Abstract
The results of previous studies investigating whether there is an association between active smoking and risk of death among breast cancer patients have been inconsistent. We investigated the association between active and passive smoking and risk of all-cause and breast cancer-specific death among female breast cancer patients in relation to menopausal and tumor estrogen/progesterone receptor (ER/PR) status. The present study included 848 patients admitted to a single hospital in Japan from 1997 to 2007. Active or passive smoking status was assessed using a self-administered questionnaire. The patients were followed until 31 December 2010. We used a Cox proportional-hazard model to estimate hazard ratios (HR). During a median follow-up period of 6.7 years, 170 all-cause and 132 breast cancer-specific deaths were observed. Among premenopausal patients, current smokers showed a non-significant higher risk of all-cause and breast cancer-specific death. A duration of smoking >21.5 years was positively associated with all-cause (HR = 3.09, 95% confidence interval [CI], 1.17–8.20) and breast cancer-specific death (HR = 3.35, 95% CI: 1.22–9.23, Ptrend = 0.035) among premenopausal patients. In premenopausal patients with ER+ or PR+ tumors, there was some suggestion that a longer duration of smoking was associated with higher risk of all-cause and breast cancer-specific death. Passive smoking demonstrated no significant risk. Our results suggest that a longer duration of active smoking is associated with an increased risk of all-cause and breast cancer-specific death among premenopausal patients, possibly with hormonal receptor-positive tumors. Breast cancer patients should be informed about the importance of smoking cessation.
Keywords: Breast cancer, hormone receptor, menopausal status, smoking, survival
Along with conventional therapy, identification of modifiable lifestyle factors that might improve the prognosis of breast cancer patients is of particular interest. A handful of epidemiological studies have investigated the relationship between active smoking and survival among breast cancer patients.1–13 However, the results have been conflicting. Some studies report that current1–5,9,12 and past1,2,9,10,12 smokers had a higher risk of all-cause death after diagnosis of breast cancer, whereas others report no association between current7,11,13 or past4,5,7,11 smoking and overall survival. As for breast cancer-specific death as an outcome, current smokers are reported to have a higher risk than those who had never smoked,2,3,6,8,9 whereas other studies fail to find such an association for current4,5 or past smoking.6 In addition, few studies have evaluated the associations between the quantity and duration of smoking and the survival of breast cancer patients.5–7,9 The associations between age at start of smoking,6 number of cigarettes smoked per day,5,6 duration of smoking,6,9 pack-years7,9 and survival have rarely been investigated together, and no previous study has evaluated the association between passive smoking and survival among breast cancer patients.
Some breast cancers express the estrogen receptor (ER) or progesterone receptor (PR). Tumor subtypes defined by these receptors present biologically different features.14 Estrogen and progesterone accelerate the growth of breast cancers expressing ER/PR. The frequency distribution of ER/PR differs across menopausal status.15 Thus, breast cancer may be a heterogeneous disease with different etiologic and biologic characteristics. An important limitation of the existing literature is a lack of information on how smoking influences the prognosis of patients with different tumor types classified by ER/PR, and with different menopausal status. A few previous studies have assessed the association between active smoking status and prognosis according to hormone receptor2,5 and menopausal status.2,3,5,11
Smoking might be one of the few potentially modifiable prognostic factors for patients with breast cancer; therefore, further characterization of the relationship between smoking and breast cancer prognosis is of importance to public health. With this goal in mind, we conducted a hospital-based prospective cohort study to investigate the relationship between smoking and the risk of all-cause and breast cancer-specific death among breast cancer patients in relation to both menopausal status and hormone receptor status. Analyses stratified according to menopausal and hormone receptor status were performed, along with analyses of the patients overall.
Materials and Methods
Study subjects
Between January 1997 and December 2007, 941 female patients aged 21 years or over at the Miyagi Cancer Center Hospital (MCCH) were newly diagnosed as having breast cancer. All of these patients were requested to complete a self-administered questionnaire upon initial admission. After diagnosis, their details were entered into the hospital-based cancer registry and the patients were followed up. This cancer registry recorded clinical and pathological findings and information on therapeutic treatments for all cancer patients admitted to the MCCH. This study was approved by the ethical review board of the Miyagi Cancer Center and was conducted in accordance with the principles specified in the Declaration of Helsinki.
Among the newly diagnosed breast cancer patients, 880 (93.5%) completed the questionnaire. After excluding 9 patients with a history of cancers other than breast cancer, the 871 remaining patients were included in the present study.
Questionnaire and clinical information
In 1997, we began a questionnaire survey in connection with hospital-based epidemiological studies of all types of cancer. The present study was performed within this survey. Details of the survey have already been described elsewhere.16–22 The questionnaire was distributed to patients on the day they made an appointment for their initial admission to the MCCH (i.e. 10–15 days before admission) and collected by nurses on the actual admission day. The purpose of the questionnaire survey is described on the cover page of the questionnaire. We considered the return of the self-administered questionnaires signed by the patients to imply their consent to participate in the study. The questionnaire included items on demographic characteristics, current height and weight, family histories of cancer and other diseases, general lifestyle factors before the development of current symptoms, including history of active smoking, history of passive smoking from spouse in the case of married patients, menopausal status, and comorbidity of other diseases.
Clinical information, including tumor stage based on the UICC TNM classification, and treatment such as chemotherapy, radiation therapy and endocrine therapy, was obtained from the MCCH hospital-based cancer registry. Information on ER/PR expression was extracted from medical records. To measure ER/PR status, enzyme immunoassay was used in the early period of the study. After mid-2003, immunohistochemistry was conducted. The concordance between the two assays was 94.3% for ER and 100% for PR in the laboratory of the MCCH.23 Receptor status was unknown for ER in 77 cases (9.1%), for PR in 87 (10.3%), and for both in 77 (9.1%); 538 (63.4%) cases were ER+ and 443 (52.2%) were PR+.
Ascertainment of exposure and follow up
At the MCCH, initial therapy is administered after admission. Therefore, data on smoking obtained from the questionnaire were considered to be pretreatment data. Information on exposure was collected from the above questionnaire survey. Exposure variables related to active smoking included history of smoking (never, past, current) and quantity and duration of smoking (i.e. age at start of smoking: never, ≤20, ≥21 years), the mean number of cigarettes smoked per day (never, ≤21, >21), duration of smoking (never, ≤21.5, ≥21.5 years) and pack-years of smoking (never, ≤13.5, >13.5). Subjects who quit smoking within 1 year before the present admission were regarded as current smokers. We combined past and current smokers into a group who had ever smoked. Duration of smoking was calculated based on the age at starting smoking, the age at quitting smoking and the age when completing questionnaire survey. Pack-years of smoking were calculated by multiplying the duration of smoking by the mean number of cigarettes smoked per day divided by 20. Cut-off points for patients’ age when they started smoking, the mean number of cigarettes smoked per day, duration of smoking and pack-years were determined by their median values. Twenty-three patients for whom information on smoking status was missing were excluded, leaving a total of 848 patients. The only exposure variable related to passive smoking was the husband’s smoking status (never, past, current). We combined past and current passive smokers into a group who had ever been passive smokers.
