Abstract
We previously identified Aurora B kinase as the only independent factor predictive of the aggressive recurrence of hepatocellular carcinoma (HCC). In this preclinical study, JNJ-28841072, a novel Aurora/vascular endothelial growth factor receptor dual kinase inhibitor, was evaluated for treatment of HCC. In vitro and in vivo effects of JNJ-28841072 were analyzed using human HCC cell cultures and xenograft models. An orthotopic liver xenograft model was used for the pharmacobiological effects on Aurora kinase and vascularization in hepatic tumors. JNJ-28841072 suppressed in vitro phosphorylation of histone H3 with induction of cell polyploidy and death in a dose-dependent manner (IC50 = 0.8–1.2 μM). In s.c. human HCC xenografts, remarkable inhibition of tumor growth was observed after JNJ-28841072 treatment (P = 0.0005). In orthotopic liver xenografts, the treatment with JNJ-28841072 significantly suppressed in vivo phosphorylation of histone H3 (P = 0.0008), vessel formation (P = 0.018), normoxic area (P = 0.0001), and hepatoma growth (P = 0.038). Our preclinical studies indicate that JNJ-28841072 is a promising novel therapeutic approach for the treatment of HCC. It might be worthy of evaluation in further studies.
Keywords: Angiogenesis, Aurora, dual kinase inhibitor, hepatocellular carcinoma, molecularly targeted medicine
Hepatocellular carcinoma (HCC) is the most common primary form of liver cancer and the third most deadly type of cancer globally, following lung and stomach cancers. With more than 750 000 new cases diagnosed every year worldwide, HCC is the fifth most common neoplasm. Patients with early-stage HCC are generally given curative treatments such as surgery or local ablation, patients with intermediate-stage HCC usually receive transarterial chemoembolization, and systemic therapies are considered in patients with advanced-stage HCC.1 A major obstacle for the treatment of HCC is the high frequency of tumor recurrence after curative resection.2 Sorafenib has shown improved overall survival in patients with advanced HCC.3 However, the median overall survival among patients with advanced HCC is still less than 1 year and the prognosis remains poor.4 None of the other drugs tested have shown positive results after sorafenib progression. Reasons for failure are heterogeneous and include lack of understanding of critical drivers of tumor progression/dissemination.5
Sorafenib is a small molecule that inhibits tumor cell proliferation and tumor angiogenesis. It acts by inhibiting the serine–threonine kinases Raf-1 and the receptor tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3 and platelet-derived growth factor receptor β. Sorafenib is effective for patients with advanced renal cell carcinoma and unresectable HCC.3 Inhibition of Raf kinase leads to a decrease in mitotic index and metaphase cell number, and a defective spindle checkpoint through Aurora B kinase inhibition.6 In our previous studies on HCC patients after curative resection, aggressive recurrence exceeding Milan criteria showed extremely poor prognosis.7 In addition, we identified overexpression of Aurora B kinase as the only independent factor predictive of aggressive recurrence,8 and inhibition of Aurora kinase as a promising novel therapeutic approach for the treatment of HCC.9
The Aurora kinase family of serine–threonine kinases control chromosome assembly and segregation during mitosis. In mammals, the Aurora kinase family consists of three members, Aurora A and B kinases and the less-characterized Aurora C kinase.10 Aberrant expression of the Aurora kinases has been reported in a variety of solid tumors including breast, colon, and pancreas.11,12 Currently there are several Aurora kinase inhibitors undergoing phase I and phase II clinical studies for blood cancer and solid cancers, and a more sufficient effect is clinically expected with Aurora inhibition.13
In recent reports, the combined inhibition of antimitotic activity and angiogenesis is receiving much attention.14,15 JNJ-28841072 is a novel molecule that inhibits Aurora and VEGFR kinases.16 The purpose of this study was to evaluate the antitumor activity of the Aurora and angiogenic kinase inhibitor JNJ-28841072 against preclinical models of HCC with identification of candidate predictive biomarkers.
