Abstract
Transport through the cell membrane can be divided into active, passive and vesicular types (exosomes). Exosomes are nano-sized vesicles released by a variety of cells. Emerging evidence shows that exosomes play a critical role in cancers. Exosomes mediate communication between stroma and cancer cells through the transfer of nucleic acid and proteins. It is demonstrated that the contents and the quantity of exosomes will change after occurrence of cancers. Over the last decade, growing attention has been paid to the role of exosomes in the development of breast cancer, the most life-threatening cancer in women. Breast cancer could induce salivary glands to secret specific exosomes, which could be used as biomarkers in the diagnosis of early breast cancer. Exosome-delivered nucleic acid and proteins partly facilitate the tumorigenesis, metastasis and resistance of breast cancer. Exosomes could also transmit anti-cancer drugs outside breast cancer cells, therefore leading to drug resistance. However, exosomes are effective tools for transportation of anti-cancer drugs with lower immunogenicity and toxicity. This is a promising way to establish a drug delivery system.
Keywords: Breast cancer, exosomes, metastasis, resistance, therapeutics
Breast cancer is the most frequently-diagnosed life-threatening cancer and the leading cause of cancer-related death in women.1 The poor prognosis of most breast cancer patients is due to late diagnosis. Markers of tissues, genes and serum are presently used in diagnosis, but these markers do not work in the diagnosis of breast cancer at an early stage.2 Emerging evidence shows that various factors, including microRNA (miRNA), and proteins participate in the development or progression of breast cancer.3–5 Distant metastatic and local recurrent tumors are the main causes of death in the clinic. Signaling pathways, growth factors and miRNA participate in distant metastasis of breast cancer.6 De novo and acquired resistance to anticancer agents remains a major obstacle in the treatment of breast cancer.
Transportation between cells and the outside environment can be divided into active, passive and vesicular types. Vesicular transport and especially exosome-mediated transport, which play a vital role in cellular transport, have been studied extensively.7 Cell-secreted exosomes communicate with the microenvironment through the delivery of proteins, nucleic acid and other substances. Deregulation of exosome-mediated transport leads to disease development.8 Exosomes function as versatile promoters in the tumorigenesis, metastasis and drug resistance of breast cancer. In this review we summarize the current knowledge on the functions of exosomes in the diagnosis, tumorigenesis, metastasis, microenvironment, drug resistance and therapy of breast cancer.
Exosomes
Exosomes are nano-sized vesicles (diameter 40–100 nm) released upon the fusion of multivesicular bodies with plasma membranes in a variety of mammalian cells.9 Exosomes were originally regarded as a form of cell surface molecule removed from reticulocytes. In fact, exosomes exist extensively in body fluids such as blood, urine, ascites and amnionic fluid. The release of exosomes is regulated by some Rab family proteins, including Rab27a and Rab27b.10 Exosomes consist of lipid bilayer membranes and numerous molecular constituents of their original cells, including proteins and nucleic acid (summarized in Fig.1).11 Exosomes export many proteins that are tumor promoters or suppressors.12 For example, heat shock proteins, p53, phosphatase and tensin homolog and adenomatous polyposis coli, which exist extensively in exosomes, are closely related with tumor development.13–16 MiRNA in exosomes account for a majority of circulatory miRNA, which have been investigated as biomarkers in different cancers.17 These findings indicate that exosomes play a critical role in the development of tumors.
Figure 1.
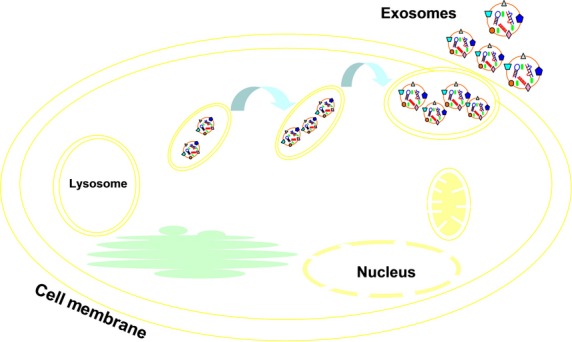
Exosomes consist of lipid bilayer membranes and numerous molecular constituents of their original cells, including proteins and nucleic acid material. The triangle, squares and circles on the membrane of exosome represent different surface molecules, such as glycoprotein and CD molecules.
