Abstract
Paclitaxel resistance is a major obstacle for the treatment of ovarian cancer. The chemoresistance mechanisms are partly related to the mitochondria. Identification of the relevant proteins in mitochondria will help in clarifying the possible mechanisms and in selecting effective chemotherapy for patients with paclitaxel resistance. In the present study, mitochondria from two paclitaxel-sensitive human ovarian cancer cell lines (SKOV3 and A2780) and their corresponding resistant cell lines (SKOV3-TR and A2780-TR) were isolated. Guanidine-modified acetyl-stable isotope labeling and liquid chromatography-hybrid linear ion trap Fourier-transform ion cyclotron resonance mass spectrometry (LC-FTICR MS) were performed to find the expressed differential proteins. Comparative proteomic analysis revealed eight differentially expressed proteins in the ovarian cancer cells and their paclitaxel-resistant sublines. Among them, mimitin and 14-3-3 ζ/δ were selected for further research. The effects of mimitin and 14-3-3 ζ/δ were explored using specific siRNA interference in ovarian cancer cell lines and immunohistochemistry in human tissue specimens. The downregulation of mimitin and 14-3-3 ζ/δ using specific siRNA in paclitaxel-resistant ovarian cancer cells led to an increase in the resistance index to paclitaxel. Multivariate analyses demonstrated that lower expression levels of the mimitin and 14-3-3 ζ/δ proteins were positively associated with shorter progression-free survival (PFS) and overall survival (OS) in patients with primary ovarian cancer (mimitin: PFS: P = 0.041, OS: P = 0.003; 14-3-3 ζ/δ: PFS: P = 0.031, OS: P = 0.011). Mimitin and 14-3-3 protein ζ/δ are potential markers of paclitaxel resistance and prognostic factors in ovarian cancer.
Keywords: Drug resistance, liquid chromatography-hybrid linear ion trap Fourier-transform ion cyclotron resonance mass spectrometry, mitochondria proteins, ovarian cancer, paclitaxel
Ovarian cancer is the most lethal disease among gynecological malignant tumors. Long-term prospective studies have confirmed combined paclitaxel and platinum chemotherapy to be the first-line therapeutic protocol for ovarian epithelial tumors.1 However, the 5-year survival rate for stage III and IV disease is approximately 30%.2 Chemoresistance is one of the major obstacles to treatment.3
Initial responsiveness to cisplatin therapy is high; however, the majority of patients ultimately relapse with resistant disease. Mechanisms of cisplatin resistance have been investigated fully in resistant cell models, including decreased cellular accumulation of drug, increased levels of glutathione, increased levels of DNA repair and increased anti-apoptotic activity.4 In contrast, although many patients will relapse with disease resistant to paclitaxel therapy, the reason for and role of paclitaxel resistance are still unclear in ovarian carcinoma.5 Possible mechanisms include P-glycoprotein export decreasing the cellular accumulation, altered expression or post-translational modification of b-tubulin, the target of paclitaxel, or other microtubule regulatory proteins.6,7 However, no biomarker has been found to predict the response to paclitaxel regimen.
Mitochondria are critical subcellular organelles responsible for ATP generation through oxidative phosphorylation in eukaryotic cells. The chemoresistance mechanisms are, in part, related to the mitochondria (e.g. drug efflux mechanisms, improved antitoxic ability and apoptotic changes in carcinoma cells).8–10 The primary component of the mitochondria is protein; therefore, proteomics can comprehensively evaluate the association between the mitochondria and ovarian chemoresistance and may lead to identifying chemoresistance targets.
In our earlier studies, we demonstrated that platinum resistance in epithelial ovarian cancer is related to the downregulation of prohibitin expression, which occurs in energy production and in the electron transfer respiratory chain in cells.11 However, the mitochondria’s exact role in chemoresistance mechanisms has not been established. In the present study, we sought to identify relevant mitochondrial proteins related to paclitaxel resistance in epithelial ovarian cancer.
Materials and Methods
Materials and reagents
Paclitaxel was purchased from Bristol-Myers Squibb (New York, NY, USA). A Cell Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular Technologies (Kumamoto, Japan). The Protease Inhibitor Cocktail was provided by Roche (Basel, Switzerland). Mouse anti-human COX4 antibody was obtained from Molecular Probes (Eugene, OR, USA). Goat anti-human lamin B and mimitin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-human flotillin-1 and rabbit anti-human β-actin antibodies were obtained from eBioscience (San Diego, CA, USA). Rabbit anti-human 14-3-3 ζ/δ antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA) and Sigma-Aldrich (St. Louis, MO, USA), respectively. The electrochemiluminescence kit was purchased from Thermo Scientific (Boston, MA, USA).
Cell lines and cell culture
SKOV3 is a human epithelial ovarian cancer cell line that was obtained from the Cell Culture Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. The paclitaxel-resistant SKOV3 (SKOV3-TR, TS) cells were generated by selecting SKOV3 cells for growth using a 2-μM paclitaxel pulse for 16 months in our laboratory, as previously described.12 The paclitaxel-sensitive human epithelial ovarian cancer cell line A2780 and paclitaxel-resistant A2780 (A2780-TR, TA) were kindly supplied by Dr Li, Department of Gynecologic Oncology, Medical University of Guangxi Cancer Institute and Hospital.13 All of the cell lines were maintained in RPMI 1640 medium containing 10% FBS, 100 U/mL penicillin and 100 U/mL streptomycin.
Drug sensitivity assay
The cells were harvested in the exponential phase. Single-cell suspensions were prepared and dispersed in 96-well plates at 2000 cells/well. Six duplicates were used for each determination. After incubation with drugs for 48 h, 10 μL of CCK-8 solution was added to each well, the plates were incubated at 37°C for 2 h, and absorbance of each well was measured at 450 nm using an enzyme-labeling instrument. The IC50 value was defined as the concentration of drugs that was required for a 50% inhibition rate relative to the controls. The resistance index (RI) of resistant cells was calculated as follows: RI = (IC50 of SKOV3-TR or A2780-TR cells)/(IC50 of SKOV3 or A2780 cells).
Isolation of mitochondria and protein extraction
Approximately 108 cells were harvested from the exponential-phase cultures for each cell line. Mitochondria isolation procedures were performed as previously described.11 Mitochondrion-enriched fractions were prepared using repeated differential centrifugation. Small portions of the fractions were fixed in 2.5% glutaraldehyde, and the morphology of isolated mitochondria was observed using SEM. The mitochondrial proteins were extracted from SKOV3, SKOV3-TR, A2780 and A2780-TR cells. The majority of the fraction samples were lysed in protein extraction buffer. After centrifugation at 13 000 g for 30 min at 4°C, the supernatant was collected as the protein sample. The total cell proteins were extracted from SKOV3 cells. The protein concentrations were determined using the Bradford Protein Assay Kit (Beyotime, Shanghai, China). The supernatants were stored at −80°C for subsequent assay.
