Abstract
Translating pathogenic insights gained from monogenic defects that cause autoinflammatory diseases into novel therapies has dramatically improved the lives of patients with these syndromes. The last 15 years have focused on the central role of IL-1 in driving autoinflammatory phenotypes and on therapies blocking IL-1 signaling. Recent discoveries from patients unresponsive to IL-1 blockade have highlighted other key inflammatory mediators and pathways. New genetic discoveries have confirmed unifying mechanisms of autoinflammation, including dysregulation of danger sensing, cell stress, and immune-receptor signaling. Recent gene discovery in novel diseases has demonstrated new concepts. First, several complex clinical syndromes, caused by mutations leading to chronic type I interferon (IFN) production present with organ manifestations different from IL-1 mediated diseases including cerebral calcifications, myositis, and interstitial lung disease and the frequent occurrence of autoantibodies. These disorders introduce type I IFN’s as inflammatory mediators that cause autoinflammatory phenotypes. Second, conditions associated with high IL-18 production may provide a direct link between autoinflammation and macrophage activation syndrome. Third, dysregulation of inflammatory and cell differentiation pathways in nonhematopoietic cells, such as aberrant calcium signaling and impaired endothelial or keratinocyte development, provide an understanding of organ specificity in autoinflammatory disorders. Many of these discoveries highlight the intricate interconnections between autoinflammation, autoimmunity, immunodeficiency, and lymphoproliferation and suggest ways in which we may better diagnose and treat autoinflammatory diseases.
Evolution of the concept of autoinflammation
Over the last 25 years, our expanding knowledge about diseases presenting with noninfectious, “sterile” inflammatory fever attacks has significantly changed our approach to treatment. In 1999, Kastner and colleagues suggested that molecular mechanisms other than autoimmunity must cause the known periodic fever syndromes, FMF and TRAPS [1], and the concept of autoinflammation was proposed. Genetic discoveries in the clinic and basic innate immune discoveries at the bench supplemented each other, resulting in the identification of several disease-causing genes associated with excessive IL-1 signaling (NLRP3, MVK, IL1RN, etc.) and the extension of IL-1 blocking therapies to autoinflammatory diseases without a known genetic cause (e.g., periodic fevers, aphthous ulcer, pharyngitis, adenitis syndrome (PFAPA) and systemic onset juvenile idiopathic arthritis (SJIA)). The discovery of IL-1 dysregulation in common inflammatory disorders, like gout and atherosclerosis, triggered investigations on the role of IL-1 in these diseases [2]. The recently discovered autoinflammatory diseases discussed in this review prompted a reevaluation of the IL-1- centric view of autoinflammation.
The clinical features of interferon-mediated autoinflammatory diseases
Excessive interferon (IFN) signaling has largely been associated with mobilizing the adaptive immune response against intracellular invaders. Given the ability of type I IFN to inhibit proliferation and “inflammatory” cytokine synthesis (most notably IL-1) [3], the discovery of IFN dysregulation causing autoinflammatory phenotypes was unexpected. A prominent, persistent gene expression pattern of transcripts known to be induced by type I IFN signaling [4], an “IFN signature,” identifies presumed “interferon-mediated diseases” or interferonopathies. The IFN signature was first described in systemic lupus and seemed to fit with the paradigm of IFN (over)stimulating adaptive immunity, but subsequent work has uncovered direct effects of IFN on innate cells in lupus [5]. The recently discovered monogenic interferon-mediated diseases elucidate the inflammatory aspects of chronic, excessive IFN production. The interferonopathies are discussed in greater detail elsewhere in this volume (below and also in Tables 1 and 2), and we review the clinical aspects of interferonopathies that help distinguish this group of disorders from IL-1-mediated diseases.
Table 1.
