Abstract
Objective
To provide an update on respiratory diseases caused by coal mine dust.
Methods
This article presents the results of a literature review initially performed for an International Conference on Occupational and Environmental Lung Disease held in summer 2013.
Results
Coal mine dust causes a spectrum of lung diseases collectively termed coal mine dust lung disease (CMDLD). These include Coal Workers’ Pneumoconiosis, silicosis, mixed dust pneumoconiosis, dust-related diffuse fibrosis (which can be mistaken for idiopathic pulmonary fibrosis), and chronic obstructive pulmonary disease. CMDLD continues to be a problem in the United States, particularly in the central Appalachian region. Treatment of CMDLD is symptomatic. Those with end-stage disease are candidates for lung transplantation. Because CMDLD cannot be cured, prevention is critical.
Conclusions
Coal mine dust remains a relevant occupational hazard and miners remain at risk for CMDLD.
Coal is an important global commodity and will remain so for the foreseeable future. Thus, mining of coal will also remain important. Despite improvements in exposure assessment and ventilation controls and the existence of protective government regulations, coal miners are still at risk for respiratory diseases caused by coal mine dust and their associated morbidity and mortality. Thus, clinicians must be prepared to diagnose these diseases and recognize their association with work in coal mining. This review provides background information such as where coal is mined and projected trends in production. It also describes respiratory conditions caused by coal mine dust, burden, and risk factors, and covers some aspects of diagnosis, treatment, and disease prevention.
GLOBAL COAL PRODUCTION
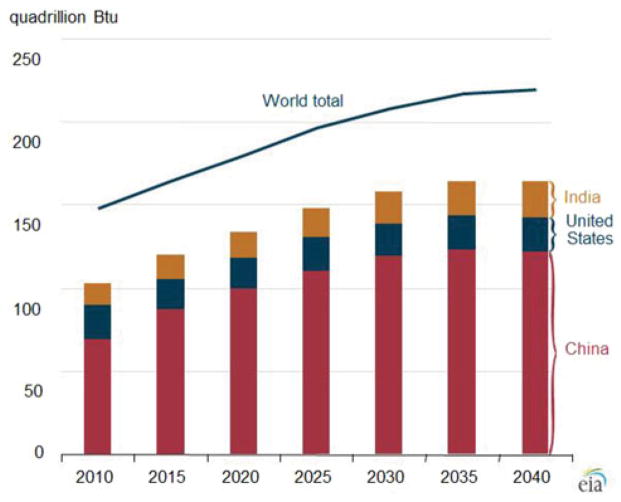
Despite controversies over greenhouse gases and competition from other energy sources such as natural gas extracted by hydraulic fracturing, coal remains the second largest energy source worldwide. Approximately 8 billion short tons were produced in 2010.1 Coal contributes about one-fourth of the world’s energy supply and more than one-third of the fuel used to generate electricity. Internationally, coal consumption has steadily increased since 2000, driven largely by increased demand in China and India.2 In 2010, China (47%), the United States (14%), and India (9%) accounted for 70% of world coal consumption. It is predicted that the share of consumption by these 3 countries will rise to 75% in 2040. Through 2040, worldwide consumption will rise at a rate of 1.8% annually (Fig. 1).1
FIGURE 1.
World coal consumption, 2010–2040. From US Energy Information Administration.1
Coal production in the United States is largely concentrated in a few states, with five accounting for 72% of production in 2011: Wyoming (40%), West Virginia (12.3%), Kentucky (9.9%), Pennsylvania (5.4%), and Texas (4.2%).2 Coal can be obtained by underground mining (primarily in the Eastern United States) or surface mining (dominant in the Western United States). Types of coal also vary by region. Anthracite coal (hard coal, highest rank), typically used in metallurgy and domestic heating, is limited geographically mainly to Appalachia and Pennsylvania. Bituminous coal (soft coal, lower ranked), typically used for electric power generation and coke for steel making, is the most abundant type of coal found in the United States and found primarily in Appalachia and in Midwestern states such as Illinois. Subbituminous coal (still lower ranked than bituminous), typically used in electric power generation and heating, is primarily found in Montana, Wyoming, Colorado, New Mexico, Washington, and Alaska. It is the most produced type of coal in the United States, accounting for 47% of coal production in 2011. Over the past several decades, there has been a shift from underground, bituminous coal production in the Eastern United States to surface, subbituminous coal production in the Western United States. Western coal production currently accounts for 58% of US coal production and is anticipated to reach 68% by 2040.