Validation of mitochondrial purity by electron microscopy and western blotting
The mitochondria preparations were fixed and processed using a standard protocol for transmission electron microscopy.14 After fixing in 1% osmium tetroxide for 1 h at 41°C, the samples were dehydrated in ascending grades of ethanol and embedded in Spurr’s resin. After overnight polymerization, ultrathin sections (70–80 nm) were cut using an ultramicrotome. The sections were laid on copper grids, stained with uranyl acetate and lead citrate, and examined using a transmission electron microscope (JEOL TEM 1010 [JEOL, Japan]).
The proteins were separated using SDS-PAGE and the purity of isolated mitochondria was assessed with western blot, and the proteins were transferred to polyvinylidene difluoride membranes. The incubation dilutions were as follows: mouse anti-human COX4 at 1:500 dilution, goat anti-human lamin B at 1:200, mouse anti-human flotillin-1 at 1:500, rabbit anti-human β-actin at 1:1000, goat anti-human mimitin at 1:200 and rabbit anti-human 14-3-3 ζ/δ at 1:1000.
Guanidine-modified acetyl-stable isotope labeling
Eighty micrograms of mitochondrial proteins in each cell line (SKOV3, SKOV3-TR, A2780 and A2780-TR) was resuspended in SDS-PAGE sample buffer and loaded on a 12% criterion gradient gel. After staining the gel using Coomassie Brilliant Blue R-250 (Bio-Rad, Hercules, CA, USA), a 1 to 2-mm broad gel slice was cut along the entire lane. Subsequently, in-gel protein was digested with trypsin, as previously described.15 The gel pieces were destained by washing three times in 25 mM NH4HCO3 in 50% CH3CN and once in 25 mM NH4HCO3 in 50% CH3OH. The gel pieces were dried in a vacuum centrifuge and incubated with digestion buffer (50 mM NH4HCO3, 10 ng/μL trypsin) at 37°C overnight. The peptides were extracted in 50% CH3CN/1% CH3COOH, and the supernatant was evaporated to dryness in a vacuum centrifuge.
The ε-amino group of lysine was guanidinated as described previously.16,17 The mitochondrial proteins of SKOV3 and A2780 cells were labeled with H6-acetic anhydride as the control group, and D6-acetic anhydride was used to label protein samples of SKOV3-TR and A2780-TR cells as the test group.
Quantitative analysis of tagged peptides by liquid chromatography-hybrid linear ion trap Fourier-transform ion cyclotron resonance mass spectrometry
The eluted peptides were analyzed using a 7-Tesla LTQ-FT mass spectrometer (Thermo Electron, San Jose, CA, USA) coupled with an Agilent 1100 nanoflow liquid chromatography system (Palo Alto, CA, USA). The MS/MS spectra were then searched against IPI human database 3.23 (ftp://ftp.ebi.ac.uk/pub/databases/IPI/old/HUMAN/ipi.HUMAN.v3.23.fasta.gz) through the local MASCOT (Version 2.1) server. The data were analyzed as previously described.12 Only peptides of seven amino acids or longer were accepted. Each peptide was identified with at least 95% confidence (Ptrend < 0.05). Each identified protein required at least one unique peptide match. The quantitative ratio of differentially labeled peptides or proteins was calculated using MS-based acetyl quantification. According to our previous research, the peak area calculation method with the reduced SD was used for extracting quantitative information.17 Using the log-logistic distribution of quantitative results based on the peak area calculation, the calculated ratio (<0.7740 and >1.2919; P < 0.05) was applied to discover significant differentially expressed proteins.
To improve the efficiency of protein validation, Peptide Mascot Score >23 (peptide with a high likelihood of matching >95%) and significant differences (ratios <0.5 and >2) of protein expression were both used as selection criteria.
Downregulation of mimitin and 14-3-3 ζ/δ expression by siRNA
We knocked down mimitin and 14-3-3ζ/δ expression by siRNA against the two genes, to confirm whether mimitin and 14-3-3ζ/δ were correlated with resistance to paclitaxel. Different siRNA separately directed against different regions of the human mimitin and 14-3-3 isoform mRNA were obtained from GenePharma (Shanghai, China). They were referred to as si Mim-1, si Mim-2, si Mim-3 and si Mim-4 for mimitin, and si 143-1, si 143-2, si 143-3 and si 143-4 for 14-3-3 ζ/δ downregulation, respectively. Scrambled siRNA nucleotides (MOCK) were used as a negative control. These RNAi oligonucleotides were transfected into cells using a Lipofectamine 2000 kit (Invitrogen, Life Technologies, Grand Island, NY, USA) for 48 h according to the manufacturer’s instructions. The downregulated expression levels of the mimitin and 14-3-3 ζ/δ were confirmed using western blotting. The constructs with the highest knockdown effciency (si Mim-1, Mim-2 for mimitin and si 143-1, si 143-2 for 14-3-3ζ/δ) were used for further experiments. Then the resistance to paclitaxel of SKOV3-TR cells after RNA interference was detected as described above. The experiments were independently performed in triplicate.
Patient selection, immunohistochemistry and survival analysis
We used ovarian cancer tissue specimens from patients who underwent surgery for ovarian epithelial cancer in the Department of Obstetrics and Gynaecology, Peking Union Medical College Hospital. The study protocol was approved by the Ethics Committee of Peking Union Medical College Hospital. All of the participants provided their written informed consent for participation in the data analysis and manuscript publication.
The first cohort including 46 patients was gathered during 2000–2005. The relevant clinical information was gathered, and tissue samples and clinical data were anonymized. All of the patients received postoperative chemotherapy using a regimen that included paclitaxel and/or platinum for at least six courses. All of the tissue specimens were divided into two groups: the sensitive group and the resistant group. The sensitive group was defined as having a complete response to chemotherapy, with a post-treatment failure-free interval of >6 months. Patients who remained stable disease or progressed with initial chemotherapy, or relapsed with a post-treatment failure-free interval of <6 months, were defined as “resistant”.18
Paraffin-embedded tissue sections (4-μm thick) were deparaffinized using xylene and dehydrated with ethanol. After immersion in 0.3% H2O2 in ethanol for 15 min, the sections were pretreated in a 10-mM citrate buffer solution (pH 6.0) at 98°C for 10 min. The sections were incubated with goat anti-human mimitin antibody (1:200) and rabbit anti-human 14-3-3 ζ/δ (1:100), respectively. HRP-conjugated secondary antibody (1:5000) was then used to stain the sections for 45 min at room temperature. For negative controls, the sections were reacted with rabbit IgG instead of the specific primary antibodies at the same dilution. The tissue sections were evaluated under a light microscope (400 × ) and scored as follows: 1, no detectable immunostaining; 2, immunostaining <25%; 3, immunostaining between 25% and <50%; 4, immunostaining 50–75%; 5, immunostaining >75%. The immunostaining intensity was evaluated by two independent observers who were blinded to the clinical data and the protein data.