Roles of Exosomes in Diagnosis of Breast Cancer
Diagnosis of breast cancer depends on imaging, biomarkers and pathology. Biomarkers are major method for breast cancer screening and early diagnosis. Molecular markers of breast cancer are classified into tissue, genetic and serum markers. Tissue markers include estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor-2 (HER-2), p53 and Ki-67.2 Negative results in ER and PR, HER-2 gene amplification, p53 mutation and increased Ki-67 status assays are significantly correlated with poorer prognosis.18–20 Serum markers cover a wide range, including carcinoembryonic antigen, CA 15.3, BR 27.29, MCA, CA 549-muc-1 family and oncoproteins.2 However, none of the markers above can be used in the diagnosis of breast cancer at an early stage.
Saliva is used as a noninvasive method to detect cancers at an early stage, including breast, pancreatic and oral cancers.21–23 As reported, salivary biomarkers (transcriptomic and proteomic signatures) are high-specificity and high-sensitivity discriminators for detection of early breast cancer.21 Exosomes derived from breast cancer (exo-BCa) can interact with salivary gland cells to alter the composition of the secreted exosomes both transcriptomically and proteomically.24 Distal breast cancer communicates with salivary glands through secretion of exosomes. In addition, the salivary biomarkers partly result from the exo-BCa-delivered proteins and mRNA. Monitoring the mRNA and protein expressions of salivary biomarkers among persons at high risk of breast cancer serves as a new efficient way for detection of breast cancer. Similarly, pancreatic cancer-derived exosomes could also lead to secretion of salivary biomarkers.25 The levels of serum exosomal Survivin (an inhibitor of apoptosis protein) and its splice variants are significantly higher in breast cancer patients compared to those who are disease-free for 5 years.26 Exosomes could play an important role as biomarkers of early breast cancer. This result may serve as a diagnostic and/or prognostic marker in early breast cancer patients.
Exosomes in Breast Cancer Tumorigenesis
It is generally supposed that cancers may originate from cancer stem cells which possess stem-like self-renewing ability and are the initial cellular components within a tumor that facilitate tumor progression.27 Breast cancer may arise from breast cancer stem cells which are able to proliferate extensively and generate differentiated breast cancer cells.28 The differentiated breast cancer cells are characterized by the ability of anti-apoptosis and cell cycle dysregulation.
Exosomes stimulated by hypoxia, heparanase and other factors are associated with angiogenesis of breast cancer, which is the most significant part of breast cancer tumorigenesis.29,30 Exosomes promote the formation of tumor blood vessels that support tumor growth and extension. Exo-BCa are proved to increase proliferation and reduce apoptosis of the surrounding normal breast cells. Exo-BCa can restrain immunological responses through regulating the expression of the NKG2D receptor by effector cells, which promotes breast cancer immune evasion.31 The NKG2D-positive cells are considerably reduced in a dose-dependent way when exo-BCa is cultured with fresh peripheral blood leukocytes. The reduction of T cells may be one way for breast cancer cells to escape from immune recognition and attack. Exosomes derived from heparanase-high breast cancer cells could enhance the spread of cancer cells.29 Heparanase-regulated exo-BCa is associated with enhanced tumor growth and angiogenesis. Exosomes- transported miRNA and proteins could promote neoplastic transformation and widely participate in different stages of breast cancer development.3,32,33 One way by which exosomes promote tumorigenesis is to convert tumor microenvironment to permissive niches. In what follows, we will elaborate on the tumor microenvironment.