Material and Methods
Reagents
JNJ-28841072 [7-[1H-indol-2-yl]-2, 3-dihydro-isoindol-1-ones] was provided by Johnson & Johnson Pharmaceutical Research and Development (Spring House, PA, USA). The compound was prepared as a 10-mg/mL stock solution in 20% hydroproxypropyl-β-cyclodextrin (Sigma-Aldrich Co., St. Louis, MO, USA), 10 mg/mL, for intraperitoneal (i.p.) and per oral formation. As the solution was cloudy, 1 N HCl was added to adjust to pH 4 with mild heating on a stirring plate. The solution became clear after 1–2 h of stirring.
Cell culture
The human HCC cell lines HuH-7 and HLF were obtained from the Human Science Research Resources Bank (Osaka, Japan). Other human HCC cell lines SK-Hep1 and Hep3B were obtained from ATCC (Manassas, VA, USA). Culture media were RPMI-1640 (HuH-7, SK-Hep1, and Hep3B) and DMEM (HLF), supplemented with 5% FBS for HLF cells or 10% FBS for the remaining cell lines. All media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin; all cell lines were cultivated in a humidified incubator at 37°C in 5% CO2 and harvested with 0.05% trypsin–0.03% EDTA. Luciferase expression plasmid pGL4.50 (luc2/CMV/Hygro) (#E131A; Promega, Madison, WI, USA) was transferred into HuH-7 and SK-Hep1 cells according to the manufacturer’s instructions and luciferase-expressing HuH-7 cells (HuH-7-Luc) and SK-Hep1 cells (SK-Hep1-Luc) were generated.
Analysis of cell proliferation and cell viability
All cell lines were cultured in the logarithmic growth phase in the presence of various concentrations of JNJ-28841072 (0.01–10 μM) for 48 h. Cells were seeded at 1 × 105 cells in 6-well plates with the appropriate control medium. After 24 h, plates were treated with compound and incubated for 48 h at 37°C. At the end of the incubation time, cells were detached from each plate, and viable cells were counted using a hemocytometer. Half-maximal inhibitory concentration (IC50) values were calculated with BioDataFit version 1.02 software, Chang Bioscience, Inc., Castro Valley, CA 94552, USA using the four-parameter logistic model. The mean values and standard deviations of IC50 were calculated in triplicate for each cell line. To investigate cell viability, triplicate samples of HuH-7, SK-Hep1, HLF, and Hep3B cells were cultured in the presence of various concentrations of JNJ-28841072 (0.01–10 μM) for 48 h. The number of non-viable cells was assessed using a hemocytometer and Trypan blue dye exclusion.
Flow cytometry
Samples of all cell lines in logarithmic growth phase were exposed to JNJ-28841072 at 3 μM for 48 h, and then fixed in 70% ethanol at 20°C overnight. Cells were rehydrated in PBS, then resuspended in PBS containing RNase 100 μg/mL (Sigma) and 10 μg/mL propidium iodide. Cellular DNA content was analyzed using FACSCaliber (BD Biosciences, San Jose, CA, USA).
Immunocytochemistry and immunohistochemistry
HuH-7, SK-Hep1, HLF, and Hep3B cells were cultured on glass slides coated with silane in the presence of various concentrations of JNJ-28841072 (0.3–3 μM) for 16 h. They were then fixed using 3.7% formalin for 10 min and permeabilized using 100% methanol for 20 min for immunocytochemical detection of phosphohistone H3 (PhH3). Xenograft tumor tissue was harvested, formalin fixed, and paraffin embedded. The primary antibody for PhH3 (catalog no. 9701; Upstate Cell Signaling Solutions, Danvers, MA, USA) was used at 1:100 dilution in PBS containing 1% BSA. The tissue sections and slides were stained with an automated immunostainer (BenchMark XT; Ventana Medical Systems, Tucson, AZ, USA) using heat-induced epitope retrieval and a standard diaminobenzidine detection kit (Ventana).