Utilizing the results of the first cohort, an additional cohort was sought for analysis and 71 patients with primary ovarian cancer in our hospital were enrolled during 2009–2012. This study group was chosen to investigate the prognostic values of mimitin and 14-3-3 ζ/δ in a larger sample size. All of these participants received cytoreductive surgery and adjuvant chemotherapy of TC regimen (paclitaxel and carboplatinum) for at least six cycles. The definition of chemoresistance was the same as that in the first cohort. Pulled slides were stained by immunohistochemistry and immunostainings were scored as defined by the first cohort. For the convenience of clinical application, we defined the expression scores of ≤2 as low expression levels and the scores of ≥3 as high expression levels. The median follow-up time was 27 months (range, 5–66 months). The patients were examined every 3 months for the first 2 years, every 6 months for the next 3 years and yearly thereafter. The date of recurrence was determined by clinical examination, imaging studies and CA 125 levels. Progression-free survival (PFS) was defined as the time interval from the date of completion of adjuvant chemotherapy to the date of documented first recurrence of disease. Overall survival (OS) was defined as the number of months from the date of completion of adjuvant chemotherapy to the date of death. Survival was censored by the closeout date (1 May 2014).
Statistical analysis
SPSS version 20.0 was used for statistical analysis. Comparison of resistance indexes was performed using Student’s t-test. The relative significance between immunohistochemical staining and drug resistance was determined by a nonparametric Mann–Whitney U-test. The clinicopathological characteristics were compared by χ2-test and the effects on survival were analyzed using the Kaplan–Meier method and the log-rank test. The multivariate analyses were conducted using the Cox proportional hazards regression model. Local significance was considered as two-sided P < 0.05.
Results
Characteristics of paclitaxel-resistant sublines
The viability of parental cell lines (SKOV3 and A2780) and paclitaxel-resistant cell lines (SKOV3-TR and A2780-TR) following treatment with different concentrations of paclitaxel are shown in Figure1. The resistance indexes of SKOV3-TR and A2780-TR cells were 18.89 ± 3.03 and 5.42 ± 1.21, respectively (Table1).
Figure 1.
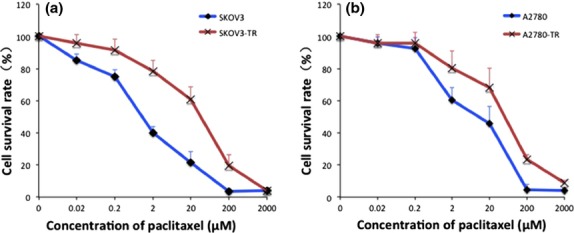
The drug sensitivity of SKOV3, A2780 cells and their resistant counterparts. Cells ([a] SKOV3; [b] A2780) were incubated with increasing concentrations of paclitaxel and cell viability was determined using the MTT assay after 48 h. All tests were performed in triplicate. Values represent the mean ± SEM.
Table 1.
Resistance of ovarian cell lines to paclitaxel
| Cell line | IC50 (μM) | Resistance index |
|---|---|---|
| Paclitaxel | ||
| SKOV3 | 1.06 ± 0.33 | 1.00 |
| SKOV3-TR | 19.40 ± 3.22 | 18.89 ± 3.03 |
| A2780 | 6.14 ± 0.45 | 1.00 |
| A2780-TR | 33.01 ± 5.80 | 5.42 ± 1.21 |
Characterization of isolated mitochondria
The purity of the isolated mitochondria was determined using electron microscopy. The examination of multiple electron microscopic fields revealed that the preparations contained few, if any, nuclear fragments or other cytosolic organelles (Fig.2a). In addition, we used western blotting to validate the purity of the isolated mitochondria. The total cell protein extraction was used as a control. Western blot results showed that mitochondrial proteins were greatly enriched in the purified samples. A mitochondrial marker COX4 was determined in total cell proteins and mitochondrial preparations were isolated from SKOV3, A2780, SKOV3-TR and A2780-TR cell lines. In contrast, lamin-B, flotillin-1 and β-actin were only detected in the cellular proteins and were absent in the mitochondrial preparations (Fig.2b).
Figure 2.
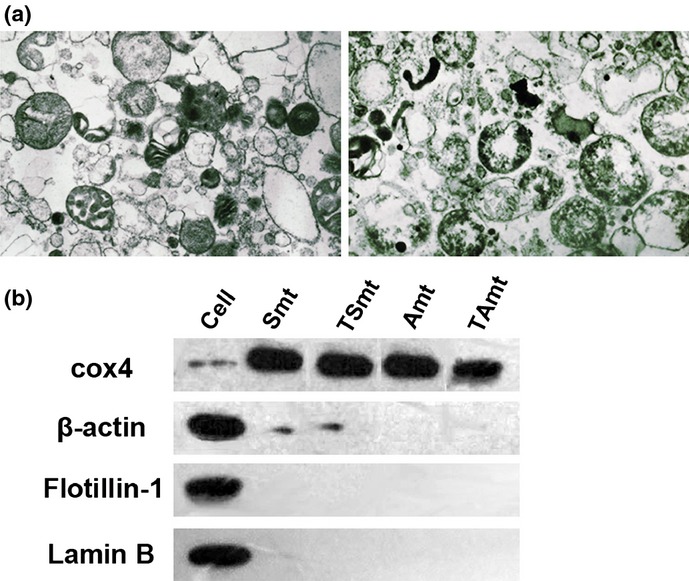
(a) Electron microscopy image of mitochondria morphology (10 000×). The purity of the mitochondria was confirmed using electron microscopy, including intactness and contamination. (b) Western blot analyses of total cell proteins (Cell) and mitochondrial preparations isolated from paclitaxel-sensitive SKOV3 cells (Smt), paclitaxel-resistant SKOV3 cells (TSmt), paclitaxel-sensitive A2780 cells (Amt), and paclitaxel-resistant A2780 cells (TAmt) for COX4 (mitochondrial marker), lamin-B (nuclear marker), flotillin-1 (cell membrane marker) and β-actin (cytoskeletal protein). There was little contamination in the mitochondria-enriched fractions.