Exosomes in Microenvironment, Invasion and Metastasis of Breast Cancer
It is generally recognized that both tumor cells and their microenvironment contribute to tumor progression and metastasis. The significance of tumor microenvironment is enhanced by the inclusion of microenvironment in “hallmarks of cancer.”34,35 The tumor microenvironment is comprised of stromal cells, soluble factors, extracellular matrix, signaling molecules, hypoxia and mechanical cues (e.g. exosomes).36 Stromal cells are comprised of fibroblasts, vascular system cells and immune cells, while soluble factors include growth factors, hormones, cytokines and chemokines.37,38 Combinations of tumor microenvironment factors can support tumor progression by helping tumor cells to escape from host immunity and drug treatments, and offer niches for metastasis.39 Hypoxia facilitates the release of exosomes by breast cancer cells and, therefore, hypoxic breast cancer cells may release more exosomes into the microenvironment to promote tumor survival.30 Exo-BCa could lead to induction of reactive oxygen species and autophagy when interacting with primary human mammary epithelial cells (HMEC).40 HMEC–exosome interactions also induce the phosphorylation of ATM, H2AX, Chk1 and p53, indicating the induction of DNA damage repair responses and stabilization of p53. The consequential transformation of microenvironment is more suitable for cancer survival. In what follows, we will discuss the role of exosomes (an important part of microenvironment) in invasion and metastasis of breast cancer.
Most breast cancer-induced deaths are due to high invasiveness and distant metastasis of breast cancer. No effective solution can cure advanced breast cancer. Researchers are still trying to understand the drivers of migratory cells.41 The roles of exosomes in invasion and metastasis of breast cancer are gradually being clarified. Amphiregulin exosomes enhance the invasiveness of recipient breast cancer cells, and exosomes contribute to breast cancer invasion through the epidermal growth factor receptor ligand signaling.42 Exosomes promote cell invasion in breast cancer through the transfer of miR-10b, which is overexpressed in MDA-MB-231 breast cancer cells as compared to non-malignant breast cells.43 In addition, exosomes derived from MDA-MB-231 cells could induce the invasion of non-malignant HMEC. Exosomes modulate tumor microenvironment by delivering miRNA. Tumor-associated macrophages promote the invasion and metastasis of breast cancer through exosome-mediated delivery of invasion-potentiating miRNA to breast cancer.44
Using fluorescent proteins as an exosome marker, Suetsugu et al. (2013) observe that exosomes produced by breast cancer cells move to other cancer cells and normal lung tissue cells in orthotopic nude mouse breast cancer models.45 This direct evidence proves that exosomes transfer from breast cancer cells to stroma at metastatic sites. Adipose tissue-derived mesenchymal stem cells (MSC) could be transformed into myofibroblasts via a Smad-mediated pathway after treatment with breast cancer exosomes.46 Tumor-associated myofibroblasts play a key role in tumor cell metastasis by participating in tumor angiogenesis and forming matrix-remodeling proteins within tumor microenvironment.47 Fibroblast-secreted exosomes facilitate the protrusive activity and motility of breast cancer cells via Wnt-planar cell polarity signaling.48 This study reveals that exosomes from stroma in tumor microenvironment play a critical role in breast cancer metastasis. In addition, MSC-derived exosomes contribute to migration of the breast cancer cell line MCF-7 through the Wnt signaling pathway.49 Breast cancer cells interact with MSC to promote breast cancer migration. Exosome-mediated transfer of miR-105 can destroy the tight junctions and the integrity of endothelial monolayers, thereby promoting the metastatic progression of breast cancer.50 MiR-105 overexpression in local breast cancer cells enhances vascular permeability and induces metastasis, but these effects are reduced by the suppression of miR-105 in metastatic breast cancer.
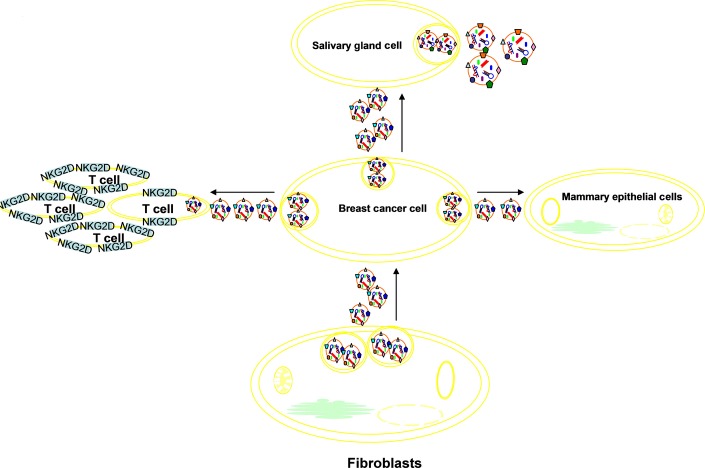
Figure2 presents a summary diagram of exosomes interacting with salivary gland cells and NKG2D-positive cells, and acting in microenvironment, invasion and metastasis of breast cancer.