Follow up was performed by reference to the MCCH Cancer Registry up to 31 December 2010. Active follow up was conducted by accessing hospital visit records, resident registration cards and permanent domicile data. Information on dates and causes of death was obtained with permission from the Ministry of Justice. During the study period, two patients (0.24%) were lost to follow up. They were treated as censored cases in the present study.
Statistical analysis
The end point of our analysis was all-cause death and breast cancer-specific death according to the International Classification of Disease for Oncology, Tenth Edition (ICD-10). Survival time was calculated for each patient from the date of diagnosis to the date of death or the end of follow up.
To investigate the risk of all-cause and breast cancer-specific death, Kaplan–Meier survival analysis and Cox proportional hazards model were used. The crude associations of exposures with survival were evaluated by Kaplan–Meier analysis. The Cox proportional hazards model was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) controlled by confounders.24 Tests for trend were employed in the Cox model for all exposure variable categories as a continuous value. Never active smoking and never passive smoking from the husband were regarded as reference categories for active and passive smoking, respectively. We considered the following variables to be potential confounders: age, body mass index (BMI) (<21.2, 21.2–<23.4, 23.4–<26.0, 26.0 ≤ , missing), tumor stage (I, II, III, IV, missing), hormone receptor status (ER+ or PR+, ER−/PR−, missing), radiation therapy (no, yes), chemotherapy (no, yes), endocrine therapy (no, yes), family history of breast cancer in father, mother, brother or sister (no, yes), and physical activity (almost no, more than 1 h per week, missing), comorbidities (no, yes), and menopausal status (premenopausal, postmenopausal, missing). Comorbidities included hypertension, ischemic heart disease, stroke and diabetes mellitus. Missing values for confounders were treated as an additional variable category, and included in the model.
For active smoking, separate analyses were conducted after dividing the patients according to menopausal status, along with analysis of the patients overall. Furthermore, stratification according to ER/PR status was performed only among premenopausal women, because there were comparatively fewer smokers among postmenopausal patients. For passive smoking, analysis for the association with all-cause and breast cancer-specific death was limited to the patients who had never smoked. Separate analyses were conducted by dividing the patients according to menopausal status along with ER/PR status. To evaluate the heterogeneity of the associations between exposure variables and all-cause death and breast cancer-specific death across menopausal status (premenopausal vs postmenopausal) and ER/PR status (ER+ or PR+ vs ER−/PR−), interaction terms (exposure variables * menopausal status, exposure variables * ER/PR status) were tested in the Cox models. Likelihood ratio tests were used to assess the significance of heterogeneity by comparing the model including the interaction term to the main-effects model. Menopause was defined as the cessation of menstrual periods due to natural or other reasons, including surgery. With regard to menopause due to other reasons, we were unable to obtain any information about history of oophorectomy; therefore, patients 44–57 years of age (defined as the mean age at natural menopause ±2 SD) were regarded as having unknown menopausal status.
Results were regarded as significant if the two-sided P-values were <0.05. All statistical analyses were performed using the SAS software package (version 9.3; SAS Institute, Cary, NC, USA).
Results
During a median follow-up period of 6.7 years, 170 all-cause and 132 breast cancer-specific deaths were observed. The characteristics of the patients at the time of breast cancer diagnosis are shown in Table1. Current smokers tended to be younger, to have a lower BMI, to have more advanced tumors, and to have fewer comorbidities than never-smokers. A total of 302 patients (35.6%) were premenopausal, 495 (58.4%) were postmenopausal and menopausal status was unknown for 51 patients (6.0%). With regard to hormone receptor status, 557 cases (65.7%) were ER+ or PR+, and 211 (24.9%) were ER−/PR−.
Table 1.
Characteristics of the study cohort
| Characteristics | Smoking status at diagnosis | Total (n = 848) | ||
|---|---|---|---|---|
| Never (n = 690) | Past (n = 40) | Current (n = 118) | ||
| All-cause death (n) | 139 | 5 | 26 | 170 |
| Breast cancer-specific death (n) | 104 | 4 | 24 | 132 |
| Age (year) | ||||
| Mean | 58.4 | 53.2 | 48.9 | 56.9 |
| SD | 11.8 | 12.7 | 10.3 | 12.2 |
| Person-years | ||||
| Sum | 4641.5 | 264.4 | 768.7 | 5674.6 |
| Mean | 6.7 | 6.6 | 6.5 | 6.7 |
| SD | 3.2 | 3.1 | 3.2 | 3.2 |
| BMI (kg/m2, %) | ||||
| <21.2 | 22.9 | 22.5 | 36.4 | 24.8 |
| 21.2–<23.4 | 24.8 | 32.5 | 23.7 | 25.0 |
| 23.4–<26.0 | 26.4 | 17.5 | 18.6 | 24.9 |
| 26.0– | 25.3 | 27.5 | 21.2 | 24.9 |
| Missing | 0.6 | — | — | 0.4 |
| Stage (%) | ||||
| I | 45.5 | 45.0 | 32.2 | 43.6 |
| II | 35.2 | 35.0 | 40.7 | 36.0 |
| III | 10.0 | 12.5 | 18.6 | 11.3 |
| IV | 7.0 | 7.5 | 7.6 | 7.1 |
| Missing | 2.3 | — | 0.9 | 2.0 |
| Hormone receptor (%) | ||||
| ER+ or PR+ | 65.1 | 72.5 | 67.0 | 65.7 |
| ER− and PR− | 25.2 | 20.0 | 24.6 | 24.9 |
| Missing | 9.7 | 7.5 | 8.5 | 9.4 |
| Radiation (%) | ||||
| No | 73.2 | 62.5 | 72.9 | 72.6 |
| Yes | 26.8 | 37.5 | 27.1 | 27.4 |
| Chemotherapy (%) | ||||
| No | 73.6 | 75.0 | 62.7 | 72.2 |
| Yes | 26.4 | 25.0 | 37.3 | 27.8 |
| Endocrine therapy (%) | ||||
| No | 69.1 | 62.5 | 71.2 | 69.1 |
| Yes | 30.9 | 37.5 | 28.8 | 30.9 |
| Family history of breast cancer in father, mother, brother or sister (%) | ||||
| No | 90.7 | 90.0 | 89.0 | 90.4 |
| Yes | 9.3 | 10.0 | 11.0 | 9.6 |
| Menopausal status (%)† | ||||
| Premenopausal | 31.3 | 45.0 | 57.6 | 35.6 |
| Postmenopausal | 62.9 | 45.0 | 36.4 | 58.4 |
| Missing | 5.8 | 10.0 | 5.9 | 6.0 |
| Physical activity (%) | ||||
| Almost no | 48.0 | 62.5 | 61.0 | 50.5 |
| More than 1 h per week | 45.2 | 30.0 | 37.3 | 43.4 |
| Missing | 6.8 | 7.5 | 1.7 | 6.1 |
| Comorbidities (%)‡ | ||||
| No | 74.5 | 77.5 | 89.8 | 76.8 |
| Yes | 25.5 | 22.5 | 10.2 | 23.2 |
Menopause was defined as the cessation of menstrual periods due to natural or other reasons, including surgery ‡Comobidities include hypertension/ischemic heart disease/stroke/diabetes mellitus. BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor.