The monogenic interferonopathies
| Disease | Gene | Protein | Defect | Cardinal features | |
|---|---|---|---|---|---|
| AGS1 | TREX1 | TREX1 | LOF, exonuclease | Deep CNS calcifications, chronic neurologic damage, chilblain or livedo rash, hepatosplenomegaly | [14, 15, 50] |
| AGS2 | RNASEH2B | RNH2B | LOF, RNAse | ||
| AGS3 | RNASEH2C | RNH2C | |||
| AGS4 | RNASEH2A | RNH2A | |||
| AGS5 | SAMHD1 | SAMH | LOF, nuclease | ||
| AGS6 | ADAR | ADAR | LOF, RNA deaminase | ||
| AGS7 | IFIH1 | MDA5 | GOF, RNA sensor | ||
| PRAAS/CANDLE | PSMB8 | PSMB5i/B5i | LOF, proteasome | Nodules, panniculitis, lipodystrophy, fevers, myositis, abdominal fat, HSM | [6–9] |
| SAVI | TMEM173 | STING | GOF, DNA sensing | CNS/small vessel infarcts, fevers, interstitial lung disease, acral skin infarcts, purpura | [11, 12] |
| ISG15 def. | ISG15 | ISG15 | LOF, protein modification | Basal ganglia calcification, mycobacterial infection | [16, 17] |
| SPENCDI | ACP5 | TRAP | LOF, phosphatase | Skeletal dysplasia, calcifications, spasticity, autoimmunity | [18, 19] |
LOF loss of function, GOF gain of function, HSM hepatosplenomegaly, CNS central nervous system
Table 2.
IL-1- versus IFN-mediated autoinflammation: a clinical comparison
| IL-1-mediated diseases | IFN-mediated diseases | |
|---|---|---|
| Systemic | ||
| CRP | Tracks closely with disease activity | Only elevated with severe flares |
| Peripheral WBC | Granulocytosis with flares | Lymphopenia or leukopenia with flares |
| Central nervous system | ||
| Meningitis | Aseptic neutrophilic infiltrate | Mild lymphocytic infiltrate |
| Imaging | Arachnoid adhesions with severe disease, cochlear inflammation | Basal ganglia calcifications, white matter disease |
| Other | ||
| Skin | Urticaria with mature neutrophil infiltrate | Panniculitis with immature neutrophils, lipodystrophy |
| MSK | Osteomyelitis, bony overgrowth | Myositis |
| CV | No primary disease | HTN, pulmonary HTN, vasculitis, vascular occlusion |
| Eye | Conjunctivitis, anterior uveitis | Keratoconjunctivitis |
| Lung | Serositis including pleuritis, pericarditis, peritonitis | Pulmonary fibrosis/interstitial lung disease |
| Autoantibodies | Infrequent, lupus anticoagulant often becomes negative with treatment | Common, autoantibody titer and presence of autoimmune-mediated organ disease are variable |
IFN interferon, CRP C-reactive protein, WBC white blood cell count, MSK musculoskeletal, CV cardiovascular, HTN hypertension
Systemic inflammation, panniculitis, and myositis due to proteasome defects (PRAAS/CANDLE)
Loss-of-function mutations in PSMB8, and other proteasome components (our unpublished data), cause a group of diseases referred to as proteasome-associated autoinflammatory syndromes (PRAAS) or chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE). The disease is referred to in the literature as joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy (JMP) syndrome and Nakajo-Nishimura syndrome [6–9]. Features can include recurrent fevers, skin manifestations ranging from annular erythema to erythema nodosum-like panniculitis, eyelid swelling, hepatomegaly, andmostly asymptomatic basal ganglia calcifications but rarely intellectual disability, myositis, and arthralgias with small joint contractures. The characteristic lipodystrophy may occur in areas of chronic rash, panniculitis, and/or myositis. Systemic inflammatory attacks occur more in childhood-onset disease. Although partially steroid-responsive, treatment of these patients is challenging and no single agent has thus far shown dramatic efficacy. Untreated, mortality is high due to uncontrolled inflammation and eventual organ failure [9].