RESPIRATORY DISEASES ARE CAUSED BY INHALING COAL MINE DUST
Inhalation of coal mine dust is known to cause several types of respiratory disease. To emphasize that there is a spectrum, these have recently been termed “coal mine dust lung disease” (CMDLD).3 Classic coal workers’ pneumoconiosis (CWP) and silicosis are the diseases most familiar to many. They have similar radiographic findings, with milder forms characterized by small (<1 cm) rounded opacities found more in the upper lung zones. A more severe form called progressive massive fibrosis (PMF) is characterized by coalescence of small opacities into large (≥1 cm) opacities. These diseases can be distinguished by pathology; their pathogenesis and pathology are reviewed elsewhere.3 Miners with combined exposures to coal and crystalline silica (quartz) dusts can also get mixed dust pneumoconiosis. Coal miners with rheumatoid arthritis and a background of pneumoconiosis are also at risk for rheumatoid pneumoconiosis, also known as Caplan syndrome. In this condition, multiple well-defined rounded nodules, classically resembling rheumatoid nodules in other locations, may occur in crops. They range in diameter from about 0.5 to several centimeters and are found predominantly at the lung periphery. The nodules often cavitate or calcify.4
A source of confusion around the term “CWP” is that in the United States, it has different meanings in clinical and statutory/legal settings. Clinically, CWP is generally referred to as interstitial disease caused by inhalation of coal mine dust. Nevertheless, 30 U.S.C. §902(b) defines pneumoconiosis in the context of coal mining as a chronic dust disease of the lung and its sequelae, including respiratory and pulmonary impairments, arising out of coal mine employment. Thus, in legal and compensation settings, “legal pneumoconiosis” includes conditions other than interstitial disease, including airways diseases such as chronic obstructive pulmonary disease (COPD). In this review, “CWP” is used narrowly in the clinical sense. The new term CMDLD is used to describe the spectrum of disease caused by coal mine dust.
In addition to the interstitial disease presentations classically associated with coal mining, coal miners are also at risk for dust-related diffuse fibrosis (DDF) and chronic airways diseases including emphysema and chronic bronchitis. All of these fall within the spectrum of CMDLD. The DDF is a form of interstitial disease occurring in coal miners that has a radiographic appearance of irregular opacities and can be mistaken for idiopathic pulmonary fibrosis (IPF) if an exposure history is not obtained.3 The occurrence of irregular opacities in coal miners has long been recognized.5–8 A recent evaluation of 30 years’ experience in the US Coal Workers’ Health Surveillance Program (CWHSP) showed that about 38% of coal miners with radiographic findings of interstitial disease had primarily irregular opacities. These miners showed a lower zone predominance (upper 20.5%, middle 38.4%, and lower 41.1%).9 A French series of coal miners evaluated for features of chronic interstitial pneumonia with honeycombing described lung pathology in eight cases. Two cases showed homogeneous interstitial fibrosis without temporal variation and with honeycombing. Six cases showed features of usual interstitial pneumonia.10
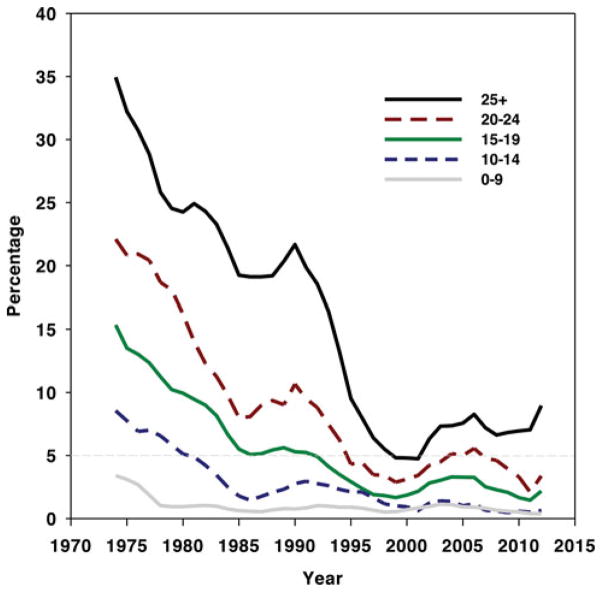
The DDF seems to occur much more frequently in long-tenured coal miners than IPF does in the general population. The prevalence rate per 100,000 in the US general population of IPF, defined by the use of the International Classification of Diseases, 9th revision, Clinical Modification [ICD-9-CM] code 516.3 in medical claims, has recently been estimated for US males at various ages. Rates per 100,000 were 3.8 at 18 to 34 years, 4.9 at 35 to 44 years, 23.3 at 45 to 54 years, 62.8 at 55 to 64 years, 148.5 at 65 to 74 years, and 276.9 at 75 or more years.11 Based on the prevalence data shown in Fig. 2, about 7% of long-tenured US coal miners participating in the CWHSP who have 25 or more years’ tenure are found to have radiographic changes consistent with CWP. As previously noted, about 38% of these long-tenured miners with radiographic changes, or about 2.7% (2700 per 100,000) of this long-tenured group, would be expected to primarily have irregular opacities. Thus, interstitial lung disease associated with irregular opacities suggestive of DDF is found considerably more frequently in long-tenured coal miners than IPF is found in the general population. This is true even among older age groups.