Kaplan–Meier analysis showed no association of smoking status with overall and breast cancer-specific survival among the patients overall. Stratification by menopausal status indicated different patterns in survival between pre-menopausal and post-menopausal patients. Among premenopausal patients, current smokers tended to have shorter survival. Shorter survival was also observed for early starters of smoking (≤20 years), heavy smokers (>21 per day) and long-term smokers (≥21.5 years), respectively (data not shown). Among them, Kaplan–Meier survival curves clearly indicated decreasing survival with increasing duration of smoking (Fig.1). However, such associations of smoking status with survival were not observed for postmenopausal patients.
Figure 1.
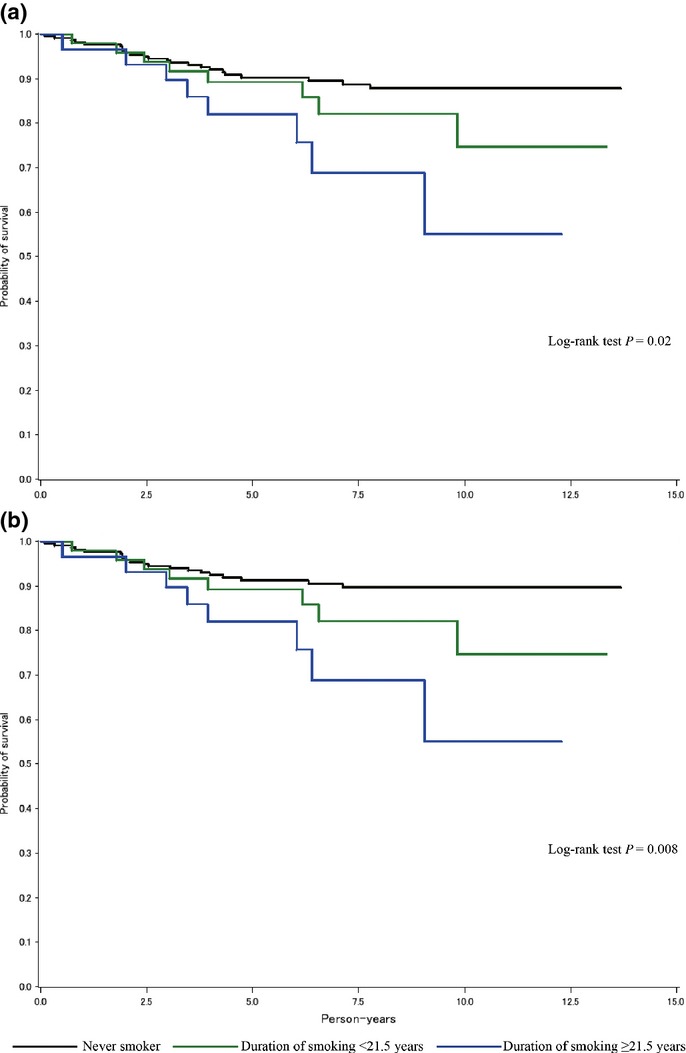
Probability of survival according to duration of smoking among premenopausal breast cancer patients: (a) overall survival and (b) breast cancer specific-survival.
Table2 shows the association of active smoking with all-cause death and breast cancer-specific death among the patients overall based on the Cox models. In comparison with patients who had never smoked, those who had ever, currently and previously smoked had no significant risk. Age at start of smoking, number of cigarettes per day, duration of smoking and pack-years were also not shown to be associated with risk.
Table 2.
HR (95% CI) of all-cause and breast cancer-specific death among overall women
| Patients | Person-years | All-cause death | Breast cancer-specific death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Death | Multivariate-adjusted | Death | Multivariate-adjusted | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| Smoking status | ||||||||||
| Never | 690 | 4641.5 | 139 | 1.00 (reference) | 104 | 1.00 (reference) | ||||
| Ever (current/past) | 158 | 1033.1 | 31 | 0.97 | 0.63–1.50 | 28 | 0.95 | 0.59–1.53 | ||
| Past | 40 | 264.4 | 5 | 0.68 | 0.27–1.67 | 4 | 0.64 | 0.23–1.77 | ||
| Current | 118 | 768.7 | 26 | 1.09 | 0.68–1.74 | 24 | 1.06 | 0.63–1.77 | ||
| Age at start of smoking (year) | ||||||||||
| 21≤ | 68 | 473.3 | 15 | 0.78 | 0.44–1.40 | 13 | 0.71 | 0.37–1.36 | ||
| ≤20 | 76 | 481.1 | 12 | 1.02 | 0.54–1.94 | 12 | 1.09 | 0.56–2.10 | ||
| P for trend | 0.76 | 0.85 | ||||||||
| Number of cigarettes per day | ||||||||||
| ≤10 | 75 | 508.3 | 14 | 0.73 | 0.40–1.33 | 13 | 0.69 | 0.36–1.32 | ||
| 11≤ | 70 | 456.4 | 13 | 1.09 | 0.60–1.98 | 12 | 1.17 | 0.62–2.19 | ||
| P for trend | 0.88 | 0.99 | ||||||||
| Duration of smoking (year) | ||||||||||
| ≤21.5 | 68 | 483.6 | 12 | 1.03 | 0.54–1.97 | 11 | 0.93 | 0.45–1.90 | ||
| 21.5< | 67 | 402.2 | 13 | 0.80 | 0.44–1.46 | 12 | 0.85 | 0.45–1.61 | ||
| P for trend | 0.51 | 0.61 | ||||||||
| Pack-years | ||||||||||
| ≤13.5 | 66 | 427.8 | 14 | 1.24 | 0.67–2.31 | 13 | 1.15 | 0.58–2.28 | ||
| 13.5< | 67 | 448.0 | 10 | 0.63 | 0.32–1.22 | 9 | 0.65 | 0.32–1.33 | ||
| P for trend | 0.28 | 0.33 | ||||||||
Adjusted by age, BMI (<21.2, 21.2–<23.4, 23.4–<26.0, 26.0–, missing), stage (I, II, III, IV, missing), hormone receptor (ER+ or PR+, ER−/PR−, missing), radiation therapy (no, yes), chemotherapy (no, yes), endocrine therapy (no, yes), family history of breast cancer in father, mother, brother or sister (no, yes), physical activity (almost no, more than 1 h per week, missing), comorbidities (no, yes), menopausal status (premenopausal, postmenopausal, missing) and passive smoking from spouse (never, ever, missing). BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; PR, progesterone receptor.