Identification of differentially expressed mitochondrial proteins by liquid chromatography-hybrid linear ion trap Fourier-transform ion cyclotron resonance mass spectrometry
The expression of different proteins was detected by guanidine-modified acetyl-stable isotope labeling and LC-FTICR MS in paclitaxel-sensitive SKOV3 and A2780 cell lines and their paclitaxel-resistant counterparts. A total of 542 proteins were quantified. Among these, 328 proteins were quantified in SKOV3-TR/SKOV3 cells and 454 in A2780-TR/A2780 cells. Seventy-nine differentially expressed proteins were selected in SKOV3-TR/SKOV3 cells and 183 in A2780-TR/A2780 cells. Eight differentially expressed proteins were shared between the two groups of cells (Table2). According to the selection criteria, mimitin and 14-3-3 ζ/δ were selected because of the high peptide matching scores at the 95% confidence level and the significant difference in the quantitative ratio. Moreover, among all eight differentially expressed proteins in Table2, the two proteins had multiple biological functions that were correlated with cell metabolism.19,20 Therefore, mimitin and 14-3-3 ζ/δ were selected as differentially expressed candidate proteins that correlated with chemoresistance in the ovarian cancer cells.
Table 2.
Eight differentially expressed proteins in mitochondria of paclitaxel-resistant and paclitaxel-sensitive cells
| Protein | Protein IPI code | TA/A | TS/S | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ratio | Mascot† | Number of peptides | Sequence coverage | Ratio | Mascot† | Number of peptides | Sequence coverage | ||
| Isoform 1 of zinc finger MYM-type protein 3 | IPI00029484.1 | 2.317 | 30 | 1 | K.CPESLRTR.N | 2.075 | 30 | 1 | K.CPESLRTR.N |
| Glutathione S-transferase P | IPI00219757.12 | 3.33 | 37 | 1 | R.TLGLYGK.D | 2.48 | 28 | 1 | R.TLGLYGK.D |
| 16 kDa protein | IPI00790740.1 | 2.089 | 33 | 1 | K.YGVSGYPTLK.I | 8.425 | 33 | 1 | K.YGVSGYPTLK.I |
| Isoform 1 of Regulator of nonsense transcripts 3A | IPI00011138.1 | 0.462 | 28 | 1 | K.QESCAPGAVVK.A | 0.48 | 28 | 1 | K.QESCAPGAVVK.A |
| Isoform Sp100-HMG of Nuclear autoantigen Sp-100 | IPI00011675.1 | 0.076 | 27 | 1 | R.AKGKPNSAK.K | 0.05 | 27 | 1 | R.AKGKPNSAK.K |
| 14-3-3 protein zeta/delta | IPI00021263.3 | 0.298 | 50 | 1 | R.NLLSVAYK.N | 0.235 | 29 | 1 | R.YLAEVAAGDDKK.G |
| Mimitin, mitochondrial precursor | IPI00031109.4 | 0.083 | 49 | 1 | K.EEPSVAPSSTGK.T | 0.135 | 54 | 1 | K.EEPSVAPSSTGK.T |
| CDNA FLJ46154 fis, clone TESTI4001348 | IPI00418801.3 | 0.393 | 33 | 1 | K.QVTEPEEK.Q | 0.243 | 33 | 1 | K.QVTEPEEK.Q |
Mascot protein scores were used to determine the order of display of the identified proteins. A, A2780; TA, A2780-TR; TA/A, differentially expressed protein between A2780-TR and A2780 cells; TS, SKOV3-TR; TS/S, differentially expressed protein between SKOV3-TR and SKOV3 cells; S, SKOV3.
Validation of mimitin and 14-3-3 ζ/δ using western blotting
To validate the expression levels of mimitin and 14-3-3 ζ/δ identified by LC-FTICR MS, the protein levels in mitochondrion-enriched fractions were measured in each cell line using western blotting. As shown in Figure3, the protein levels of mimitin and 14-3-3 ζ/δ were higher in the SKOV3 and A2780 cell lines than in their paclitaxel-resistant counterparts. The results were consistent with those of the LC-FTICR MS.
Figure 3.
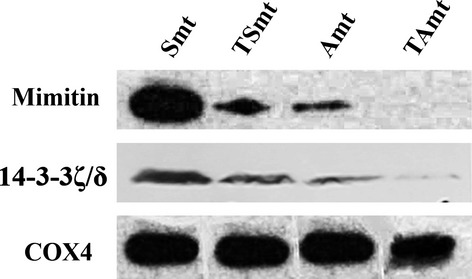
Western blotting was used to validate the expression levels of mimitin and 14-3-3 ζ/δ in two paclitaxel-resistant cell lines, SKOV3-TR (TSmt) and A2780-TR (TAmt), and their parental sensitive cell lines, SKOV3 (Smt) and A2780 (Amt). The proteins were located at 20 and 28 kDa. COX4 was the loading control.
Increased paclitaxel-resistance by downregulated expression levels of mimitin and 14-3-3 ζ/δ
To further detect whether low expressed mimitin and 14-3-3 ζ/δ could be associated with paclitaxel resistance of ovarian cancer cells, we downregulated the expression levels of mimitin and 14-3-3 ζ/δ through RNA interference in SKOV3-TR cell lines, which were verified using western blot analysis, and then measured the paclitaxel resistance (Fig.4). The resistance indexes of SKOV3-TR cells were significantly increased compared with the negative control after the expression level of mimitin was downregulated by two separate siRNA (si Mim-1 and si Mim-2) (P = 0.049 and P = 0.043, respectively). Similarly, the resistance indexes were increased after 14-3-3 ζ/δ knockdown by two separate siRNAs (si 143-1 and si 143-2) (P = 0.031 and P = 0.022, respectively).
Figure 4.