FIGURE 2.
Percentage of examined US underground miners with coal workers’ pneumoconiosis (ILO category 1/0+), 1970–2012. Data are shown as 5-year moving average, with separate plots for various tenures in coal mining. Data are from NIOSH CWHSP.19
It has been recognized for decades that coal mine dust exposure can cause COPD.3,12 Recent studies have added to the strength of evidence. For example, a study of 722 autopsied coal miners and nonminers in the United States showed that cumulative dust exposure was a significant predictor of pathological emphysema severity and had a similar additive effect on emphysema severity as smoking.13 In a prospective study following 260 newly hired Chinese coal miners, 48 developed bronchitic symptoms, which were associated with a sharp early decline in forced expiratory volume in 1 second (FEV1).14 The FEV1 decline over the first 3 years of employment was nonlinear, with a sharp decline in the first year and plateauing or even recovery thereafter.15 An analysis of underground coal miners’ surveillance data from the CWHSP showed that abnormal spirometry in coal miners was associated with radiographic evidence of CWP. Also, these two health outcomes had similar geographic distributions. The overall prevalence of lung function impairment was 13.1% in actively working underground coal miner participants.16
BURDEN AND RISK FACTORS FOR CWP
The National Institute for Occupational Safety and Health (NIOSH) has operated the CWHSP and tracked the burden of CWP in underground coal miners since 1970. For the purposes of the CWHSP, CWP is defined as a final determination of small opacities on a chest radiograph of a profusion of 1/0 or greater, or any large opacity, using the International Labor Office classification system.17 Detailed descriptions of the CWHSP methods are available elsewhere.18,19 As shown in Fig. 2, the prevalence of CWP declined after implementation of the 1969 Federal Coal Mine Health and Safety Act, which put into place regulations to protect coal miners. The prevalence of the most severe form of CWP, PMF, reached a low of 0.08% of miners surveyed for the period 1995 to 2000. Nevertheless, since 2000, rates of CWP have increased and most troubling is the increase in severity. For example, in West Virginia alone, 138 miners with PMF were approved for compensation between 2000 and 2009. Their mean age was only 52.6 years.20 The most up-to-date data for percentage of underground coal miners participating in CWHSP found to have radiographic evidence of CWP are presented in Fig. 2. It suggests that after a nadir in the mid- to late 1990s, prevalence of CWP subsequently increased among CWHSP participants with 15 or more years of tenure in underground coal mining. Data from the CWHSP have also demonstrated geographical clustering of disease and rapid disease progression,21 increased prevalence of radiographic abnormalities suggestive of silicosis,22,23 and associations between disease prevalence and small mine size24–26 during this same period. A subsequent analysis of potential sources of bias in CWHSP data suggested that the finding of an increase in CWP in underground coal miners since 2000 was broadly accurate.27
