings as the gold standard.17 Given the limitations of all nonresectional diagnostic techniques for pancreatic cysts the accuracy of testing should be based on the gold standard of resectional diagnosis. Second, the current study used noninvasive IPMN (primarily branch duct) and MCN as the comparison group to SCA. These are the lesions where diagnostic uncertainty may exist (Table 3). Invasive (malignant) IPMN is typically not a diagnostic dilemma as imaging often reveals a solid mass component, jaundice, or significant main pancreatic ductal dilatation. Cyst fluid analysis is generally unnecessary for clinical decision-making in these patients. Finally, the results from the current study are in agreement with single biomarker studies. CEA was the strongest marker in the current study, and CEA and CA 72.4 were the only markers significantly elevated in mucinous cysts compared with SCA. Single marker studies have reported similar findings.11,14,18
In conclusion, these preliminary results suggest that there is significant differential protein expression in the cyst fluid of patients with SCA, IPMN, and MCN. These differences may allow the development of a multimarker model that has a greater accuracy than CEA alone. Additional markers are currently being added to the platform in an effort to further improve the discriminatory power of the prevalidation model, and a validation study using endoscopically obtained samples is being developed.
Acknowledgments
Supported, in part, by the Marshall and Therese Sonenshine Foundation, the Society of Surgical Oncology, and the National Cancer Institute, Early Detection Research Network (U01 CA08496).
References
- 1.Allen PJ, Jaques DP, D’Angelica M, et al. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970–977. doi: 10.1016/j.gassur.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Del CC, Warshaw AL. Current management of cystic neoplasms of the pancreas. Adv Surg. 2000;34:237–248. [PubMed] [Google Scholar]
- 3.Fernandez-Del CC, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am. 1995;75:1001–1016. doi: 10.1016/s0039-6109(16)46742-3. [DOI] [PubMed] [Google Scholar]
- 4.Le Borgne J, De Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152–161. doi: 10.1097/00000658-199908000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999;178:269–274. doi: 10.1016/s0002-9610(99)00186-5. [DOI] [PubMed] [Google Scholar]
- 6.Ooi LL, Ho GH, Chew SP, et al. Cystic tumours of the pancreas: a diagnostic dilemma. Aust N Z J Surg. 1998;68:844–846. doi: 10.1046/j.1440-1622.1998.01481.x. [DOI] [PubMed] [Google Scholar]
- 7.Tibshirani R, Hastie T, Narasimhan B, et al. Sample classification from protein mass spectrometry, by “peak probability contrasts”. Bioinformatics. 2004;20:3034–3044. doi: 10.1093/bioinformatics/bth357. [DOI] [PubMed] [Google Scholar]
- 8.Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99–103. doi: 10.2214/ajr.175.1.1750099. [DOI] [PubMed] [Google Scholar]
- 9.Procacci C, Biasiutti C, Carbognin G, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906–912. doi: 10.1097/00004728-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Minami M, Itai Y, Ohtomo K, et al. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171:53–56. doi: 10.1148/radiology.171.1.2928546. [DOI] [PubMed] [Google Scholar]
- 11.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 12.Maker AV, Lee LS, Raut CP, et al. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. 2008;15:3187–3192. doi: 10.1245/s10434-008-0110-0. [DOI] [PubMed] [Google Scholar]
- 13.Walsh RM, Henderson JM, Vogt DP, et al. Prospective preoperative determination of mucinous pancreatic cystic neoplasms. Surgery. 2002;132:628–633. doi: 10.1067/msy.2002.127543. [DOI] [PubMed] [Google Scholar]
- 14.Lewandrowski KB, Southern JF, Pins MR, et al. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg. 1993;217:41–47. doi: 10.1097/00000658-199301000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Gorelik E, Landsittel DP, Marrangoni AM, et al. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 17.Khalid A, McGrath KM, Zahid M, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–973. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 18.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383–389. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]