In vivo studies in s.c. tumor xenograft model
An s.c. tumor model was used to analyze the in vivo activity of JNJ-28841072, as described previously.9 Five-week-old female NOD.CB17-PRkdcScid/J mice were purchased from Charles River Laboratories (Kanagawa, Japan) and kept under pathogen-free conditions, fed standard food, and given free access to sterilized water. In all experiments, mice were anesthetized by 100 mg/kg pentobarbital (Nembural; Abbott Laboratories, North Chicago, IL, USA) i.p. injection. Subcutaneous xenografts were established by inoculating 1 × 107 HuH-7 cells into the right dorsal flank. Palpable tumors were confirmed on day 7 following inoculation, and mice were randomized into treatment groups to receive JNJ-28841072 or the control Tris-buffered saline. JNJ-28841072 was prepared in Tris-buffered saline (pH 4) and given by i.p. injection. Tumor size was measured using calipers as frequently as every 2 days for 2 weeks, and tumor volumes were calculated as AB2 × 0.5 (A, length; B, width). The Animal Care Committee of Tokyo Medical and Dental University School of Medicine (Tokyo, Japan) approved the experimental protocols in accordance with its institutional guidelines.
In vivo studies in orthotopic tumor xenograft model
An orthotopic xenograft model was created by direct intrahepatic inoculation of HuH-7–Luc cells, as essentially described in our studies.9 With the mice fully anesthetized, a small transverse incision was made below the sternum to expose the liver. Then, 5 × 106 cells suspended in 25 μL RPMI-1640 and 25 μL Matrigel (BD Biosciences, San Jose, CA, USA) were slowly injected at a 30° angle into the upper left lobe of the liver using a 28-gauge needle. After injection, a small piece of sterile gauze was placed on the injection site, and light pressure was applied for 1 min to prevent bleeding. The abdomen was then closed with a 6–0 silk suture. The pharmacobiological effects of JNJ-28841072 treatment in the orthotopic liver tumors were assessed by immunohistochemical analysis of PhH3 expression in control tumors and in those harvested 1 day after a single 100 mg/kg JNJ-28841072-cyclodextrin i.p. injection. Immunohistochemical analysis of CD31 (BD Biosciences) and pimonidazole (Hypoxyprobe-1; Cosmo Bio Co., Ltd., Tokyo, Japan) expression was carried out in tumors harvested 7 days after i.p. injection of JNJ-28841072-cyclodextrin (100 mg/kg) or control.
Results
In vitro effects of JNJ-28841072 in human HCC cells
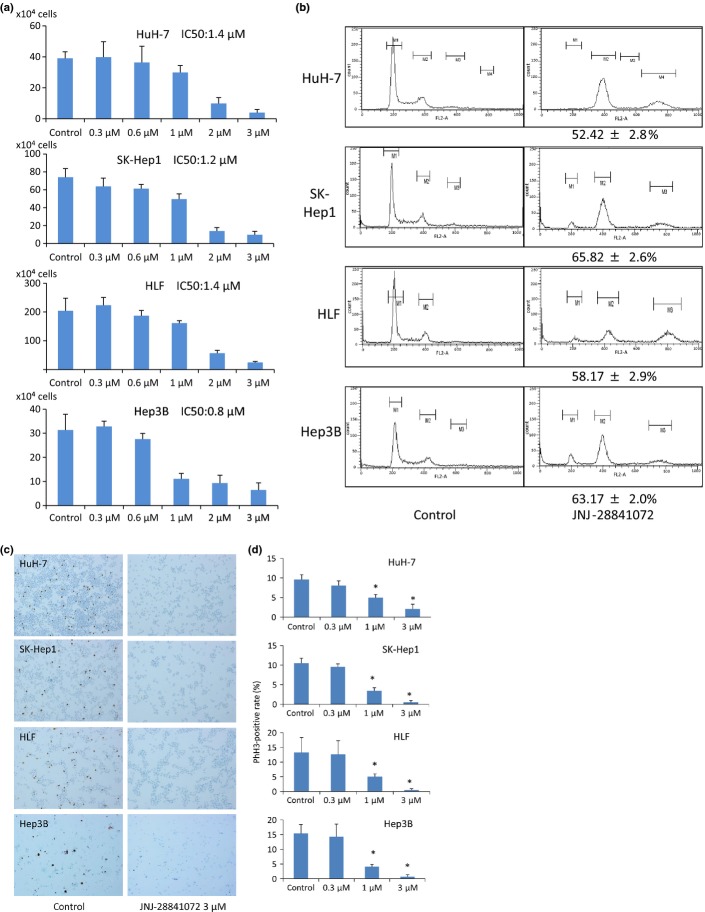
To evaluate the growth inhibitory effects of JNJ-28841072, cell proliferation assays were carried out in HCC cell lines (HuH-7, SK-Hep1, HLF, and Hep3B). JNJ-28841072 showed potent antiproliferative activity in all HCC cell types with the following IC50 values: HuH-7, 1.4 ± 0.3 μM; SK-Hep1, 1.2 ± 0.2 μM; HLF, 1.4 ± 0.2 μM; and Hep3B, 0.75 ± 0.15 μM (Fig.1a). Alterations in DNA ploidy in the human HCC cell lines were analyzed by flow cytometry. Accumulation of cells with >4N DNA content was observed in all of the cell lines following 24-h incubation with 3 μM JNJ-28841072 (HuH-7, 52.42 ± 2.8%; SK-Hep1, 65.82 ± 2.6%; HLF, 58.17 ± 2.9%; Hep3B, 63.17 ± 2.0%). The accumulation of polyploid cells is consistent with failed cytokinesis following inhibition of Aurora B kinase activity (Fig.1b).