PRAAS/CANDLE patients have a persistently high elevation of the IFN signature as well as high serum IL-6. Various autoantibodies are often detectable without a pattern between patients and in the absence of autoimmunity [7–9]. PSMB8 is a subunit of the immunoproteasome: a protein degradation complex induced by IFNs and essential for antigen presentation among other functions. The molecular mechanisms linking proteasome dysfunction to the high IFN signature remain unknown, but the unfolded protein response, other cellular stress responses, and abnormal cell death are all proposed mechanisms (refer to Brehm, Krueger review in this volume). By contrast, proteasome dysfunction caused by mutations in TPP2 results in T cell dysfunction and predominantly presents with immunodeficiency and infections [10]. Given the poor responses to known treatments, therapies blocking IFN signaling are being used in clinical studies (see below).
Vasculopathy, vasculitis, and interstitial lung disease with STING hyperactivity (SAVI)
We recently described a novel vasculopathy/vosculitis syndrome caused by gain-of-function mutations in TMEM173/STING [11, 12]. STING-associated vasculopathy with onset in infancy (SAVI) patients develop severe small dermal vessel vasculitis/and microangiopathic thrombosis often early in life. A telangiectatic, ulcerative, or pustular rash develops mostly on acral surfaces, including the digits, earlobes, and nose, and often results in digital ischemia and auto- or surgical amputation. Many patients also develop progressive and potentially fatal interstitial lung disease. Myositis can develop and autoantibody production is common. CNS disease and cerebral calcifications are not typically seen in SAVI. Autoantibody production varies widely and is not associated with disease severity, which is likely modulated by additional genetic factors [10].
STING is an adaptor molecule of the cytosolic DNA danger sensing machinery. It responds to the enzymatic product of the DNA sensor cGAS (but may also respond to DNA directly) by mobilizing a signaling program that results in IRF3 activation and IFNβ transcription. SAVI patients uniformly show persistently high IFN signatures in the blood.
A compassionate use study blocking IFN signaling in PRAAS/CANDLE and SAVI with the Janus Kinase (JAK) inhibitor baricitinib is ongoing (www.clinicaltrials.gov NCT01724580).
Subacute encephalomyelitis with cerebral calcifications and white matter disease due to cytosolic nucleotide dysregulation (AGS)
In Aicardi-Goutières syndrome (AGS), patients are rarely seen in autoinflammatory disease clinics. Their disease presentation usually mimics intrauterine/congenital infections. Patients can develop cerebrospinal fluid pleocytosis and basal ganglia calcification, resultant subacute weakness, spasticity, paresthesias, and long-term neurologic and cognitive defects [13, 14]. The genetic causes of AGS include lost enzymatic activities important for regulating intracellular DNA and RNA metabolism (reviewed elsewhere in this issue). The resulting accumulation of cytosolic nucleotides promotes cell stress and triggers danger sensing and type I interferon production. In addition to these loss-of-function mutations that cause AGS 1–6, gain-of-function mutations in a cytosolic RNA sensor IFIH1 (encoding MDA5) induce a highly variable AGS-like phenotype [15]. Non-CNS manifestations of AGS include chilblains-like rash or livedo reticularis and often occur after the onset of CNS disease [14].
Although basal ganglion calcifications are seen in PRAAS/CANDLE patients, CANDLE patients lack white matter disease and rarely present with seizure, suggesting that upregulation of the IFN pathway may vary in different organs in different interferonopathies.
Other recently identified interferonopathies
Two other recently described interferonopathies, ISG15 deficiency and spondyloenchondrodysplasia with immune dysregulation (SPENCDI), illustrate how interferon-induced phenotypes can present with clinical features of immunodeficiency and autoimmunity, respectively. ISG15 is an IFN-responsive gene important for preventing IFN amplification loops, and its deficiency has been associated with excessive IFN signaling and variably symptomatic basal ganglia calcifications akin to AGS [16, 17]. However, a subset of patients lacking ISG15 who were immunized with Bacillus Calmette– Guérin (BCG) vaccine also showed a striking lack of response to IFNγ and developed recurrent, severe mycobacterial infections [16]. By contrast, patients bearing loss-of-function mutations in ACP5 (encoding tartrate-resistant acid phosphatase, or TRAP) develop a syndrome of axial bone dysplasia, cerebral calcifications, and immune dysregulation [18, 19]. These patients’ peripheral blood also bears a strong IFN signature, and although they can develop childhood fevers, their inflammatory phenotype is dominated by autoantibody-mediated pathology (e.g., hemolytic anemia, autoimmune thyroiditis, systemic lupus).