Stratification by menopausal status yielded inconsistent results (Table3). Among premenopausal patients, ever and current smokers had a non-significantly higher risk of all-cause and breast cancer-specific death. In terms of smoking duration, subjects who had smoked for more than 21.5 years showed a significantly higher risk for all-cause (HR = 3.09, 95% CI: 1.17–8.20) and breast cancer-specific (HR = 3.35, 95% CI: 1.22–9.23, Ptrend = 0.035) death than those who had never smoked. Patients who had started smoking at the age of 20 years or younger, those who smoked 11 or more cigarettes per day and those who had more than 13.5 pack-years showed a non-significantly higher risk of both all-cause and breast cancer-specific death. Postmenopausal patients tended to show an inverse association with the exposures listed; however, statistical analysis showed that this was not significant. Age at start of smoking (Pheterogeneity = 0.005 for all-cause death, Pheterogeneity = 0.005 for breast cancer-specific death), number of cigarettes per day (Pheterogeneity = 0.013 and 0.015), duration of smoking (Pheterogeneity = 0.02 and 0.018) and pack-years (Pheterogeneity = 0.042 and 0.035) were heterogeneously associated with risk according to menopausal status.
Table 3.
HR (95% CI) of all-cause and breast cancer-specific death by menopausal status
| Patients | Person-years | All-cause death | Breast cancer-specific death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Death | Multivariate-adjusted | Death | Multivariate-adjusted | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| Premenopausal | ||||||||||
| Smoking status | ||||||||||
| Never | 216 | 1560.3 | 23 | 1.00 (reference) | 20 | 1.00 (reference) | ||||
| Ever (current/past) | 86 | 556.3 | 17 | 1.54 | 0.71–3.35 | 17 | 1.74 | 0.77–3.93 | ||
| Past | 18 | 116.3 | 2 | 1.04 | 0.19–5.87 | 2 | 1.02 | 0.17–6.16 | ||
| Current | 68 | 440.0 | 15 | 1.62 | 0.73–3.56 | 15 | 1.85 | 0.81–4.23 | ||
| Age at start of smoking (year) | ||||||||||
| 21≤ | 25 | 164.2 | 6 | 0.98 | 0.31–3.10 | 6 | 1.11 | 0.34–3.62 | ||
| ≤20 | 56 | 360.0 | 11 | 2.08 | 0.87–4.95 | 11 | 2.31 | 0.94–5.71 | ||
| P for trend | 0.12 | 0.076 | ||||||||
| Number of cigarettes per day | ||||||||||
| ≤10 | 40 | 263.7 | 7 | 0.98 | 0.35–2.75 | 7 | 1.12 | 0.38–3.28 | ||
| 11≤ | 41 | 268.3 | 9 | 2.16 | 0.85–5.49 | 9 | 2.30 | 0.88–6.01 | ||
| P for trend | 0.14 | 0.1 | ||||||||
| Duration of smoking (year) | ||||||||||
| ≤21.5 | 48 | 330.7 | 8 | 0.95 | 0.35–2.63 | 8 | 1.10 | 0.39–3.15 | ||
| 21.5< | 29 | 172.2 | 8 | 3.09 | 1.17–8.20 | 8 | 3.35 | 1.22–9.23 | ||
| P for trend | 0.054 | 0.035 | ||||||||
| Pack-years | ||||||||||
| ≤13.5 | 44 | 287.2 | 9 | 1.29 | 0.50–3.35 | 9 | 1.52 | 0.57–4.09 | ||
| 13.5< | 32 | 215.0 | 6 | 1.87 | 0.67–5.22 | 6 | 1.96 | 0.68–5.65 | ||
| P for trend | 0.23 | 0.19 | ||||||||
| Postmenopausal | ||||||||||
| Smoking status | ||||||||||
| Never | 434 | 2823.9 | 102 | 1.00 (reference) | 71 | 1.00 (reference) | ||||
| Ever (current/past) | 61 | 404.1 | 13 | 0.76 | 0.41–1.39 | 10 | 0.68 | 0.34–1.38 | ||
| Past | 18 | 124.3 | 2 | 0.33 | 0.08–1.37 | 1 | 0.18 | 0.02–1.30 | ||
| Current | 43 | 279.8 | 11 | 1.01 | 0.52–1.98 | 9 | 1.01 | 0.48–2.15 | ||
| Age at start of smoking (year) | ||||||||||
| 21≤ | 37 | 269.6 | 9 | 0.79 | 0.39–1.63 | 7 | 0.76 | 0.33–1.75 | ||
| ≤20 | 15 | 88.0 | 0 | — | — | 0 | — | — | ||
| P for trend | 0.05 | 0.046 | ||||||||
| Number of cigarettes per day | ||||||||||
| ≤10 | 28 | 190.4 | 7 | 0.75 | 0.33–1.70 | 6 | 0.77 | 0.31–1.93 | ||
| 11≤ | 25 | 169.7 | 3 | 0.45 | 0.14–1.46 | 2 | 0.33 | 0.08–1.43 | ||
| P for trend | 0.14 | 0.11 | ||||||||
| Duration of smoking (year) | ||||||||||
| ≤21.5 | 17 | 144.4 | 3 | 0.72 | 0.22–2.35 | 2 | 0.67 | 0.16–2.85 | ||
| 21.5< | 31 | 178.0 | 5 | 0.53 | 0.20–1.36 | 4 | 0.46 | 0.16–1.35 | ||
| P for trend | 0.16 | 0.14 | ||||||||
| Pack-years | ||||||||||
| ≤13.5 | 20 | 132.9 | 5 | 1.14 | 0.45–2.93 | 4 | 1.14 | 0.38–3.38 | ||
| 13.5< | 27 | 180.2 | 3 | 0.33 | 0.10–1.08 | 2 | 0.25 | 0.06–1.07 | ||
| P for trend | 0.09 | 0.08 | ||||||||
| Heterogeneity of premenopausal vs postmenopausal for; | ||||||||||
| Age at first smoking (year) | 0.005 | 0.005 | ||||||||
| Number of cigarettes per day | 0.013 | 0.015 | ||||||||
| Duration of smoking (year) | 0.02 | 0.018 | ||||||||
| Pack-years | 0.042 | 0.035 | ||||||||
Adjusted by age, BMI (<21.2, 21.2–<23.4, 23.4–<26.0, 26.0–, missing), stage (I, II, III, IV, missing), hormone receptor (ER+ or PR+, ER−/PR−, missing), radiation therapy (no, yes), chemotherapy (no, yes), endocrine therapy (no, yes), family history of breast cancer in father, mother, brother or sister (no, yes), physical activity (almost no, more than 1 h per week, missing), comorbidities (no, yes) and passive smoking from spouse (never, ever, missing). BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; PR, progesterone receptor.