Figure 1.
In vitro effects of JNJ-28841072 in human hepatocellular carcinoma (HCC) cells. (a) Representative bar graphs show cell viability rates (%) after incubation with various concentrations of JNJ-28841072 in each cell line. Vertical bars indicate SD. (b) Cellular DNA content was analyzed by flow cytometry in four human HCC cell lines after 24 h of incubation with 3 μM JNJ-28841072 or control DMSO buffer; the increasing rate of >4N DNA (%) is indicated. (c) Immunocytochemistry of phosphohistone H3 (PhH3) in human HCC cells after 16 h of incubation with 3 μM JNJ-28841072 or control DMSO buffer. Magnification, ×40. (d) PhH3-positive rate (%) of high power field. Vertical bars indicate SE. Statistical analysis used two-tailed Student’s t-test (*P < 0.05).
In vitro effects of JNJ-28841072 on phosphorylation of histone H3 in human HCC cell lines
The inhibition of Aurora B kinase is determined by its specific cellular substrate histone H3. We investigated whether JNJ-28841072 was able to inhibit PhH3 in the sensitive HuH-7, SK-Hep1, HLF, and Hep3B cells. JNJ-28841072 (3 μM) yielded a substantial reduction in the level of PhH3 (Fig.1c,d).
In vivo effects of JNJ-28841072 on s.c. xenografts of human HCC cells
To investigate in vivo antitumor activity, JNJ-28841072-cyclodextrin 100 mg/kg per day was given to NOD.CB17-PRkdcScid/J mice bearing established HuH-7 s.c. xenografts on two consecutive days per week for 2 weeks (n = 8). Tumor volumes were measured every other day. As shown in Figures2 and S1, significant regression of HuH-7 tumors was observed in the group of mice that received JNJ-28841072 compared with the control. The mean tumor volumes were substantially decreased by treatment with JNJ-28841072 on day 14 following treatment, and tumor volumes in treated mice were 19% of those in control mice. Similar growth inhibition was observed in SK-Hep1 s.c. xenografts by administration of JNJ-28841072 (Figs2 and S2). None of the JNJ-28841072-treated mice showed signs of wasting or other toxicity relative to control mice. JNJ-28841072 was tolerated at the dose at which antitumor efficacy was observed.
Figure 2.
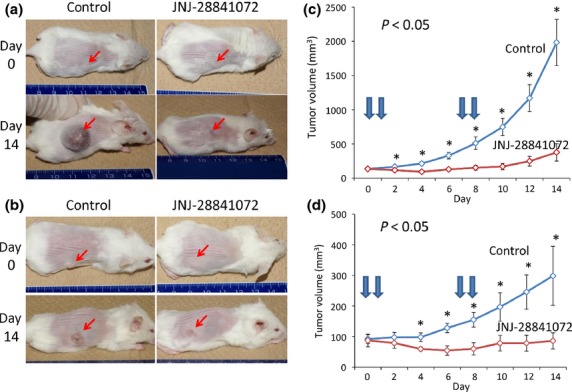
In vivo effects of JNJ-28841072 on human hepatocellular carcinoma growth in s.c. xenograft models. Established s.c. xenografts were treated with 100 mg/kg JNJ-28841072-cyclodextrin i.p. or control Tris buffer on two consecutive days per week for 2 weeks. (a,b) Subcutaneous tumors in mice on days 0 or 14 following treatment with JNJ-28841072 (right) or control (left). (c,d) Tumor volumes were measured and plotted every other day in JNJ-28841072-treated or control mice (n = 8). (a,c) HuH-7 cells. (b,d) SK-Hep1 cells. Arrows, treatment time; vertical bars indicate SE. Statistical analysis used two-tailed Student’s t-test (*P < 0.05).