A role for IL-18 in macrophage activation syndrome and autoinflammation
Twenty-five years ago, Kumar and colleagues connected a syndrome of fulminant macrophage activation with impaired perforin-granzyme-mediated cytotoxicity [20]. Since then, the disease known as hemophagocytic lymphohistiocytosis (HLH) includes several monogenic defects directly linked to defective cytotoxicity. Familial HLH (FHL) is generally interpreted as an immunodeficiency, as the sepsis-like symptoms are generally triggered by persistent viral infection, often Epstein-Barr virus (EBV) [21]. A clinically similar disorder, macrophage activation syndrome (MAS) that complicates rheumatic/autoinflammatory diseases like systemic juvenile idiopathic arthritis (sJIA) and Still’s disease, shares common clinical features with HLH. Though partial impairment of cytotoxicity has been associated with MAS [22, 23], the clinical assessment of NK function is hampered by wide assay variability and lack of specificity [24]. MAS flares are not clearly triggered by infection; thus, how perforin/granzyme mediated killing promotes MAS remains poorly understood.
Episodic fevers, enteropathy, and MAS due to NLRC4 hyperactivity (NLRC4-MAS)
The recent association of gain-of-function mutations in NLRC4 with MAS suggests common pathogenic pathways that lead to macrophage activation in HLH and MAS [25, 26]. The reported NLRC4-MAS patients have had variable, early-onset enterocolitis followed by recurrent febrile episodes. Severe episodes were triggered by sleep deprivation, emotional stress, and infection and were classic for MAS with pancytopenia, hepatitis, splenomegaly, and hyperferritinemia. Hemophagocytosis was variable, and defects in cytotoxicity were apparent only during disease flares. Episodes were responsive to corticosteroids and possibly inhibition of IL-1 [26]. All NLRC4-MAS patients had extraordinary elevation of serum IL-18 even during clinical quiescence, a finding also seen in sJIA/Still’s disease patients at risk for MAS [27, 28].
NLRC4 is a cytosolic innate immune sensor that upon activation triggers the formation of an inflammasome. NLRC4 is homologous to NLRP3; the NLRP3 gene is mutated in cryopyrin-associated periodic syndromes (CAPS). Hyperactivity of either protein results in spontaneous inflammasome activation and excessive caspase-1-dependent cell death (pyroptosis). However, serum levels of IL-18 are 10- to 100-fold higher than in CAPS, and NLRC4-MAS macrophages appear uniquely primed for spontaneous IL-18 production [25, 26]. Notably, a mild familial cold-induced autoinflammatory syndrome (FCAS, a type of CAPS) phenotype was also associated with an activating NLRC4 mutation [29]. IL-18 levels in these patients were not tested.
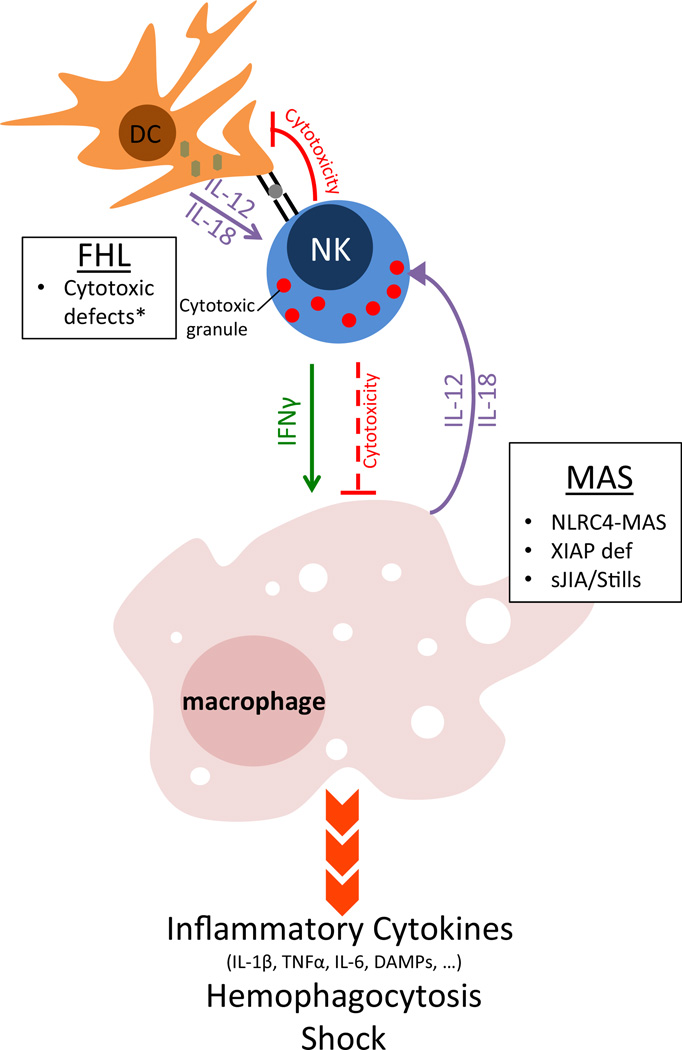