As already noted, the CWHSP has previously been limited to underground coal miners. Thus, less data exists documenting the burden of pneumoconiosis in US surface miners. Still, it is well known that surface miners are at risk for pneumoconiosis. In 1983, a sentinel case of acute silicosis was reported in a surface coal mine driller.28 The case report included reanalysis of surveillance conducted in US surface coal miners in the early 1970s showing that drill crew workers were at significantly increased risk for pneumoconiosis relative to other surface coal miners. Medical examinations of surface coal miners in Pennsylvania conducted in 1984 to 1985 documented radiographic evidence of pneumoconiosis in 4.5% of men with no history of dusty work other than surface coal mining. Drilling work was a risk factor for the presence and severity of pneumoconiotic disease.29 More contemporary national surveillance of surface coal miners conducted across the United States in 2010 to 2011 documented radiographic evidence of pneumoconiosis in 2% and PMF in 0.5%. Miners from Central Appalachia had substantially higher rates of pneumoconiosis (3.7%) and PMF (1.2%) than miners from other parts of the country.30
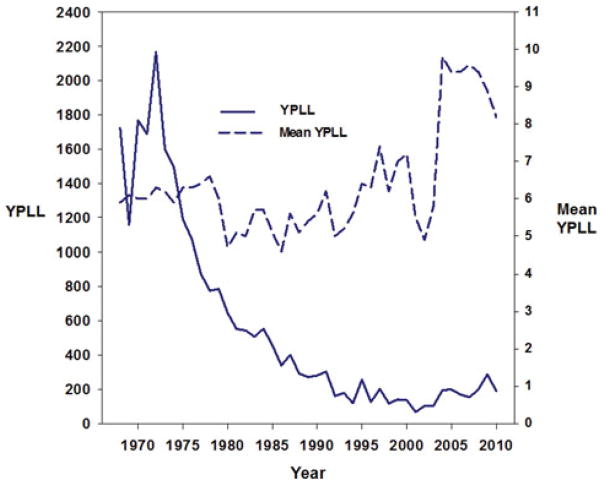
Years of potential life lost (YPLL) is a measure of premature mortality based on the Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS) multiple cause-of-death data files. It is influenced by the number of people dying of a disease and their age at death, because death of a younger person results in a greater YPLL than death of an older person. The annual trend in YPLL is similar to the trends in CWP prevalence. It shows a decline from the 1970s through 2000 and a subsequent increase since that period. As shown in Fig. 3, in 2010, mortality from CWP led to an average 8.2 YPLL among decedents of CWP (data updated from 2009 MMWR).31
FIGURE 3.
Years of potential life lost (YPLL) before the age of 65 years and mean YPLL per decedent for decedents 25 years or older with coal workers’ pneumoconiosis as the underlying cause of death—United States, 1968–2010. Based on annual underlying cause of death from multiple cause-of-death files, National Center for Health Statistics, Centers for Disease Control and Prevention (updated from figure in Amandus et al29).
A 2011 review by NIOSH summarized current trends.32 It found that those surveillance data indicated that the prevalence of CWP was rising and that coal miners were developing severe CWP at relatively young ages (<50 years). The increase in CWP was especially concentrated in the central Appalachian region of southern West Virginia, eastern Kentucky, and western Virginia. Multiple factors were felt to potentially be causative, including excessive coal mine dust levels, increased duration of exposure (due to longer working hours), and potentially increased crystalline silica exposure due to the need to cut rock to mine thinner coal seams. Workers in smaller mines appeared to be at special risk.
DIAGNOSIS OF CMDLD
Diagnosis of CMDLD-related interstitial disease has recently been reviewed.3 Diagnosis can generally be made clinically, on the basis of a combination of an appropriate exposure history, radiological or pathological findings consistent with the diagnosis, and a lack of alternative explanations for the patient’s lung disease. The exposure history can explore intensity of exposure by asking about high-exposure jobs, especially working near the face in underground mines or near drilling operations in surface mines. Duration of exposure is also important, because cumulative exposure is what is most related to risk of disease. In general, pneumoconiosis typically takes at least 10 years after initial exposure to develop in the United States. It is important to ask about jobs associated with silica exposure, generally from generating quartz dust through activities such as cutting or drilling rock. Examples include roof bolting in underground mines and drilling in surface mines.
With regard to chest imaging, it is important to remember that not all patients have “classic patterns” of simple pneumoconiosis (small rounded opacities, upper zone predominance) or of PMF.