In vivo effects of JNJ-28841072 on orthotopic liver xenografts of human HCC cells
An orthotopic xenograft model of liver tumors with Matrigel was used to explore tumor growth inhibition in situ, as essentially described in our studies.9 JNJ-28841072-cyclodextrin (100 mg/kg) was given to mice bearing HuH-7 orthotopic xenografts on two consecutive days per week for 2 weeks (n = 3). Histological analysis of the liver tumors was carried out within 4 weeks after treatment. Growth of liver tumors was found to be suppressed in all of the mice that had been treated with JNJ-28841072 (Figs3 and S3). After drug treatment, the mean liver tumor weight in those animals that had received JNJ-28841072 was 13% of that in the control mice. Similar growth inhibition was observed in SK-Hep1 orthotopic xenografts by treatment with JNJ-28841072 (Figs3 and S3). These results show that JNJ-28841072 was able to significantly inhibit in vivo growth of human HCC tumor in the liver microenvironment in mice. All of the host tissues examined, including liver, bone marrow, kidney, intestine, and lung, were histologically normal in all experiments.
Figure 3.
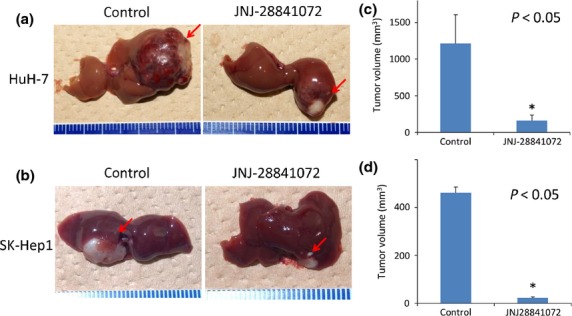
In vivo effects of JNJ-28841072 on human hepatocellular carcinoma growth in orthotopic xenograft models. Established orthotopic xenografts were treated with 100 mg/kg JNJ-28841072-cyclodextrin i.p. or control Tris buffer on two consecutive days per week for 2 weeks. (a,b) Liver tumors in mice on days 14 following treatment with JNJ-28841072 (right) or control (left). (c,d) Liver tumor volumes were measured in JNJ-28841072-treated or control mice (n = 3). (a,c) HuH-7 cells. (b,d) SK-Hep1 cells. Arrows, treatment time; vertical bars indicate SE. Statistical analysis used two-tailed Student’s t-test (*P < 0.05).
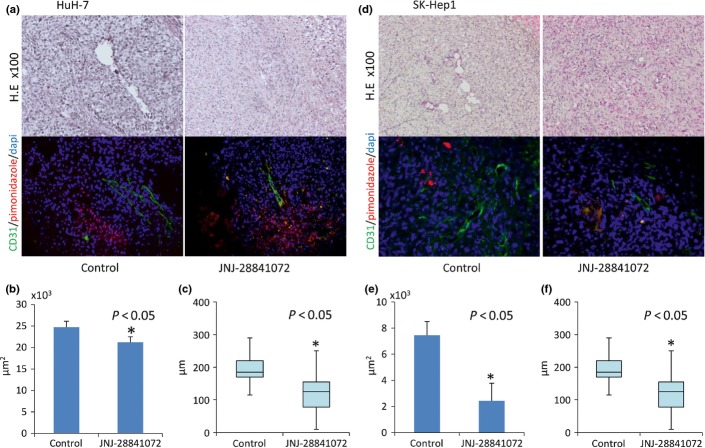
Pharmacobiological effects of JNJ-28841072 on orthotopic liver xenografts of human HCC cells
The liver xenograft model in HuH-7 described above was subjected to histological analysis by immunostaining to investigate the pharmacobiological effects of JNJ-28841072 in the hepatic microenvironment. Twenty-four hours after treatment with JNJ-28841072, there was a substantial decrease in PhH3 compared with the control (Fig.4). One week after treatment with JNJ-28841072, the total blood vessel area were substantially decreased by treatment with JNJ-28841072 and distance between tumor vessels and adjacent ischemic area compared with the control (Fig.5). Similar results were observed in SK-Hep1 orthotopic xenografts by treatment with JNJ-28841072 (Figs4c,d and 5d,e). The hepatocytes from the host liver were histologically normal at all points following JNJ-28841072 administration.