Figure 2.
Exosomes derived from breast cancer (exo-BCa) interact with salivary gland cells, altering the composition of their secreted exosomes, which could be biomarkers of early breast cancer. Exo-BCa reduce NKG2D receptor expression of T cells to protect breast cancer cells from immune recognition and attack. Exo-BCa could induce the invasion ability of non-malignant human mammary epithelial cells. Exosomes secreted by fibroblasts in microenvironment facilitate breast cancer cells’ protrusive activity and motility.
Exosomes in Breast Cancer Resistance
De novo and acquired resistances to radiation, chemotherapy or targeted therapies are significant challenges in the treatment of breast cancer.51 Emerging evidence indicates that cancer stem cells or cancer-initiating cells may contribute to radiation resistance of breast cancer.52,53 Deregulation of tumor-associated miRNA, proteins and signal transduction pathways is involved in breast cancer chemoresistance.54,55 Trastuzumab resistance is partly induced by cancer-associated fibroblasts through increasing cancer stem cells and activating multiple pathways in HER2-positive breast cancer.56 The findings above suggest that various factors participate in development of breast cancer resistance.
Exosomes transfer RNA and proteins to mediate the communication between stromal cells and cancer cells, which can influence treatment response. Exosomes transferred from stromal to breast cancer cells contribute to chemotherapy and radiation resistance through antiviral and NOTCH3 pathways.57 These exosomes increase the interferon-related DNA damage resistance signature and enhance the transcription of NOTCH target genes, thereby promoting the resistance through expanding the spectrum of therapy-resistant breast cancer cells. Release of exosomes can be promoted by hypoxia and exosomes that are associated with the radiation resistance of tumor cells under hypoxic conditions.58,30 Chronic hypoxia can alter DNA damage repair pathways and thereby induce DNA replication errors and genetic instability, which contribute to radiation resistance.59 Therefore, hypoxia-induced radiation resistance may partly be due to the delivery of exosomes. Exosomes from drug-resistant breast cancer cells transmit chemoresistance through the delivery of p-gp and miRNA.60,61 Drug-sensitive variant MCF-7 cell line (MCF-7/S) can acquire drug resistance in the presence of exosomes from docetaxel (DOC/exo)-resistant MCF-7 breast cancer cells.60 Meanwhile, p-gp expression of MCF-7/S increases and is influenced by the dose of exosomes. These results point to the fact that docetaxel-resistant breast cancer cells assimilate other breast cancer cells to acquire chemoresistance via exosome delivery of p-gp. After coculture with DOC/exo, the general resistance of MCF-7/S is enhanced and levels of some miRNAs are distinctively increased, and thereby, the general resistance of MCF-7/S is enhanced after coculture.61 In addition, DOC/exo is capable of altering gene expression in MCF-7/S. Accumulation of anticancer drugs in exosomes/vesicles that shed out of cancer cells is a drug efflux mechanism involved in drug resistance.62 Docetaxel resistance is related to the enhancement of exosome secretion in a prostate cancer model, probably due to docetaxel efflux through exosomes.63 However, this hypothesis has not been proven in any breast cancer model, and should be verified through further studies. Figure3 presents a summary diagram of exosomes in breast cancer resistance.
Figure 3.
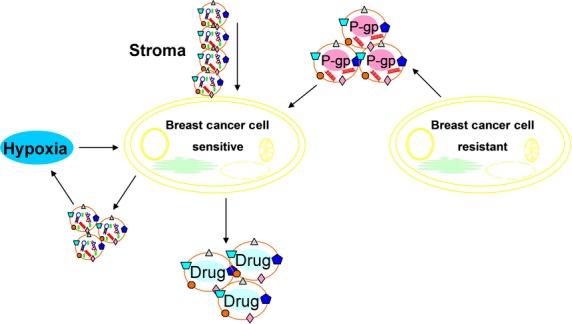
Exosomes lead to transferred from stroma to breast cancer cells contributed to chemotherapy and radiation resistance. Release of exosomes is promoted by hypoxia and exosomes are associated with radiation resistance under hypoxic conditions. Exosomes from drug-resistant breast cancer cells transmit chemoresistance through delivering p-gp and miRNA. Accumulation of anticancer drugs in exosomes/vesicles that shed out of breast cancer cells is a drug efflux mechanism involved in drug resistance.