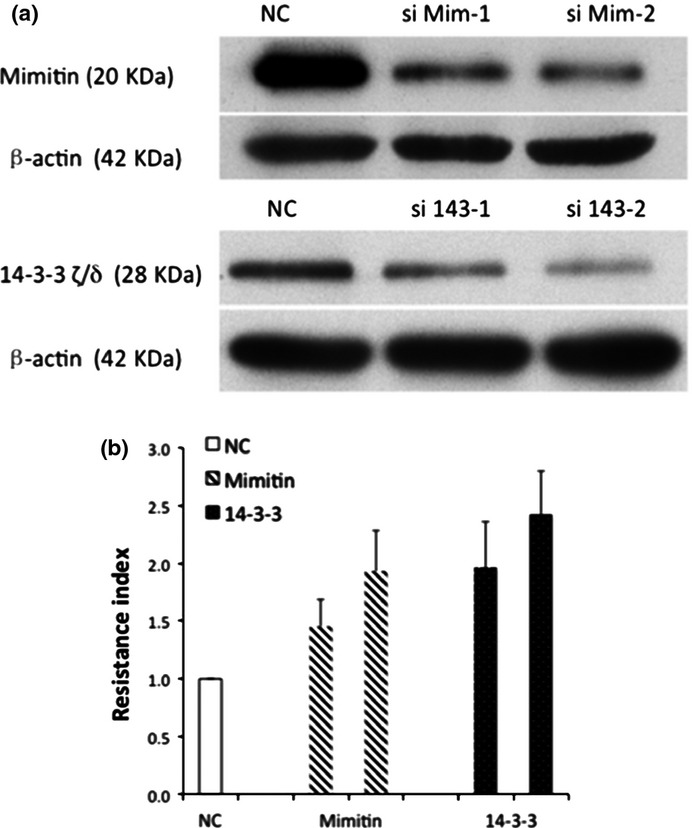
(a) Loss-of-function screening was performed using small interfering RNA targeting mimitin and 14-3-3 ζ/δ in SKOV3-TR cells. The protein expression levels were confirmed using western blotting. The constructs with the highest knockdown efficiency (si Mim-1, Mim-2 for mimitin and si 143-1, si 143-2 for 14-3-3ζ/δ) were used for further experiments. (b) For measurements of the with control scramble siRNA (NC), mimitin or 14-3-3 ζ/ siRNA paclitaxel resistance index, the numbers of viable cells after transfection were assessed using the Cell Counting Kit-8 assay. The resistance index for cells with lower expressed mimitin was 1.45, 1.93 and for 14-3-3 ζ/δ-transfection was 1.96, 2.42 (si Mim-1: P = 0.049, si Mim-2: P = 0.043, si 143-1: P = 0.031, and si 143-2: P = 0.022, respectively).
Relationship between chemoresistance and Mimitin/14-3-3 ζ/δ expression in ovarian cancer tissues
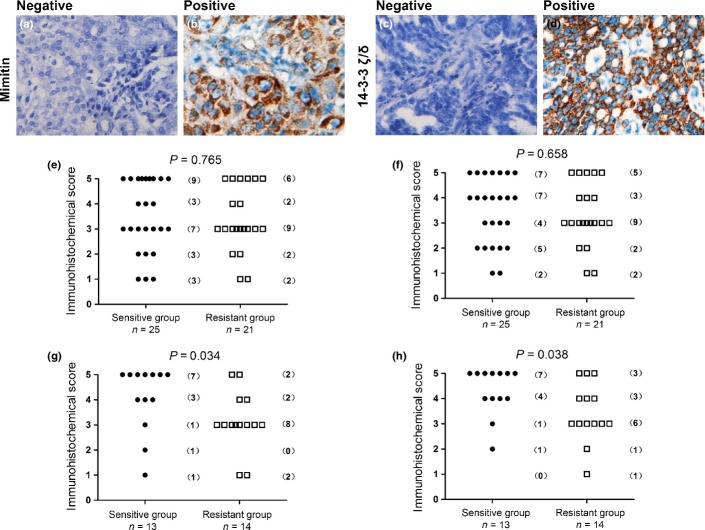
Among all 46 patients of the first cohort, 25 patients were chemosensitive and the remaining 21 patients were chemoresistant (Table3). The expression levels of mimitin and 14-3-3 ζ/δ were detected using immunohistochemistry (Fig.5a–d). The patient numbers are shown in Figure5e and f, according to different mimitin or 14-3-3 ζ/δ protein levels. No significant differences of the mimitin or 14-3-3 ζ/δ expression level were observed between the sensitive and resistant groups (mimitin: P = 0.765, Fig.5e; 14-3-3 ζ/δ: P = 0.658, Fig.5f).
Table 3.
Clinical and pathological characteristics of 46 patients
| Characteristic | Sensitive (n = 25) | Resistant (n = 21) |
|---|---|---|
| FIGO stage | ||
| I | 3 | 0 |
| II | 5 | 1 |
| III | 15 | 19 |
| IV | 2 | 1 |
| Histological type | ||
| Serous | 13 | 11 |
| Clear cell | 3 | 3 |
| Other† | 9 | 7 |
Other, all the other ovarian epithelial cancers excluding serous and clear cells.
Figure 5.
Immunohistochemistry was used to detect the expression of mimitin and 14-3-3 ζ/δ in the first cohort of 46 ovarian cancer patients. In specimens not expressing mimitin and 14-3-3 ζ/δ (negative control), there was no brown staining in the cytoplasm (a,c). Mimitin and 14-3-3 ζ/δ were stained brown in the cytoplasm (b,d). Different immunohistochemical staining scores were recorded and the plots represented the numbers of patients (e–h). When patients with different regimens of chemotherapy enrolled, no significant correlation of immunohistochemical staining and chemotherapy response was observed (Mann–Whitney U-test: mimitin, P = 0.765; 14-3-3 ζ/δ, P = 0.658, Fig.5e,f). While 27 patients with only regimens containing paclitaxel enrolled, a significant difference was observed in the expression levels of mimitin and 14-3-3 ζ/δ between paclitaxel-sensitive and paclitaxel-resistant patients (Mann-Whitney U-test: mimitin, P = 0.034; 14-3-3 ζ/δ, P = 0.038, Fig.5g,h).
Considering that the cell lines used in the study were resistant to paclitaxel, we excluded the patients whose chemotherapy regimen did not include paclitaxel. Twenty-seven patients received treatment containing paclitaxel after surgery, 14 of whom were chemoresistant (Table4). Their expressions in the paclitaxel-resistant group were significantly downregulated compared to those in paclitaxel-sensitive group (mimitin: P = 0.034, Fig.5g; 14-3-3 ζ/δ: P = 0.038, Fig.5 h).
Table 4.
Clinical and pathological characteristics of 27 patients in the first cohort
| Characteristic | Sensitive (n = 13) | Resistant (n = 14) |
|---|---|---|
| FIGO stage | ||
| I | 1 | 0 |
| II | 2 | 2 |
| III | 10 | 11 |
| IV | 0 | 1 |
| Histological type | ||
| Serous | 8 | 9 |
| Clear cell | 2 | 2 |
| Other† | 3 | 3 |
Other: all the other ovarian epithelial cancers excluding serous and clear cell.