As previously described, distribution across multiple lung zones or lower zone predominance and irregular small opacities are not uncommon. High-resolution computed tomographic scans of the chest are more sensitive in identifying small and large opacities in those with a history of coal mine dust exposure than plain chest images. High-resolution computed tomographic scans are most useful in assessment of symptomatic patients and those with borderline or atypical radiographic findings.
It is important to rule out other causes for patients’ lung disease, especially if there has been less than 10 years of exposure to coal mine dust. Differential diagnoses include mycobacterial infection, silica-associated granulomatosis with polyangiitis, and other interstitial pneumonias.
If a patient has had at least 10 years’ of exposure to coal mine dust and has a clinical appearance typical for CWP, biopsy is not typically needed for diagnosis. Bilateral symmetrical elongated mass lesions in those with advanced CWP are only rarely malignant. If there is concern about malignancy based on clinical symptoms or in the setting of a unilateral large opacity, a positive positron emission tomography scan is of uncertain significance, because PMF lesions are often metabolically active and show uptake suggestive of malignancy. In this type of setting, a biopsy may be needed to resolve diagnostic uncertainty.
Patients should undergo pulmonary function testing at baseline and in follow-up to document level of impairment and to track progression over time. Pulmonary function testing also plays an important role in assessment for CMDLD-related airways disease in those with substantial exposure histories.
TREATMENT OF CMDLD
There is no specific curative treatment for CWP or other types of CMDLD.3 Ideally, additional exposure should be limited. This may be difficult for patients without alternative means of support, who may not wish to change their occupations or for their employers to become aware of their health status. Patients should be seen periodically to evaluate for progression and to provide symptomatic support. Complications such as airflow obstruction, respiratory tract infection, respiratory failure/ hypoxemia, cor pulmonale, arrhythmias, and pneumothorax may occur. If there has been significant crystalline silica exposure, clinicians should be alert to the possibility of Mycobacterial infection as a complication. Supportive treatment also includes good general respiratory care. Patients should receive influenza and pneumococcal vaccinations as appropriate. They should also be asked about tobacco use at every clinic visit and those who are tobacco users urged to quit. Those willing to make a quit attempt should be assisted in doing so.33
Lung transplantation has been used in the setting of end-stage disease. Lung transplantation for occupational lung disease has historically been rare. In fact, the first case of lung transplantation for CWP in the United States documented in United Network of Organ Sharing data was reported in 1996.34 Two reports published in 2012 evaluating lung transplantation for CWP reported somewhat different results. The experience in 143 patients undergoing unilateral or bilateral lung transplantation at the University of Kentucky was of generally good outcomes and comparable survivability versus other medical conditions.35 In contrast, other investigators observed shorter lung graft survivability compared with transplants for COPD, IPF, and silicosis.36 Nevertheless, given the lack of other options for miners with CWP and end-stage lung disease, those caring for such miners who are appropriate candidates for transplantation should consider referring them for transplant evaluation.
PREVENTION OF CMDLD
Because CMDLD cannot be cured, the best way to control it is through prevention. NIOSH has provided comprehensive recommendations for prevention.32,37 The most important prevention measures are in the workplace and thus not under the control of the clinician. NIOSH recommends limiting exposures to respirable coal mine dust to 1 mg/m3, and to respirable crystalline silica to 0.05 mg/m3, as time-weighted average concentrations for up to a 10-hour day during a 40-hour work week. Worker exposures should be kept as far below these recommended exposure limits as feasible through the use of engineering controls and work practices. It is also important to frequently monitor worker exposures so interventions can be made if overexposures are detected. In this regard, NIOSH has worked with partners to develop and implement the use of a real-time personal respirable dust monitor in coal mines.38 The personal respirable dust monitor allows miners to monitor the concentration of respirable dust in their breathing zone, allowing the individual to know if and when overexposure is occurring. This feedback can lead to immediate corrective action to reduce the exposure source or timely removal of the miner from excessive exposures.
Clinicians may become involved in another aspect of a comprehensive prevention program, medical screening, and surveillance. Medical screening can benefit the screened coal miner if CMDLD is detected early and interventions are made to prevent progression by limiting further exposure. This is called secondary prevention. Medical screening can also benefit others in the screened population if evaluation of population health data shows particular jobs or work areas as trouble spots and interventions are made to correct exposures before they cause health effects in additional workers. This is called primary prevention.