Figure 4.
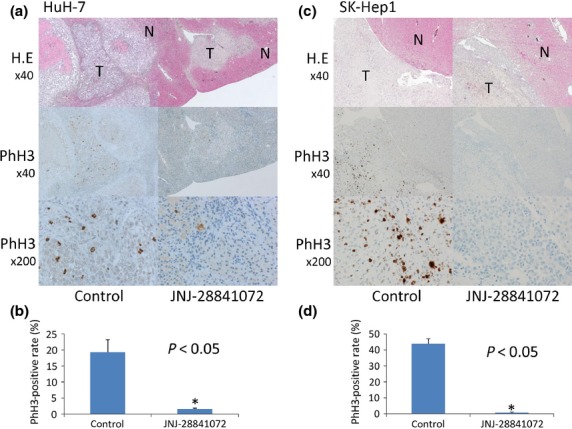
Pharmacobiological analysis of orthotopic xenograft models of hepatocellular carcinoma using HuH-7 cells (a,b) and SK-Hep1 cells (c,d). (a,c, upper) Transverse sections of liver tumor (T) or host normal liver (N) were stained with H&E. Magnification, ×40. The same sections were analyzed for expression of phosphohistone H3 (PhH3). (a,c, middle) magnification, ×40; (a,c, lower) magnification, ×200. (b,d) PhH3-positive rate (%) of high power field. Vertical bars indicate SE. Statistical analysis used two-tailed Student’s t-test (*P < 0.05).
Figure 5.
Pharmacobiological analysis of orthotopic xenograft models of hepatocellular carcinoma using HuH-7 cells (a–c) and SK-Hep1 cells (d–f). (a,d, upper) H&E staining; magnification, ×100. The same sections were analyzed for expression of CD31 (green) and pimonidazole (red). (a,d, lower) magnification, ×100. (b,e) Vascular area by immunohistochemical analysis of CD31 of high power field. (c,f) Distance between tumor vessels and adjacent ischemic area by immunohistochemical analysis of pimonidazol of high power field. Vertical bars indicate SE. Statistical analysis used two-tailed Student’s t-test (*P < 0.05).
Discussion
JNJ-28841072 has been developed as a novel dual kinase inhibitor affecting tumor progression and vascularization that inhibits both Aurora and VEGFR kinases. JNJ-28841072 showed potent antiproliferative activity in all HCC cell types. Our study showed a cell proliferation suppressant effect in vitro and tumor suppressant effect using human liver cancer cell strain in vivo with the phosphorylation inhibition of histone H3, which is a specific substrate of Aurora. In addition, JNJ-28841072 caused a vascularization suppressant effect with hypoxia in a liver tumor model and was able to show a remarkable liver tumor suppressant effect.
Active angiogenesis and frequent metastasis are responsible for rapid recurrence and poor survival of patients with HCC.17 Tumor vascularization is important as a treatment target of HCC.18 Sunitinib (Pfizer, New York, NY, USA) promotes VEGFR and platelet-derived growth factor receptor inhibition and is a molecularly targeted medicine with a strong vascularization suppressant effect. A clinical trial of sunitinib versus sorafenib in HCC was carried out, but sunitinib did not show results superior to sorafenib.19 Controlling vascularization of neoplasms by VEGFR inhibition can induce tumor shrinkage, but also hypoxic conditions in the tumor environment that activates and exacerbates cancer cells.20,21 According to previous studies,22 hypoxia stimulates Aurora activity and consequently increases the malignant potential of HCC cells. JNJ-28841072 is structurally optimized to inhibit the activities of Aurora and VEGFR. It might cut off the negative feedback loop induced by anti-angiogenic treatments. In our study, s.c. tumor volumes in treated mice were 5–13%, and orthotopic xenografts were 19–29% of those in control mice.