Association between mimitin and 14-3-3 ζ/δ protein levels and clinicopathological characteristics in primary ovarian cancer cases
The baseline data of the validation cohort including 71 cases of patients who received paclitaxel and carboplatinum chemotherapy from 2009 to 2012 are summarized in Table5. The numbers of chemosensitive and chemoresistant patients were 56 and 15, respectively. Among the 15 chemoresistant patients, 10 presented with low expression of mimitin, whereas 9 of 56 participants presented with low expression of mimitin in the chemosensitive group (P < 0.001). Meanwhile, 11 of 15 paclitaxel-resistant patients presented with low expression of 14-3-3 ζ/δ compared to 7 of 56 cases with low expression of 14-3-3 ζ/δ in the paclitaxel-sensitive group (P 0.001). According to the Kaplan–Meier survival curve, mimitin and 14-3-3 ζ/δ protein expression levels had a significant impact on PFS and OS (mimitin: PFS: P = 0.002, OS: P = 0.001; 14-3-3 ζ/δ: PFS: P = 0.028; OS: P = 0.014) (Fig.6).
Table 5.
Clinical and pathological characteristics of 71 patients in the validation cohort
| Characteristic | Sensitive (n = 56) | Resistant (n = 15) | P |
|---|---|---|---|
| FIGO stage | |||
| I | 3 | 0 | 0.630 |
| II | 7 | 3 | |
| III | 42 | 10 | |
| IV | 4 | 2 | |
| Histological type | |||
| Serous | 38 | 11 | 0.533 |
| Clear cell | 10 | 1 | |
| Other | 8 | 3 | |
| Mimitin expression | |||
| Low | 9 | 10 | <0.001 |
| High | 47 | 5 | |
| 14-3-3 ζ/δ expression | |||
| Low | 7 | 11 | <0.001 |
| High | 49 | 4 | |
| PFS (median, months) | 20 | 4 | <0.001 |
| OS (median, months) | 31 | 11 | <0.001 |
Significant values appear in boldface type. P < 0.05 was considered to be statistically significant. OS, overall survival; PFS, progression-free survival.
Figure 6.
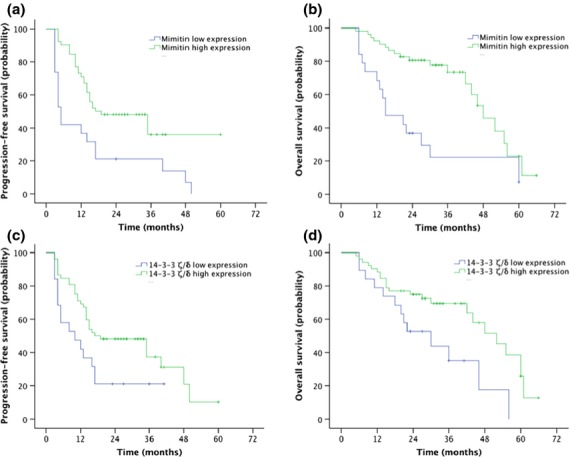
Comparison of progression-free survival (PFS) and overall survival (OS) in the validation cohort of 71 patients, stratified by mimitin (a,b) or 14-3-3 ζ/δ (c,d) expression levels (mimitin: PFS: P = 0.002, OS: P = 0.001; 14-3-3 ζ/δ: PFS: P = 0.028; OS: P = 0.014).
When the data were stratified in the multivariate analysis using stepwise Cox regression procedures, mimitin and 14-3-3 ζ/δ immunoreactivity, residual disease ≥1 cm and paclitaxel-resistance were significant at P < 0.05 for PFS and OS in all patients. These findings suggest that mimitin and 14-3-3 ζ/δ expression levels are independent predictors of survival outcomes (mimitin: PFS: HR 2.11, 95% CI 1.03–4.33, P = 0.041, OS: HR 3.96, 95% CI 1.59–9.90, P = 0.003; 14-3-3 ζ/δ: PFS: HR 2.29, 95% CI 1.14–4.62, P = 0.031, OS: HR 2.88, 95% CI 1.28–6.51, P = 0.011; Table6).
Table 6.
Multivariate analyses predicting survival in the validation cohort
| Risk factors | n | PFS | P | OS | P |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||||
| Age | 0.876 | 0.267 | |||
| ≤50 years | 23 | Referent | Referent | ||
| >50 years | 48 | 0.95 (0.47–1.91) | 0.61 (0.25–1.47) | ||
| FIGO stage | 0.045 | 0.013 | |||
| I–II | 13 | Referent | Referent | ||
| III–IV | 58 | 1.407 (1.17–1.98) | 2.26 (1.09–3.75) | ||
| Histotype | 0.838 | 0.113 | |||
| Type I | 21 | Referent | Referent | ||
| Type II | 49 | 1.08 (0.54–2.16) | 1.49 (1.21–3.18) | ||
| Grade | 0.609 | 0.234 | |||
| Low | 11 | Referent | Referent | ||
| Medium–high | 60 | 1.78 (1.30–3.04) | 1.389 (1.08–4.84) | ||
| Residual disease | <0.001 | <0.001 | |||
| ≤1 cm | 58 | Referent | Referent | ||
| >1 cm | 13 | 2.19 (1.59–4.43) | 2.46 (1.89–4.16) | ||
| Mimitin expression | 0.041 | 0.003 | |||
| High | 52 | Referent | Referent | ||
| Low | 19 | 2.11 (1.03–4.33) | 3.96 (1.59–9.90) | ||
| 14-3-3 ζ/δ expression | 0.021 | 0.011 | |||
| High | 53 | Referent | Referent | ||
| Low | 18 | 2.29 (1.14–4.62) | 2.88 (1.28–6.51) |
Significant values appear in boldface type. P < 0.05 was considered to be statistically significant. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Discussion
The nature of this drug resistance remains a major obstacle for the successful treatment of ovarian cancer. In the present study, we used comparative proteomic methods for screening the mitochondrial protein expression profiles, and we identified for the first time the two paclitaxel-resistant associated proteins, mimitin and 14-3-3 ζ/δ, in ovarian cancer cells.
Using LC-FTICR MS, we successfully identified 542 proteins, and 8 differentially expressed proteins were detected between the two groups of cells. Among these proteins, glutathione S-transferase P was highly expressed in SKOV3-TR and A2780-TR cells. Glutathione S-transferase P (GSTP1, EC 2.5.1.18) is known to be one of the most abundantly expressed genes in ovarian tumors and tumor cell lines.21 It is a polymorphic phase II drug-metabolising enzyme, which conjugates the antioxidant tri-peptide glutathione with many toxic hydrophobic and electrophilic xenobiotics to facilitate elimination. GSTP1 expression is increased in human tumor cell lines either inherently or made resistant to chemotherapy drugs, including cisplatin and various alkylating agents.22,23 Hence, our proteomics analysis is consistent with the literature. Moreover, we assessed mimitin and 14-3-3 ζ/δ in detail because they were significantly differentially expressed in paclitaxel-resistant cell lines and, according to the published literature, they might anticipate the regulation of cellular processes.