NIOSH recommends the following program of medical surveillance for coal miners37:
A spirometric examination and plain chest radiograph as soon as possible after beginning employment (“preplacement,” within 3 months for spirometric examination and within 3 to 6 months for chest radiograph).
A spirometric examination each year for the first 3 years of coal mining and every 2 to 3 years after that if the miner remains engaged in coal mining.
A chest radiograph every 4 to 5 years for the first 15 years of coal mining and every 3 years after that if the miner remains engaged in coal mining.
A chest radiograph and spirometric examination at the end of employment in coal mining if more than 6 months have passed since the last examination.
A standardized respiratory symptom questionnaire and a standardized occupational history questionnaire administered at the first examination and updated at each subsequent periodic examination.
In addition to the above NIOSH recommendations, the World Health Organization recommends, “Ideally, health surveillance, particularly for workers exposed to silica dust, should be lifelong.”39(p32)
As previously noted in the section on treatment, miners should be asked at each examination about tobacco use and those who are tobacco users urged to quit. Those willing to make a quit attempt should be assisted in doing so.33 Spirometry should be interpreted consistent with international standards.40,41 Chest radiographs should be classified according to the International Labour Organization classification and a small opacity profusion of 1/0 or greater or the presence of large opacities viewed as suggestive of pneumoconiosis.17 Miners should be notified in a timely manner about the results of their examinations, including whether or not any abnormalities were detected. Appropriate medical counseling and counseling about any legal implications of findings should be provided. In addition, aggregate data from the screened population should be analyzed and reviewed regularly to identify trouble spots where improvements in primary prevention are needed to better protect miners.
CONCLUSION
Coal continues to be an important global commodity and mining it will continue to be an important activity for the foreseeable future. In the United States, coal mining in the East is characterized by more underground mining of bituminous coal and in the West by more surface mining of subbituminous coal. Exposure to coal mine dust during mining can cause a spectrum of disease termed CMDLD. This spectrum includes classic forms of CWP and silicosis, mixed dust pneumoconiosis, DDF (which can be mistaken for IPF), and chronic obstructive airways disease, including emphysema and chronic bronchitis. In recent years, the United States has seen an increasing burden of CMDLD-interstitial diseases, particularly in the central Appalachian region. Those affected have included younger miners younger than 50 years with severe disease. Proposed explanations include increased levels and duration of dust exposure, increased silica content of dust, and issues related to working in smaller operations. Because CMDLD can only be treated symptomatically, prevention is critical. Clinicians may be called upon to participate in medical screening and surveillance programs, which are a part of comprehensive prevention programs. Data from medical screening can be useful for both primary and secondary preventions.
Acknowledgments
The authors thank Dr Daniel Banks, Dr Ware Kuschner, and the staff of the American College of Chest Physicians for organizing the 2013 Conference on Occupational and Environmental Lung Disease, where this review was presented in part.
Footnotes
Disclosures: No commercial disclosures.
Conflict of interest/funding source: The authors are full-time US government employees. No other funding supported this work. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.US Department of Energy, E.I.A., Office of Coal, Nuclear, Electric and Alternate Fuels. [Accessed November 29, 2013];International energy outlook. Available at http://www.eia.gov/forecasts/ieo/coal.cfm. Published 2013.
- 2.Humphries M, Sherlock MF. U.S. and world coal production, federal taxes, and incentives. [Accessed November 29, 2013];CRS Report R43011. Available at http://www.fas.org/sgp/crs/misc/R43011.pdf. Published 2013.