Based on a limited number of patients (Table4), there was some suggestion that a smoking duration of more than 21.5 years showed higher all-cause and breast cancer-specific death among premenopausal patients with ER+ or PR+ tumors by the further stratification according to ER/PR status. Kaplan–Meier survival curves also showed shorter overall and breast cancer-specific survival among these premenopausal patients with longer duration of smoking (not shown in figures). In comparison to patients with ER+ or PR+ tumors, patients with ER−/PR− tumors tended to have no significant risk associated with smoking status.
Table 4.
HR (95%CI) of all-cause and breast cancer-specific death by ER/PR status among premenopausal women
| Patients | Person-years | All-cause death | Breast cancer-specific death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Death | Multivariate-adjusted | Death | Multivariate-adjusted | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| ER+ or PR+ | ||||||||||
| Smoking status | ||||||||||
| Never | 151 | 1084.2 | 8 | 1.00 (reference) | 6 | 1.00 (reference) | ||||
| Ever (current/past) | 64 | 435.7 | 8 | 2.23 | 0.65–7.68 | 8 | 2.83 | 0.73–11.02 | ||
| Past | 16 | 109.5 | 1 | 2.42 | 0.25–23.10 | 1 | 3.02 | 0.27–33.47 | ||
| Current | 48 | 326.2 | 7 | 2.20 | 0.61–7.94 | 7 | 2.80 | 0.69–11.41 | ||
| Age at start of smoking (year) | ||||||||||
| 21≤ | 20 | 128.8 | 4 | 3.92 | 0.82–18.61 | 4 | 5.14 | 0.86–30.90 | ||
| ≤20 | 39 | 274.7 | 4 | 1.55 | 0.34–7.09 | 4 | 2.12 | 0.43–10.38 | ||
| P for trend | 0.41 | 0.28 | ||||||||
| Number of cigarettes per day | ||||||||||
| ≤10 | 30 | 199.7 | 4 | 3.35 | 0.86–13.09 | 4 | 4.70 | 1.04–21.18 | ||
| 11≤ | 30 | 212.3 | 4 | 1.32 | 0.25–6.89 | 4 | 1.46 | 0.24–8.82 | ||
| P for trend | 0.47 | 0.4 | ||||||||
| Duration of smoking (year) | ||||||||||
| ≤21.5 | 36 | 260.7 | 3 | 0.46 | 0.07–3.13 | 3 | 0.51 | 0.07–3.87 | ||
| 21.5< | 21 | 128.5 | 5 | 10.86 | 2.19–53.80 | 5 | 17.32 | 2.63–113.84 | ||
| P for trend | 0.021 | 0.014 | ||||||||
| Pack-years | ||||||||||
| ≤13.5 | 34 | 230.1 | 6 | 3.08 | 0.71–13.45 | 6 | 4.05 | 0.81–20.26 | ||
| 13.5< | 23 | 159.1 | 2 | 1.64 | 0.27–9.77 | 2 | 1.90 | 0.27–13.31 | ||
| P for trend | 0.32 | 0.25 | ||||||||
| ER− and PR− | ||||||||||
| Smoking status | ||||||||||
| Never | 49 | 384.4 | 10 | 1.00 (reference) | 10 | 1.00 (reference) | ||||
| Ever (current/past) | 18 | 106.2 | 6 | 1.56 | 0.33–7.45 | 6 | 1.56 | 0.33–7.45 | ||
| Past | 1 | 6.1 | 0 | – | – | 0 | – | – | ||
| Current | 17 | 100.1 | 6 | 1.52 | 0.32–7.09 | 6 | 1.52 | 0.32–7.09 | ||
| Age at start of smoking (year) | ||||||||||
| 21≤ | 4 | 28.8 | 1 | 0.48 | 0.03–6.78 | 1 | 0.48 | 0.03–6.78 | ||
| ≤20 | 14 | 77.4 | 5 | 2.80 | 0.44–17.72 | 5 | 2.80 | 0.44–17.72 | ||
| P for trend | 0.36 | 0.36 | ||||||||
| Number of cigarettes per day | ||||||||||
| ≤10 | 9 | 57.4 | 2 | 1.52 | 0.18–12.82 | 2 | 1.52 | 0.18–12.82 | ||
| 11≤ | 8 | 48.1 | 3 | 1.26 | 0.19–8.21 | 3 | 1.26 | 0.19–8.21 | ||
| P for trend | 0.78 | 0.78 | ||||||||
| Duration of smoking (year) | ||||||||||
| ≤21.5 | 10 | 56.9 | 4 | 2.12 | 0.29–15.78 | 4 | 2.12 | 0.29–15.78 | ||
| 21.5< | 7 | 43.2 | 2 | 1.10 | 0.14–8.36 | 2 | 1.10 | 0.14–8.36 | ||
| P for trend | 0.78 | 0.78 | ||||||||
| Pack-years | ||||||||||
| ≤13.5 | 8 | 44.0 | 2 | 1.57 | 0.19–12.73 | 2 | 1.57 | 0.19–12.73 | ||
| 13.5< | 8 | 55.4 | 3 | 1.21 | 0.19–7.84 | 3 | 1.21 | 0.19–7.84 | ||
| P for trend | 0.8 | 0.8 | ||||||||
| Heterogeneity of ER+ or PR+ vs ER− and PR− for; | ||||||||||
| Age at first smoking (year) | 0.90 | 0.49 | ||||||||
| Number of cigarettes per day | 0.87 | 0.51 | ||||||||
| Duration of smoking (year) | 0.67 | 0.38 | ||||||||
| Pack-years | 0.91 | 0.55 | ||||||||
Adjusted by age, BMI (<21.2, 21.2–<23.4, 23.4-<26.0, 26.0–, missing), stage (I, II, III, IV, missing), radiation therapy (no, yes), chemotherapy (no, yes), endocrine therapy (no, yes), family history of breast cancer in father, mother, brother or sister (no, yes), physical activity (almost no, more than 1 h per week, missing), comorbidities (no, yes), and passive smoking from spouse (never, ever, missing). BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; PR, progesterone receptor.