Mimitin is a myc-induced mitochondrial protein that was first reported by Tsuneoka et al. in 2005.24 The transcription of mimitin is directly stimulated by c-myc, which is a ubiquitous mediator of cell proliferation that transactivates the expression of various genes through E-box sites. Patients with a higher c-myc expression level had a better 5-year survival rate (69.8 vs 43.5%; n = 101 patients).25 The expression of mimitin is correlated with c-myc and cell proliferation.19 14-3-3 proteins are a highly conserved acidic protein family that is present in all eukaryotic species, which was first identified in the late 1960s by Moore and Perez.26 The 14-3-3 family comprises the β, γ, ε, η, σ, τ (or θ) and ζ isoforms. The activation of 14-3-3 association generally inhibits the cell cycle and prevents apoptosis.27,28 The loss of 14-3-3 expression sensitizes cancer cells to conventional anticancer agents.
The correlation of mimitin, 14-3-3 ζ/δ and paclitaxel resistance has not been discussed previously. In our experiment, LC-FTICR MS showed that mimitin protein level was downregulated 7.4-fold in the paclitaxel-resistant SKOV3 cell line and 12-fold in A2780 cells compared with the corresponding paclitaxel-sensitive cell lines, while 14-3-3 ζ/δ protein was downregulated 4.3-fold in SKOV3/SKOV3-TR cells and 3.4-fold in A2780/A2780-TR cell lines. Furthermore, mimitin and 14-3-3 ζ/δ silencing by siRNA efficiently increased the resistance to paclitaxel in ovarian cancer cells. Therefore, it was hypothesized that the underexpression of mimitin and 14-3-3 ζ/δ may promote tumor cell proliferation and/or survival in ovarian cancer. To test our hypothesis, we examined the expression status of mimitin and 14-3-3 ζ/δ in patients with primary ovarian cancer. The immunohistochemitry results demonstrated that in paclitaxel-resistant patients, mimitin was underexpressed in 66.7% (10/15) of ovarian cancer patients while 14-3-3 ζ/δ was underexpressed in 73.3% (11/15) of ovarian cancer patients. Among ovarian cancer patients receiving carboplatin and paclitaxel regimen, the lower expressions of mimitin and 14-3-3 ζ/δ were poor prognosticators independent of other prognostic factors.
How mimitin and 14-3-3 ζ/δ influence paclitaxel resistance in ovarian cancer is complicated and the mechanism remains unclear. Paclitaxel is a cytotoxic microtubule stabilizing agent. It could cause the formation of unusually stable microtubules and trigger the mitotic spindle checkpoint, resulting in apoptosis.7 Wegrzyn et al. found that the mimitin gene was activated by the proinflammatory cytokines IL-1 and IL-6 in HepG2 cells. In addition, mimitin could interact with a microtubular protein (MAP1S) and may indirectly participate in apoptosis.19 Hanzelka et al. observed that mimitin prevented mitochondrial stress upon exposure to cytokines, and this protective effect was delivered independent of a suppression of the NF-κB pathway.29 Thus, the underexpression of mimitin might induce the dysfunction of microtubule and abnormal apoptosis of cancer cells, altering their response to paclitaxel. The effects of 14-3-3 ζ/δ seem to be more complex.30–33 The role of 14-3-3 ζ/δ in ovarian cancer is also controversial. Hatzipetros found that serum 14-3-3 zeta protein levels did not significantly differ in healthy postmenopausal patients versus epithelial ovarian cancer (EOC) patients.34 However, Kobayashi demonstrate that 14-3-3 zeta was secreted by ascitic monocytes/macrophages from EOC patients and was present in malignant ascites of EOC patients, but the functional role for 14-3-3 zeta as a secreted protein was unclear.35 Waldemarson et al. (2012) found a gradual upregulation of 14-3-3 ζ/δ protein when going from normal to benign to borderline to malignant tumors using iTRAQ technology, making it a biomarker of early stage ovarian cancer.36 During the development of ovarian cancer, the loss of 14-3-3 ζ/δ might make the ovarian cancer cells mimic the behavior of borderline tumor and become less sensitive to paclitaxel, resulting in the failure of chemotherapy. However, the sensitivity to chemotherapy is critical to the survival of ovarian cancer patients, especially for those at advanced stages.37
Our study is significant because differentially expressed mitochondrial proteins were detected in paclitaxel-resistant ovarian cancer cell lines and their parental cells using comparing proteomic techniques. In addition, it is the first report to demonstrate the correlation of mimitin, 14-3-3 ζ/δ and paclitaxel resistance in ovarian cancer. The limitations of our study included the definition of paclitaxel resistance. It’s difficult to eliminate the interference of carboplatin since the standard chemotherapy for ovarian cancer contains both carboplatin and paclitaxel. Thus it is impossible to collect one cohort of patients receiving only paclitaxel as adjuvant chemotherapy. However, we tested the drug sensitivity to cisplatin, carboplatin and epirubicin in SKOV3-TR cells and found that their resistance indexes had not been affected by downregulation of mimitin or 14-3-3 ζ/δ (data no shown). Moreover, in our first cohort of immunohistochemistry, we verified that the level of mimitin or 14-3-3 ζ/δ only related to the failure of chemotherapy containing paclitaxel but not those regimens without paclitaxel. Therefore, we believe that the decrease of these two proteins is a specific mechanism of paclitaxel resistance.
In conclusion, we found that mimitin and 14-3-3 ζ/δ proteins were downregulated in the paclitaxel-resistant SKOV3 and A2780 cell lines compared with their sensitive counterparts using LC-FTICR MS and western blotting. The lower expressions of mimitin and 14-3-3 ζ/δ were related to paclitaxel resistance and were poor prognosticators independent of other prognostic factors in epithelial ovarian patients. We believe that the identification of mimitin and 14-3-3 ζ/δ may help in predicting the prognosis of ovarian cancer patients and in applying more individualized chemotherapy.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (30571944).
Disclosure Statement
The authors have no conflicts of interest.