Exosomes in Breast Cancer Therapeutics
Surgical treatment, chemotherapy, radiotherapy, hormone therapy and target therapy are currently recognized as effective methods for treatment of breast cancer. Rapidly-dividing cells, such as breast cancer cells, hair follicle cells, gastrointestinal tract cells and bone marrow cells, are targets of chemotherapy and radiotherapy.64 Hormone therapy has been used in patients with positive estrogen receptor and progestrone receptor.65 However, the therapeutic effects and palliative/unpredictable responses of the two treatments are limited by the side effects on normal cells. Drug resistance is also responsible for treatment failure. Thus, more attention should be devoted to research on novel therapies for breast cancer. In what follows, we discuss the role of exosomes in breast cancer therapeutics.
Exosomes that are endogenous nano-sized membrane vesicles can be used to carry drugs with low immunogenicity and toxicity.66 Exosomes after modification to target breast cancer cells could be used to deliver doxorubicin (dox).67 Purified exosomes loaded with dox via electroporation, produced by mouse immature dendritic cells to reduce immunogenicity and toxicity, are highly efficient at targeting and dox delivery, leading to significant inhibition of breast cancer without obvious toxicity. As reported, exosomes derived from epigallocatechin gallate are able to inhibit infiltration of tumor-associated macrophage and polarization of M2 macrophages, thereby suppressing breast cancer growth.68 Exosomes have the potential to worsen the tumor microenvironment for cancer growth. Immunity escape is an important characteristic of breast cancer. Curcumin-pretreated exo-BCa could reverse immune suppression of NK cell activation, which may account for anti-cancer properties of curcumin. In other words, exosomes are able to transmit anti-tumor substances to recover the surveillance of immune system. Exosomes released by adenoviral vector (AdVHER2)-transfected dendritic cells proved capable of inducing HER2-specific cytotoxic T lymophocyte responses and protective immunity against trastuzumab-resistant breast cancer in vitro.69 This novel HER2-TEXO vaccine may offer a better therapeutic choice for HER2-positive breast cancer patients, especially trastuzumab-resistant patients. Nevertheless, there are also some unsolved problems in the clinical application of exosomes. The acquisition of plenty of exosomes is inconvenient owing to the low number of exosomes. Thus, researchers must seek better methods to produce plenty of exosomes without great cost. The way in which exosomes should enter the body is also controversial. Intravenous injection is convenient but the exosomes should be targeted to breast cancer cells, which is a challenge for exosome design. Local injection avoids the problem of targeted delivery design, but it faces new problems. Whether locally injected exosomes are absorbed effectively and cover all breast cancer cells remains unresolved. Figure4 presents a summary diagram of exosomes in breast cancer therapeutics.
Figure 4.
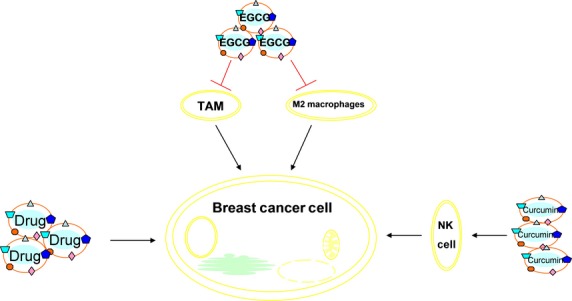
Exosomes derived from epigallocatechin gallate were able to suppress breast cancer growth by inhibiting tumor-associated macrophage infiltration and M2 macrophage polarization. Exo-BCa pretreated with curcumin could reverse immune suppression of NK cell activation. Exosomes loaded with anti-cancer drugs showed highly efficient targeting and drug delivery, leading to significant inhibition of breast cancer without obvious toxicity and immunogenicity.