For tumor progression in vivo, various pathways involving the tumor cell and the microenvironment function synergistically.23 The role of the organ microenvironment in cancer is particularly important for HCC, an organotropic cancer in which the liver-specific microenvironment may play a critical role in HCC tumor development, angiogenesis, cellular apoptosis, and drug sensitivity. In this study of JNJ-28841072 for HCC, the in vivo efficacy was evaluated using not only an s.c. model but also orthotopic liver cancer model that more closely mimics clinical tumorigenicity of HCC. Treatment to inhibit only one pathway by monotargeted drugs might adversely activate other pathways, and drug resistance by these interactions emerges quickly.24 Inhibition of multiple targets, including a tumor factor and a host factor, is important to cancer treatment through combined molecularly target medicine, and is thought to be effective for advanced cancer.25 For HCC, targeting only for antitumor effects is inadequate. Treatment for HCC must combine targeting of the tumor environment along with an antitumor effect. Toward that end, we proved a liver tumor suppressant effect using the dual kinase inhibitor JNJ-28841072. To support the clinical evaluation of JNJ-28841072, more data and further study is required for drug-related adverse events and biomarkers. We used PhH3 and CD31 in tumor tissue as markers to assess Aurora kinase inhibition and anti-angiogenesis, respectively. Because both markers sensitively reflected the disease, we expect them to be superior biomarkers. However, further study is necessary to develop non-invasive methods of measurement. In addition, drug-related adverse events were not observed in JNJ-28841072-treated mice in our study, and all of the host tissues examined, including liver and bone marrow, were histologically normal in all experiments. Of course, additional toxicological studies must be carried out prior to clinical trials to predict drug-related adverse events and organ disorder. Nevertheless, our preclinical work showed that further studies are justified in preparation for clinical applications of JNJ-28841072 in HCC.
Acknowledgments
This work was supported by the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Grant-in-Aid for Scientific Research on Innovative Areas, Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a Health and Labor Sciences Research Grant from the Ministry of Health, Labor and Welfare of Japan. We thank Johnson & Johnson Pharmaceutical Research and Development (Spring House, PA, USA) for kindly providing us with JNJ-28841072 for experimental studies. We also thank Dr. Peter Connolly (Janssen Research & Development LLC, Spring House, PA, USA) for helpful review on our manuscript.
Disclosure Statement
The authors have no conflict of interest.
Supporting Information
Fig. S1. Subcutaneous hepatocellular carcinoma tumor models of Huh7 cells on day 14. Representative images are s.c. tumors derived from Huh7 in mice on day 14 following treatment with JNJ-28841072 or control. Bar = 10 mm.
Fig. S2. Subcutaneous hepatocellular carcinoma tumor models of SK-Hep1 cells on day 14. Representative images are s.c. tumors derived from SK-Hep1 in mice on day 14 following treatment with JNJ-28841072 or control. Bar = 10 mm.
Fig. S3. Orthotopic models of hepatocellular carcinoma on day 14. Representative images of liver tumors in mice on day 14 following treatment with JNJ-28841072 or control. Lower, SK-Hep1; upper, HuH-7. Bar = 10 mm.