- 3.Petsonk EL, Rose C, Cohen R. Coal mine dust lung disease. New lessons from old exposure. Am J Respir Crit Care Med. 2013;187:1178–1185. doi: 10.1164/rccm.201301-0042CI. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber J, Koschel D, Kekow J, Waldburg N, Goette A, Merget R. Rheumatoid pneumoconiosis (Caplan’s syndrome) Eur J Intern Med. 2010;21:168–172. doi: 10.1016/j.ejim.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Amandus HE, Lapp NL, Jacobson G, Reger RB. Significance of irregular small opacities in radiographs of coalminers in the USA. Br J Ind Med. 1976;33:13–17. doi: 10.1136/oem.33.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft A, Lyons JP, Andersson N, Saunders MJ. Prevalence and relation to underground exposure of radiological irregular opacities in South Wales coal workers with pneumoconiosis. Br J Ind Med. 1983;40:169–172. doi: 10.1136/oem.40.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins HP, Dick JA, Bennett JG, et al. Irregularly shaped small shadows on chest radiographs, dust exposure, and lung function in coalworkers’ pneumoconiosis. Br J Ind Med. 1988;45:43–55. doi: 10.1136/oem.45.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young RC, Jr, Rachal RE, Carr PG, Press HC. Patterns of coal workers’ pneumoconiosis in Appalachian former coal miners. J Natl Med Assoc. 1992;84:41–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Laney AS, Petsonk EL. Small pneumoconiotic opacities on U.S. coal worker surveillance chest radiographs are not predominantly in the upper lung zones. Am J Ind Med. 2012;55:793–798. doi: 10.1002/ajim.22049. [DOI] [PubMed] [Google Scholar]
- 10.Brichet A, Tonnel AB, Brambilla E, et al. Chronic interstitial pneumonia with honeycombing in coal workers. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:211–219. [PubMed] [Google Scholar]
- 11.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 12.Coggon D, Taylor AN. Coal mining and chronic obstructive pulmonary disease: a review of the evidence. Thorax. 1998;53:398–407. doi: 10.1136/thx.53.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuempel ED, Wheeler MW, Smith RJ, Vallyathan V, Green FH. Contributions of dust exposure and cigarette smoking to emphysema severity in coal miners in the United States. Am J Respir Crit Care Med. 2009;180:257–264. doi: 10.1164/rccm.200806-840OC. [DOI] [PubMed] [Google Scholar]
- 14.Wang ML, Wu ZE, Du QG, et al. Rapid decline in forced expiratory volume in 1 second (FEV1) and the development of bronchitic symptoms among new Chinese coal miners. J Occup Environ Med. 2007;49:1143–1148. doi: 10.1097/JOM.0b013e31814b8d51. [DOI] [PubMed] [Google Scholar]
- 15.Wang ML, Wu ZE, Du QG, et al. A prospective cohort study among new Chinese coal miners: the early pattern of lung function change. Occup Environ Med. 2005;62:800–805. doi: 10.1136/oem.2005.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ML, Beeckman-Wagner LA, Wolfe AL, Syamlal G, Petsonk EL. Lung-function impairment among US underground coal miners, 2005 to 2009: Geographic patterns and association with coal workers’ pneumoconiosis. J Occup Environ Med. 2013;55:846–850. doi: 10.1097/JOM.0b013e31828dc985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Labour Office. Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses. Geneva: International Labour Office; 2011. [Google Scholar]
- 18.Attfield MD, Althouse RB. Surveillance data on US coal miners’ pneumoconiosis, 1970 to 1986. Am J Public Health. 1992;82:971–977. doi: 10.2105/ajph.82.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Occupational Safety and Health. [Accessed December 1, 2013];Coal Workers’ Health Surveillance Program (CWHSP) Available at http://www.cdc.gov/niosh/topics/surveillance/ords/CoalWorkersHealthSurvProgram.html.