Table5 shows the association of passive smoking with all-cause death and breast cancer-specific death among married patients. Analyses stratified according to menopausal status and ER/PR status demonstrated no significant change in risk for passive smoking from the husband associated with all-cause and breast cancer-specific death.
Table 5.
HR (95%CI) of all-cause and breast cancer-specific death associated with passive smoking status
| Passive smoking status | Patients | Person-years | All-cause death | Breast cancer-specific death | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Death | Multivariate-adjusted | Death | Multivariate-adjusted | ||||||||
| HR | 95% CI | P | HR | 95% CI | P | ||||||
| All | |||||||||||
| Never | 207 | 1307.1 | 39 | 1.00 (reference)† | 32 | 1.00 (reference)† | |||||
| Ever (current/past) | 379 | 2686.0 | 70 | 0.87 | 0.58–1.31 | 52 | 0.85 | 0.54–1.36 | |||
| Premenopausal | |||||||||||
| Never | 69 | 469.4 | 7 | 1.00 (reference)‡ | 6 | 1.00 (reference)‡ | |||||
| Ever (current/past) | 118 | 903.8 | 11 | 0.97 | 0.31–3.02 | 10 | 0.94 | 0.25–3.50 | |||
| ER+ or PR+ | |||||||||||
| Never | 49 | 335.1 | 4 | 1.00 (reference) | 3 | 1.00 (reference) | |||||
| Ever (current/past) | 82 | 604.6 | 4 | 0.58 | 0.10–3.46 | 3 | 1.05 | 0.12–8.98 | |||
| ER− and PR− | |||||||||||
| Never | 17 | 114.1 | 3 | 1.00 (reference) | 3 | 1.00 (reference) | |||||
| Ever (current/past) | 28 | 224.9 | 5 | – | – | 5 | – | – | |||
| Heterogeneity of ER+ or PR+ vs ER− and PR− | – | – | |||||||||
| Postmenopausal | |||||||||||
| Never | 127 | 769.6 | 28 | 1.00 (reference)‡ | 22 | 1.00 (reference)‡ | |||||
| Ever (current/past) | 237 | 1617.8 | 51 | 0.79 | 0.49–1.29 | 35 | 0.75 | 0.42–1.33 | |||
| ER+ or PR+ | |||||||||||
| Never | 80 | 505.9 | 9 | 1.00 (reference) | 6 | 1.00 (reference) | |||||
| Ever (current/past) | 150 | 1038.7 | 26 | 1.00 | 0.44–2.24 | 12 | 0.67 | 0.22–2.07 | |||
| ER− and PR− | |||||||||||
| Never | 37 | 224.4 | 13 | 1.00 (reference) | 12 | 1.00 (reference) | |||||
| Ever (current/past) | 61 | 429.8 | 15 | 0.97 | 0.40–2.40 | 13 | 0.79 | 0.30–2.04 | |||
| Heterogeneity of ER+ or PR+ vs ER− and PR− | 0.46 | 0.65 | |||||||||
| Heterogeneity of premenopausal vs postmenopausal | 0.91 | 0.67 | |||||||||
All analyses are adjusted by age, BMI (<21.2, 21.2-<23.4, 23.4–<26.0, 26.0–, missing), stage (I, II, III, IV, missing), radiation therapy (no, yes), chemotherapy (no, yes), endocrine therapy (no, yes), family history of breast cancer in father, mother, brother or sister (no, yes), physical activity (almost no, more than 1 h per week, missing), and comorbidities (no, yes).
Additionally adjusted by hormone receptor (ER+ or PR+, ER−/PR−, missing), and menopausal status (premenopausal, postmenopausal, missing).
Additionally adjusted by hormone receptor. BMI, body mass index; CI, confidence interval; ER, estrogen receptor; HR, hazard ratio; PR, progesterone receptor.
Discussion
This study showed that a longer duration of smoking was associated with a significant higher risk of all-cause and breast cancer-specific death among premenopausal patients. These findings were also supported by the Kaplan–Meier survival analysis. Stratification by hormone receptor status indicated that a longer duration of smoking appeared to be associated with a higher risk of all-cause and breast cancer-specific death among premenopausal patients with ER+ or PR+ tumors. No significant association was observed among postmenopausal patients. Previous studies investigating the relationship between active smoking and patient outcome have showed conflicting results.1–13 Furthermore, only four of these studies considered menopausal status2,3,5,11 and only two considered hormone receptor status.2,5 In Japan, one study has assessed the association between active smoking and overall survival among 398 patients; however, stratification by menopausal and hormone receptor status has never been performed.25 No previous study has evaluated the association between passive smoking and survival among breast cancer patients. Our study is of importance in having assessed the relationship between active and passive smoking and all-cause or breast cancer-specific death to the point of taking into consideration multiple risk factors for breast cancer in addition to menopausal status and hormone receptor status among Japanese breast cancer patients.
Some studies, mainly from Western countries, have shown that current1–5,9,12 and past1,2,9,10,12 smokers were at higher risk of all-cause death after diagnosis of breast cancer and that current smokers had a higher risk of breast cancer-specific death than individuals who had never smoked.2,3,6,8,9 Current smoking was reported to be associated with a higher risk of all-cause3 and breast cancer-specific death among premenopausal patients.2,3 In our study, in comparison to never smokers, current smokers and individuals who had ever smoked in the past had a non-significantly higher risk of all-cause and breast cancer-specific death among premenopausal patients. One previous study showed that earlier age at start of smoking, a higher number of cigarettes smoked per day and a longer duration of smoking were associated with a higher risk of all-cause death.6 The results in our study were generally consistent with the previous study of premenopausal women; in particular, long-term premenopausal smokers (duration of smoking >21.5 years) had a higher risk of all-cause and breast cancer-specific death. In contrast, postmenopausal patients had no significant risk associated with active smoking. One possible reason for this higher risk of death among premenopausal patients is that lifestyles related to smoking might also influence the prognosis of breast cancer patients. In comparison with never smokers, current smokers among women in the Miyagi cohort, whose residential area was roughly the same as that of patients in our study, were less educated, and consumed fewer green vegetables and oranges.26 In the present study, premenopausal long-term smoking patients tended to be physically inactive and to consume little fruit (data not shown). These specific lifestyles among long-term smoking patients might have affected their prognosis.27,28 However, more studies to clarify the association between survival and smoking-related lifestyles are clearly needed.