References
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- Wong KH, Mang OW, Au KH, Law SC. Incidence, mortality, and survival trends of ovarian cancer in Hong Kong, 1997 to 2006: a population-based study. Hong Kong Med J. 2012;18:466–74. [PubMed] [Google Scholar]
- Stordal B, Pavlakis N, Davey R. A systematic review of platinum and taxane resistance from bench to clinic: an inverse relationship. Cancer Treat Rev. 2007;33:688–703. doi: 10.1016/j.ctrv.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. doi: 10.1016/s0027-5107(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Eno ML, Im DD, Rosenshein NB, Sood AK. Clinical relevance of extent of extreme drug resistance in epithelial ovarian carcinoma. Gynecol Oncol. 2010;116:61–5. doi: 10.1016/j.ygyno.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh H, Wiesen K, Simpkins H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem Pharmacol. 1997;53:461–70. doi: 10.1016/s0006-2952(97)83383-7. [DOI] [PubMed] [Google Scholar]
- Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–95. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio JB, Cardoso CM, Santos MS, Almeida LM, Vicente JA, Fernandes MA. Cisplatin impairs rat liver mitochondrial functions by inducing changes on membrane ion permeability:prevention by thiol group protecting agents. Toxicology. 2009;259:18–24. doi: 10.1016/j.tox.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Saitou M, Isonishi S, Hamada T, et al. Mitochondrial ultrastructure-associated chemotherapy response in ovarian cancer. Oncol Rep. 2009;21:199–204. [PubMed] [Google Scholar]
- Alvero AB, Montagna MK, Holmberg JC, Craveiro V, Brown D, Mor G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol Cancer Ther. 2011;10:1385–93. doi: 10.1158/1535-7163.MCT-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai ZQ, Yin J, He HJ, et al. Mitochondrial comparative proteomics of human ovarian cancer cells and their platinum-resistant sublines. Proteomics. 2010;10:3789–99. doi: 10.1002/pmic.200900685. [DOI] [PubMed] [Google Scholar]
- Yan XD, Li M, Yuan Y, Mao N, Pan LY. Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol Rep. 2007;17:1163–9. [PubMed] [Google Scholar]
- Li L, Luan Y, Wang G, et al. Development and characterization of five cell models for chemoresistance studies of human ovarian carcinoma. Int J Mol Med. 2004;14:257–64. [PubMed] [Google Scholar]
- Takeya K, Koike M, Mori R, Toda T. Light and electron microscope studies of mycobacterium–mycobacteriophage interactions. III. Further studies on the ultrathin sections. J Biophys Biochem Cytol. 1961;11:441–7. doi: 10.1083/jcb.11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Ji C, Guo N, Li L. Differential dimethyl labeling of N-termini of peptides after guanidination for proteome analysis. J Proteome Res. 2005;4:2099–108. doi: 10.1021/pr050215d. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang J, Zheng Z, et al. A systematic N-terminal peptide quantitative labeling strategy for differential proteomic analysis. Proteomics Clin Appl. 2010;4:633–43. doi: 10.1002/prca.200900065. [DOI] [PubMed] [Google Scholar]
- Swenerton K, Muss HB, Robinson EG, editors. Salvage Chemotherapy for Refractory Disease in Ovarian Cancer Controversies on Management. New York: Churchill Livingstone; 1998. [Google Scholar]
- Wegrzyn P, Yarwood SJ, Fiegler N, et al. Mimitin - a novel cytokine-regulated mitochondrial protein. BMC Cell Biol. 2009;10:23. doi: 10.1186/1471-2121-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin NO, Bass MD, Warwood S, et al. An integrin-alpha4-14-3-3zeta- paxillin ternary complex mediates localised Cdc42 activity and accelerates cell migration. J Cell Sci. 2009;122:1654–64. doi: 10.1242/jcs.049130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- Masanek U, Stammler G, Volm M. Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol. 2002;2:37–41. doi: 10.1046/j.1359-4117.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- Sawers L, Ferguson MJ, Ihrig BR, et al. Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br J Cancer. 2014;111:1150–8. doi: 10.1038/bjc.2014.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneoka M, Teye K, Arima N, et al. A novel Myc-target gene, mimitin, that is involved in cell proliferation of esophageal squamous cell carcinoma. J Biol Chem. 2005;280:19977–85. doi: 10.1074/jbc.M501231200. [DOI] [PubMed] [Google Scholar]
- Iba T, Kigawa J, Kanamori Y, et al. Expression of the c-myc gene as a predictor of chemotherapy response and a prognostic factor in patients with ovarian cancer. Cancer Sci. 2004;95:418–23. doi: 10.1111/j.1349-7006.2004.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BE, Perez VJ. Specific acidic proteins of the nervous systems. In: Carlson FD, editor. Physiological and Biochemical Aspects of Nervous Integration. Englewood Cliffs: Prentice–Hall; 1967. pp. 343–59. [Google Scholar]
- Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Curr Opin Cell Biol. 2002;14:237–45. doi: 10.1016/s0955-0674(02)00312-5. [DOI] [PubMed] [Google Scholar]
- Samuel T, Weber HO, Rauch P, et al. The G2/M regulator 14-3-3sigma prevents apoptosis through sequestration of Bax. J Biol Chem. 2001;276:45201–206. doi: 10.1074/jbc.M106427200. [DOI] [PubMed] [Google Scholar]
- Hanzelka K, Skalniak L, Jura J, Lenzen S, Gurgul-Convey E. Effects of the novel mitochondrial protein mimitin in insulin-secreting cells. Biochem J. 2012;445:349–59. doi: 10.1042/BJ20111920. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Komatsu S, Ichikawa D, et al. Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer. 2013;108:1324–31. doi: 10.1038/bjc.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Takayama K, Urano T, et al. 14-3-3ζ, a novel androgen-responsive gene, is upregulated in prostate cancer and promotes prostate cancer cell proliferation and survival. Clin Cancer Res. 2012;18:5617–27. doi: 10.1158/1078-0432.CCR-12-0281. [DOI] [PubMed] [Google Scholar]
- Masui O, White NM, DeSouza LV, et al. Quantitative proteomic analysis in metastatic renal cell carcinoma reveals a unique set of proteins with potential prognostic significance. Mol Cell Proteomics. 2013;12:132–44. doi: 10.1074/mcp.M112.020701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liang Q, Wen YQ, et al. Comparative proteomics analysis of human osteosarcomas and benign tumor of bone. Cancer Genet Cytogenet. 2010;198:97–106. doi: 10.1016/j.cancergencyto.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Hatzipetros I, Gocze P, Koszegi T, et al. Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res. 2013;6:79. doi: 10.1186/1757-2215-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Deavers M, Patenia R, et al. 14-3-3 zeta protein secreted by tumor associated monocytes/macrophages from ascites of epithelial ovarian cancer patients. Cancer Immunol Immunother. 2009;58:247–58. doi: 10.1007/s00262-008-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldemarson S, Krogh M, Alaiya A, et al. Protein expression changes in ovarian cancer during the transition from benign to malignant. J Proteome Res. 2012;11:2876–89. doi: 10.1021/pr201258q. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–7. doi: 10.1002/ijc.24654. [DOI] [PubMed] [Google Scholar]