Conclusions and Prospects
Exosomes are nano-sized vesicles that mediate inter-cellular and intra-cellular communication. There is growing evidence that exosomes play an essential role in the process of pathological states. Exosomes could function as biomarkers of various cancers, including breast cancer. The contents and quantity of exosomes released by breast cancer cells change compared to normal breast cells. Dysregulation of exosomes in body fluid indicates that diseases may lack sensitivity and specificity. Exosomes are potential carriers of drugs targeting breast cancer cells. In this article, we review studies on how exosomes act as a drug delivery tool in vitro. However, there is a long way to go before exosomes can be utilized to establish a drug delivery system in vivo. Further studies are required to better understand the role of exosomes in the occurrence of breast cancer. In conclusion, we summarize the main roles of exosomes in the occurrence and progression of breast cancer, and provide a new perspective on the drug delivery system.
Acknowledgments
We thank Shan-Liang Zhong and Wei-Xian Chen for useful discussions and help in revision of the present paper. This study was supported by the National Natural Science Foundation of China (81272470).
Disclosure Statement
The authors have no conflict of interest to declare.
References
- DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- Donepudi MS, Kondapalli K, Amos SJ, Venkanteshan P. Breast cancer statistics and markers. J Cancer Res Ther. 2014;10:506–511. doi: 10.4103/0973-1482.137927. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Ma SL. miRNAs in breast cancer tumorigenesis (Review) Oncol Rep. 2012;27:903–910. doi: 10.3892/or.2011.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Kao HY. Alpha-actinin 4 and tumorigenesis of breast cancer. Vitam Horm. 2013;93:323–351. doi: 10.1016/B978-0-12-416673-8.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia LP, Zhou FF, Yang MT, Liu Q. Roles of Aurora-A in tumorigenesis and prognosis of breast cancer. Ai Zheng. 2009;28:668–672. [PubMed] [Google Scholar]
- Rahim F, Hajizamani S, Mortaz E, et al. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014;2014:405920. doi: 10.1155/2014/405920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staals RH, Pruijn GJ. The human exosome and disease. Adv Exp Med Biol. 2010;702:132–142. [PubMed] [Google Scholar]
- Kosaka N, Yoshioka Y, Tominaga N, Hagiwara K, Katsuda T, Ochiya T. Dark side of the exosome: the role of the exosome in cancer metastasis and targeting the exosome as a strategy for cancer therapy. Future Oncol. 2014;10:671–681. doi: 10.2217/fon.13.222. [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. 1–13. [DOI] [PubMed] [Google Scholar]
- van den Boorn JG, Daßler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65:331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Begne M, Lu B, Han X, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem J. 1995;308:823–830. doi: 10.1042/bj3080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- Putz U, Howitt J, Doan A, et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:a70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- Lim JW, Mathias RA, Kapp EA, et al. Restoration of full-length APC protein in SW480 colon cancer cells induces exosome-mediated secretion of DKK-4. Electrophoresis. 2012;33:1873–1880. doi: 10.1002/elps.201100687. [DOI] [PubMed] [Google Scholar]
- Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura Y, Nishimura R, Nakatsukasa K, et al. Change in estrogen receptor, HER2, and Ki-67 status between primary breast cancer and ipsilateral breast cancer tumor recurrence. Eur J Surg Oncol. 2015;41:548–552. doi: 10.1016/j.ejso.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Alco G, Bozdogan A, Selamoglu D, et al. Clinical and histopathological factors associated with Ki-67 expression in breast cancer patients. Oncol Lett. 2015;9:1046–1054. doi: 10.3892/ol.2015.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JS, Fletcher JA, Linette GP, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xiao H, Karlan S, et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One. 2010;5:e15573. doi: 10.1371/journal.pone.0015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Farrell JJ, Zhou H, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138:949–957. doi: 10.1053/j.gastro.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali SB. Proteomics for oral cancer. Oral Oncol. 2014;50:e67. doi: 10.1016/j.oraloncology.