One previous study showed that only current, and not past, smokers had a significantly higher risk of breast cancer-specific death among women with ER+ tumors.2 However, another study showed no association between current and past smoking and breast cancer-specific death among women with ER+/PR+ or ER−/PR− breast cancers.5 In our study with a limited number of patients, there was some suggestion that those with a longer duration of smoking might have a higher risk of all-cause and breast cancer-specific death by stratification of hormone receptor status among premenopausal women. It has been suggested that this potential relationship might be related to the estrogen-like substances in active tobacco smoke, which can exert estrogenic effects.29 According to the detailed analysis of our data, premenopausal ER+ or PR+ cancer patients with a longer duration of smoking tended to have more advanced tumors compared to those who had never smoked or had only smoked from a short duration. Furthermore, the magnitude of the risk of death was essentially unchanged in the analysis among patients with stage I–III cancer (data not shown). These observations suggest that long-term exposure to the estrogen-like substances in tobacco smoke might accelerate the progression of hormone receptor-positive tumors.30–32 Smoking-related ER+ or PR+ tumors might be of high grade. In addition, long-term smoking might cause immunological deterioration.33 Such effects of smoking on hormonal and immune systems and the abovementioned lifestyle factors could contribute to the increased risk of all-cause and breast cancer-specific death among premenopausal patients with ER+ or PR+ breast cancer. Further large-scale studies are needed to confirm or refute our results.
Our study demonstrated no association between passive smoking as a result of inhaling the husband’s tobacco smoke and survival of breast cancer patients. No previous studies have demonstrated an association between passive smoking and the survival of breast cancer patients. Some prospective studies in the USA34,35 and in Japan22,36–38 have demonstrated the association between passive smoking and the risk of breast cancer, but these results have not been consistent; therefore, an association between passive smoking and the incidence risk of breast cancer remains to be clarified, let alone an association between passive smoking and survival of breast cancer patients.
Several limitations of our present study need to be considered. First, some patients might have ceased active and passive smoking after developing diseases, and this might have led to misclassification of active and passive smoking status. However, because our questionnaire was given to each patient on the day they made an appointment for their first admission to the MCCH before any definite diagnosis or treatment, any information bias would likely have been minimal. Second, only information about passive smoking from the husband’s tobacco smoke among married women was included in the analysis. The role of passive smoking might not have been fully evaluated due to the lack of data on exposure to occupational passive smoking. Third, active and passive smoking may cause comorbidities and increase mortality. We carried out a sensitivity analysis among patients without comorbidities (n = 649), but the results remained almost unchanged (data not shown). Fourth, stratification by hormone receptor status among premenopausal patients may have resulted in false positive or false negative results. The 95% CI were wide for HR according to hormone receptor status among premenopausal cases, suggesting that the statistical power might have been limited due to the relatively small number of patients and all-cause and breast cancer-specific deaths. However, smoking prevalence among Japanese women has been lower than among Western populations.39–41 To obtain reliable results with this stratification, subsequent recruitment of patients and follow up will be required. Fifth, the generalizability of our results may have been limited because our study was conducted among a population living in a rural area of Japan. More studies are needed to verify our results and to assess their generalizability.
One of the strengths of the present study was that only two of the patients were lost to follow up during the study period. The MCCH Cancer Registry conducts active follow up by accessing hospital visit records, resident registration cards and permanent domicile data. In cases of death occurring outside the hospital, information on the date and cause of death were obtained with permission from the Ministry of Justice. Another strength was that our study gave consideration not only to clinical stage but also treatments such as chemotherapy, endocrine therapy and radiation therapy from an epidemiological viewpoint. Thus, smoking is regarded as an independent risk factor for all-cause and breast cancer-specific death. Based on our findings, clinicians will be able to advise breast cancer patients to cease smoking. Furthermore, our study may well provide important information also for public health policy. Considering the higher risk of death for premenopausal patients with more than 21.5-year duration of smoking, smoking control targeting of young people is essential. This smoking control will contribute not only to the reduction of breast cancer mortality but also the prevention of smoking-related cancers.42
In conclusion, factors associated with active smoking, such as a longer duration of smoking, were shown to be associated with a higher risk of all-cause and breast cancer-specific death in premenopausal patients. Higher risk also appeared to be present in premenopausal patients with ER+ or PR+ tumors. No such significant association was observed among postmenopausal patients. Passive smoking was not associated with any risk. To improve the prognosis of breast cancer, breast cancer patients should be informed about the importance of smoking cessation in the clinical setting. Moreover, considering the higher risk of death for premenopausal patients with a long duration of smoking, smoking control targeting young people is urgently needed.
Acknowledgments
This work was supported by Grants-in-Aid from the JSPS for Scientific Research (B) (23390169).
Disclosure Statement
The authors have no conflict of interest to declare.