References
- Marquardt JU, Thorgeirsson SS. SnapShot: hepatocellular carcinoma. Cancer Cell. 2014;25:550.e1. doi: 10.1016/j.ccr.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Worns MA, Galle PR. HCC therapies–lessons learned. Nat Rev Gastroenterol Hepatol. 2014;11:447–52. doi: 10.1038/nrgastro.2014.10. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–9. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- Eves EM, Shapiro P, Naik K, Klein UR, Trakul N, Rosner MR. Raf kinase inhibitory protein regulates aurora B kinase and the spindle checkpoint. Mol Cell. 2006;23:561–74. doi: 10.1016/j.molcel.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Noguchi N, Ochiai T, et al. Outcomes and recurrence of initially resectable hepatocellular carcinoma meeting milan criteria: rationale for partial hepatectomy as first strategy. J Am Coll Surg. 2007;204:1–6. doi: 10.1016/j.jamcollsurg.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Arii S, Yasen M, et al. Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. Br J Surg. 2008;95:611–9. doi: 10.1002/bjs.6011. [DOI] [PubMed] [Google Scholar]
- Aihara A, Tanaka S, Yasen M, et al. The selective Aurora B kinase inhibitor AZD1152 as a novel treatment for hepatocellular carcinoma. J Hepatol. 2010;52:63–71. doi: 10.1016/j.jhep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Keen N, Taylor S. Aurora-kinase inhibitors as anticancer agents. Nat Rev Cancer. 2004;4:927–36. doi: 10.1038/nrc1502. [DOI] [PubMed] [Google Scholar]
- Lee EC, Frolov A, Li R, Ayala G, Greenberg NM. Targeting Aurora kinases for the treatment of prostate cancer. Cancer Res. 2006;66:4996–5002. doi: 10.1158/0008-5472.CAN-05-2796. [DOI] [PubMed] [Google Scholar]
- Li D, Zhu J, Firozi PF, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–7. [PubMed] [Google Scholar]
- Kollareddy M, Zheleva D, Dzubak P, Brahmkshatriya PS, Lepsik M, Hajduch M. Aurora kinase inhibitors: progress towards the clinic. Invest New Drugs. 2012;30:2411–32. doi: 10.1007/s10637-012-9798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin ML, Frey RR, Heyman HR, et al. Thienopyridine ureas as dual inhibitors of the VEGF and Aurora kinase families. Bioorg Med Chem Lett. 2012;22:3208–12. doi: 10.1016/j.bmcl.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Diamond JR, Eckhardt SG, Tan AC, et al. Predictive biomarkers of sensitivity to the aurora and angiogenic kinase inhibitor ENMD-2076 in preclinical breast cancer models. Clin Cancer Res. 2013;19:291–303. doi: 10.1158/1078-0432.CCR-12-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TV, Emanuel SL, O’Grady HR, et al. 7-1H-Indol-2-yl]-2,3-dihydro-isoindol-1-ones as dual Aurora-A/VEGF-R2 kinase inhibitors: design, synthesis, and biological activity. Bioorg Med Chem Lett. 2008;18:5130–3. doi: 10.1016/j.bmcl.2008.07.090. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Arii S. Current status and perspective of antiangiogenic therapy for cancer: hepatocellular carcinoma. Int J Clin Oncol. 2006;11:82–9. doi: 10.1007/s10147-006-0566-5. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–75. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- Ellis LM, Reardon DA. Cancer: the nuances of therapy. Nature. 2009;458:290–2. doi: 10.1038/458290a. [DOI] [PubMed] [Google Scholar]
- Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–70. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Cui SY, Huang JY, Chen YT, et al. The role of Aurora A in hypoxia-inducible factor 1alpha-promoting malignant phenotypes of hepatocelluar carcinoma. Cell Cycle. 2013;12:2849–66. doi: 10.4161/cc.25916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Lu JJ, Pan W, Hu YJ, Wang YT. Multi-target drugs: the trend of drug research and development. PLoS ONE. 2012;7:e40262. doi: 10.1371/journal.pone.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Subcutaneous hepatocellular carcinoma tumor models of Huh7 cells on day 14. Representative images are s.c. tumors derived from Huh7 in mice on day 14 following treatment with JNJ-28841072 or control. Bar = 10 mm.
Fig. S2. Subcutaneous hepatocellular carcinoma tumor models of SK-Hep1 cells on day 14. Representative images are s.c. tumors derived from SK-Hep1 in mice on day 14 following treatment with JNJ-28841072 or control. Bar = 10 mm.
Fig. S3. Orthotopic models of hepatocellular carcinoma on day 14. Representative images of liver tumors in mice on day 14 following treatment with JNJ-28841072 or control. Lower, SK-Hep1; upper, HuH-7. Bar = 10 mm.