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Lau CS, Wong DT. Breast cancer exosome-like microvesicles and salivary gland cells interplay alters salivary gland cell-derived exosome-like microvesicles in vitro. PLoS One. 2012;7:e33037. doi: 10.1371/journal.pone.0033037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Kim Y, Chia D, et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013;288:26888–26897. doi: 10.1074/jbc.M113.452458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, Bennit HF, Turay D, et al. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC Cancer. 2014;14:176. doi: 10.1186/1471-2407-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj M. Cancer stem cells and oncology therapeutics. Curr Opin Oncol. 2007;19:61–64. doi: 10.1097/CCO.0b013e328011a8d6. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene. 2004;23:7274–7282. doi: 10.1038/sj.onc.1207947. [DOI] [PubMed] [Google Scholar]
- Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288:10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol Dis. 2005;34:206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Jiang H, Li Z, Li X, Xia J. Intercellular transfer of messenger RNAs in multiorgan tumorigenesis by tumor cell-derived exosomes. Mol Med Rep. 2015;11:4657–4663. doi: 10.3892/mmr.2015.3312. [DOI] [PubMed] [Google Scholar]
- Ahmed SF1, Das N1, Sarkar M1, Chatterjee U2, Chatterjee S2, Ghosh MK. Exosome-mediated delivery of the intrinsic C-terminus domain of PTEN protects it from proteasomal degradation and ablates tumorigenesis. Mol Ther. 2015;23:407. doi: 10.1038/mt.2014.202. Corrigendum to “ ”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Kalluri R. The role of the microenvironment in mammary gland development and cancer. Cold Spring Harb Perspect Biol. 2010;2:a3244. doi: 10.1101/cshperspect.a003244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Warshall C, Bandyopadhyay C, Dutta D, Chandran B. Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells. PLoS One. 2014;9:e97580. doi: 10.1371/journal.pone.0097580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- Higginbotham JN, Demory BM, Gephart JD, et al. Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 2011;21:779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65:383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes Cancer. 2011;2:1139–1145. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Lin R, Wang S, Zhao RC. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol Cell Biochem. 2013;383:13–20. doi: 10.1007/s11010-013-1746-z. [DOI] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25:501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Lagadec C, Vlashi E, Della DL, et al. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res. 2010;12:R13. doi: 10.1186/bcr2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Tekiner TA, Basaga H. Role of microRNA deregulation in breast cancer cell chemoresistance and stemness. Curr Med Chem. 2013;20:3358–3369. doi: 10.2174/09298673113209990003. [DOI] [PubMed] [Google Scholar]
- Kuo MT. Roles of multidrug resistance genes in breast cancer chemoresistance. Adv Exp Med Biol. 2007;608:23–30. doi: 10.1007/978-0-387-74039-3_2. [DOI] [PubMed] [Google Scholar]
- Mao Y, Zhang Y, Qu Q, et al. Cancer-associated fibroblasts induce trastuzumab resistance in HER2 positive breast cancer cells. Mol BioSyst. 2015;11:1029–1040. doi: 10.1039/c4mb00710g. [DOI] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159:499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldh M, Ekstrom K, Valadi H, et al. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5:e15353. doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Lv MM, Zhu XY, Chen WX, et al. Exosomes mediate drug resistance transfer in MCF-7 breast cancer cells and a probable mechanism is delivery of P-glycoprotein. Tumour Biol. 2014;35:10773–10779. doi: 10.1007/s13277-014-2377-z. [DOI] [PubMed] [Google Scholar]
- Chen WX, Liu XM, Lv MM, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9:e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- Corcoran C, Rani S, O’Brien K, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- Hussain HA, Harvey AJ. Evolution of breast cancer therapeutics: breast tumour kinase’s role in breast cancer and hope for breast tumour kinase targeted therapy. World J Clin Oncol. 2014;5:299–310. doi: 10.5306/wjco.v5.i3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Kim H, Liu C, et al. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xie Y, Ahmed KA, et al. Exosomal pMHC-I complex targets T cell-based vaccine to directly stimulate CTL responses leading to antitumor immunity in transgenic FVBneuN and HLA-A2/HER2 mice and eradicating trastuzumab-resistant tumor in athymic nude mice. Brest Cancer Res TR. 2013;140:273–284. doi: 10.1007/s10549-013-2626-7. [DOI] [PubMed] [Google Scholar]