References
- Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP. Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev. 1997;21:497–509. [PubMed] [Google Scholar]
- Braithwaite D, Izano M, Moore DH, et al. Smoking and survival after breast cancer diagnosis: a prospective observational study and systematic review. Breast Cancer Res Treat. 2012;136:521–33. doi: 10.1007/s10549-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. Int J Cancer. 2013;132:401–10. doi: 10.1002/ijc.27617. [DOI] [PubMed] [Google Scholar]
- Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010;19:366–73. doi: 10.1097/CEJ.0b013e32833b4828. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer. 2007;120:2672–7. doi: 10.1002/ijc.22575. [DOI] [PubMed] [Google Scholar]
- Calle EE, Miracle-McMahill HL, Thun MJ, Heath CW., Jr Cigarette smoking and risk of fatal breast cancer. Am J Epidemiol. 1994;139:1001–7. doi: 10.1093/oxfordjournals.aje.a116939. [DOI] [PubMed] [Google Scholar]
- Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–6. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- Manjer J, Andersson I, Berglund G, et al. Survival of women with breast cancer in relation to smoking. Eur J Surg. 2000;166:852–8. doi: 10.1080/110241500447227. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst. 2014;106:359. doi: 10.1093/jnci/djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Maso L, Zucchetto A, Talamini R, et al. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int J Cancer. 2008;123:2188–94. doi: 10.1002/ijc.23747. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Gaudet MM, Eng SM, et al. Active and passive cigarette smoke and breast cancer survival. Ann Epidemiol. 2007;17:385–93. doi: 10.1016/j.annepidem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Padron-Monedero A, Tannenbaum SL, Koru-Sengul T, et al. Smoking and survival in female breast cancer patients. Breast Cancer Res Treat. 2015;150:395–403. doi: 10.1007/s10549-015-3317-3. [DOI] [PubMed] [Google Scholar]
- Ewertz M, Gillanders S, Meyer L, Zedeler K. Survival of breast cancer patients in relation to factors which affect the risk of developing breast cancer. Int J Cancer. 1991;49:526–30. doi: 10.1002/ijc.2910490409. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Iwase H, Toyama T, et al. Estrogen receptor-positive breast cancer in Japanese women: trends in incidence, characteristics, and prognosis. Ann Oncol. 2011;22:1318–25. doi: 10.1093/annonc/mdq596. [DOI] [PubMed] [Google Scholar]
- Minami Y, Tateno H. Associations between cigarette smoking and the risk of four leading cancers in Miyagi Prefecture, Japan: a multi-site case-control study. Cancer Sci. 2003;94:540–7. doi: 10.1111/j.1349-7006.2003.tb01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Tochigi T, Kawamura S, et al. Height, urban-born and prostate cancer risk in Japanese men. Jpn J Clin Oncol. 2008;38:205–13. doi: 10.1093/jjco/hym170. [DOI] [PubMed] [Google Scholar]
- Kawai M, Minami Y, Nishino Y, Fukamachi K, Ohuchi N, Kakugawa Y. Body mass index and survival after breast cancer diagnosis in Japanese women. BMC Cancer. 2012;12:149. doi: 10.1186/1471-2407-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Kakugawa Y, Nishino Y, Hamanaka Y, Ohuchi N, Minami Y. Anthropometric factors, physical activity, and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women: a case-control study. Cancer Causes Control. 2013;24:1033–44. doi: 10.1007/s10552-013-0181-5. [DOI] [PubMed] [Google Scholar]
- Kawai M, Kakugawa Y, Nishino Y, Hamanaka Y, Ohuchi N, Minami Y. Reproductive factors and breast cancer risk in relation to hormone receptor and menopausal status in Japanese women. Cancer Sci. 2012;103:1861–70. doi: 10.1111/j.1349-7006.2012.02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Nishino Y, Kawai M, Kakugawa Y. Being breastfed in infancy and adult breast cancer risk among Japanese women. Cancer Causes Control. 2012;23:389–98. doi: 10.1007/s10552-011-9888-3. [DOI] [PubMed] [Google Scholar]
- Nishino Y, Minami Y, Kawai M, et al. Cigarette smoking and breast cancer risk in relation to joint estrogen and progesterone receptor status: a case-control study in Japan. Springerplus. 2014;3:65. doi: 10.1186/2193-1801-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakugawa Y, Minami Y, Tateno H, Inoue H, Fujiya T. Relation of serum levels of estrogen and dehydroepiandrosterone sulfate to hormone receptor status among postmenopausal women with breast cancer. Breast Cancer. 2007;14:269–76. doi: 10.2325/jbcs.14.269. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables (with discussion) J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- Tominaga K, Andow J, Koyama Y, et al. Family environment, hobbies and habits as psychosocial predictors of survival for surgically treated patients with breast cancer. Jpn J Clin Oncol. 1998;28:36–41. doi: 10.1093/jjco/28.1.36. [DOI] [PubMed] [Google Scholar]
- Hozawa A, Ohkubo T, Yamaguchi J, et al. Cigarette smoking and mortality in Japan: the Miyagi Cohort Study. J Epidemiol. 2004;14(Suppl 1):S12–7. doi: 10.2188/jea.14.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushi LH, Kwan ML, Lee MM, Ambrosone CB. Lifestyle factors and survival in women with breast cancer. J Nutr. 2007;137:236S–42S. doi: 10.1093/jn/137.1.236S. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28:4074–80. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek MD, Finch GL. Diluted mainstream cigarette smoke condensates activate estrogen receptor and aryl hydrocarbon receptor-mediated gene transcription. Environ Res. 1999;80:9–17. doi: 10.1006/enrs.1998.3872. [DOI] [PubMed] [Google Scholar]
- Wells AJ. Breast cancer, cigarette smoking, and passive smoking. Am J Epidemiol. 1991;133:208–10. doi: 10.1093/oxfordjournals.aje.a115859. [DOI] [PubMed] [Google Scholar]
- Zhu BQ, Heeschen C, Sievers RE, et al. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell. 2003;4:191–6. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
- Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–9. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Margolis KL, Wactawski-Wende J, et al. Association of active and passive smoking with risk of breast cancer among postmenopausal women: a prospective cohort study. BMJ. 2011;342:d1016. doi: 10.1136/bmj.d1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med. 2011;171:125–33. doi: 10.1001/archinternmed.2010.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaoka T, Yamamoto S, Sobue T, Sasaki S, Tsugane S. Active and passive smoking and breast cancer risk in middle-aged Japanese women. Int J Cancer. 2005;114:317–22. doi: 10.1002/ijc.20709. [DOI] [PubMed] [Google Scholar]
- Lin Y, Kikuchi S, Tamakoshi K, et al. Active smoking, passive smoking, and breast cancer risk: findings from the Japan Collaborative Cohort Study for Evaluation of Cancer Risk. J Epidemiol. 2008;18:77–83. doi: 10.2188/jea.18.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino Y, Tsubono Y, Tsuji I, et al. Passive smoking at home and cancer risk: a population-based prospective study in Japanese nonsmoking women. Cancer Causes Control. 2001;12:797–802. doi: 10.1023/a:1012273806199. [DOI] [PubMed] [Google Scholar]
- Forey B. International Smoking Statistics: A Collection of Historical Data from 30 Economically Developed Countries. 2nd edn. London, Oxford; New York, NY: Wolfson Institute of Preventive Medicine; Oxford University Press; 2002. [Google Scholar]
- Ministry of Health, Labour and Welfare. Report on Tobacco or Health. Adult prevalence of tobacco smoking. Tokyo: Japan Health Promotion & Fitness Foundation; 2008. (in Japanese) [Google Scholar]
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–9. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005–Systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–9. doi: 10.1093/annonc/mdr437. [DOI] [PubMed] [Google Scholar]