- 20.Wade WA, Petsonk EL, Young B, Mogri I. Severe occupational pneumoconiosis among West Virginian coal miners: one hundred thirty-eight cases of progressive massive fibrosis compensated between 2000 and 2009. Chest. 2011;139:1458–1462. doi: 10.1378/chest.10-1326. [DOI] [PubMed] [Google Scholar]
- 21.Antao VC, Petsonk E, Sokolow L, et al. Rapidly progressive coal workers’ pneumoconiosis in the United States: geographic clustering and other factors. Occup Environ Med. 2005;62:670–674. doi: 10.1136/oem.2004.019679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laney AS, Attfield MD. Quartz exposure can cause pneumoconiosis in coal workers. J Occup Environ Med. 2009;51:867. doi: 10.1097/JOM.0b013e3181abb035. author reply 868. [DOI] [PubMed] [Google Scholar]
- 23.Laney AS, Petsonk EL, Attfield MD. Pneumoconiosis among underground bituminous coal miners in the United States: is silicosis becoming more frequent? Occup Environ Med. 2010;67:652–656. doi: 10.1136/oem.2009.047126. [DOI] [PubMed] [Google Scholar]
- 24.Laney AS, Attfield MD. Coal workers’ pneumoconiosis and progressive massive fibrosis are increasingly more prevalent among workers in small underground coal mines in the United States. Occup Environ Med. 2010;67:428–431. doi: 10.1136/oem.2009.050757. [DOI] [PubMed] [Google Scholar]
- 25.Laney AS, Petsonk EL, Hale JM, Wolfe AL, Attfield MD. Potential determinants of coal workers’ pneumoconiosis, advanced pneumoconiosis, and progressive massive fibrosis among underground coal miners in the United States, 2005–2009. Am J Public Health. 2012;102(Suppl 2):S279–S283. doi: 10.2105/AJPH.2011.300427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suarthana E, Laney AS, Storey E, Hale JM, Attfield MD. Coal workers’ pneumoconiosis in the United States: regional differences 40 years after implementation of the 1969 Federal Coal Mine Health and Safety Act. Occup Environ Med. 2011;68:908–913. doi: 10.1136/oem.2010.063594. [DOI] [PubMed] [Google Scholar]
- 27.Laney AS, Attfield MD. Examination of potential sources of bias in the US coal workers’ health surveillance program. Am J Public Health. 2014;104(1):165–170. doi: 10.2105/AJPH.2012.301051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banks DE, Bauer MA, Castellan RM, Lapp NL. Silicosis in surface coalmine drillers. Thorax. 1983;38:275–278. doi: 10.1136/thx.38.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amandus HE, Petersen MR, Richards TB. Health status of anthracite surface coal miners. Arch Environ Health. 1989;44:75–81. doi: 10.1080/00039896.1989.9934379. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Pneumoconiosis and advanced occupational lung disease among surface coal miners—16 states, 2010–2011. MMWR Morb Mortal Wkly Rep. 2012;61:431–434. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Coal workers’ pneumoconiosis-related years of potential life lost before age 65 years—United States, 1968–2006. MMWR Morb Mortal Wkly Rep. 2009;58:1412–1416. [PubMed] [Google Scholar]
- 32.National Institute for Occupational Safety and Health; DHHS, editor. Coal Mine Dust Exposures and Associated Health Outcomes: A Review of Information Published Since 1995. Current Intelligence Bulletin 64. Washington, DC: DHHS (NIOSH); 2011. [Google Scholar]
- 33.Clinical Practice Guideline Treating Tobacco, U, L. Dependence Update Panel, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enfield KB, Floyd S, Peach P, Sifri CD, Lau C, Tobbins M. Transplant outcome for coal workers pneumoconiosis. American Transplant Congress; San Diego, CA. 2010. [Google Scholar]
- 35.Hayes D, Jr, Diaz-Guzman E, Davenport DL, et al. Lung transplantation in patients with coal workers’ pneumoconiosis. Clin Transplant. 2012;26:629–634. doi: 10.1111/j.1399-0012.2011.01590.x. [DOI] [PubMed] [Google Scholar]
- 36.Enfield KB, Floyd S, Barker B, et al. Survival after lung transplant for coal workers’ pneumoconiosis. J Heart Lung Transplant. 2012;31:1315–1318. doi: 10.1016/j.healun.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 37.National Institute for Occupational Safety and Health. Criteria for a Recommended Standard, Occupational Exposure to Respirable Coal Mine Dust. Cincinnati: NIOSH Publications Dissemination; 1995. DHSS (NIOSH) Publication No. 95–106. [Google Scholar]
- 38.Page SJ, Volkwein JC, Vinson RP, et al. Equivalency of a personal dust monitor to the current United States coal mine respirable dust sampler. J Environ Monit. 2008;10:96–101. doi: 10.1039/b714381h. [DOI] [PubMed] [Google Scholar]
- 39.Wagner GR. Screening and Surveillance of Workers Exposed to Mineral Dust. Geneva: World Health Organization; 1996. [Google Scholar]
- 40.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 41.Redlich CA, Tarlo SM, Hankinson JL, et al. Official American Thoracic Society technical standards: spirometry in the occupational setting. Am J Respir Crit Care Med. 2014;189:983–993. doi: 10.1164/rccm.201402-0337ST. [DOI] [PubMed] [Google Scholar]