NLRC4 hyperactivity may promote MAS in any of several ways (Fig. 1). Excess inflammasome activity could directly promote macrophage-derived inflammation in the form of cytokine production, pyroptosis, and/or persistence. Chronic IL-18 overstimulation could promote excessive Th1-type responses by lymphocytes. Chronic IL-18 could also, paradoxically, impair NK cell development and function.
Fig. 1. Mechanisms to macrophage activation syndrome.
loss-of-function mutations that impair perforin/granzyme-mediated cytotoxicity lead to FHL. FHL patients are well until infected dendritic cells presenting viral antigens and expressing Th1-cytokines cannot be killed by NK and cytotoxic T cells. This unchecked dendritic cell activity causes massive lymphocyte proliferation, interferon gamma secretion, and systemic macrophage activation. Cytotoxicity may also be important for limiting macrophage activation directly. In MAS, primary defects in macrophages or dendritic cells may promote the phenotype by several mechanisms: Spontaneous inflammasome activity or XIAP-deficiency may activate macrophages directly, while chronic IL-18 over-secretion may prime for exaggerated lymphocyte responses and/or impair cytotoxicity (as in FHL). Caused by loss-of-function mutations in PRF1, UNC13D, STX11, and STXBP2 (asterisk). FHL familial hemophagocytic lymphohistiocytosis, DC dendritic cell, MAS macrophage activation syndrome, sJIA systemic juvenile idiopathic arthritis, DAMPs damage associated molecular patterns
MAS, Crohn’s disease, and other autoinflammatory phenotypes in XIAP deficiency
Deficiency of XIAP was originally described as an X-linked risk factor for EBV-triggered HLH and was associated with NKT cell immunodeficiency [30]. However, several recent reports have suggested that XIAP deficiency may be better characterized as autoinflammatory. Only about half of XIAP-related HLH is triggered by EBV [31]. Likewise, defective cytotoxicity and other lymphocyte defects identified in FHL are not present in XIAP deficiency [31]. Furthermore, phenotypes suggestive of autoinflammation (particularly inflammatory bowel disease but also arthritis, erythema nodosum, periodic fevers, and uveitis) have recently been identified [32, 33]. Finally, as in MAS, patients with XIAP-related HLH have constitutive and extreme elevation of serum IL-18 [34]. XIAP performs many functions, and no single disease-associated pathway seems to correlate with its deficiency [35]. XIAP was originally found to inhibit apoptosis, but it is also critical for NOD2 signaling [36] and Crohn’s disease and early-onset sarcoidosis/Blau syndrome have been associated with putative loss- and gain-of-function mutations in NOD2, respectively [37]. Additionally, murine studies suggest that XIAP-deficiency may promote inflammasome formation and hyperinflammation [38]. Thus, deficiency of XIAP results in a spectrum of clinical phenotypes with features of both immunodeficiency and autoinflammation, and attacks resulting in MAS.
New clinical phenotypes in diseases with mutations that largely affect tissue differentiation and/or immunity
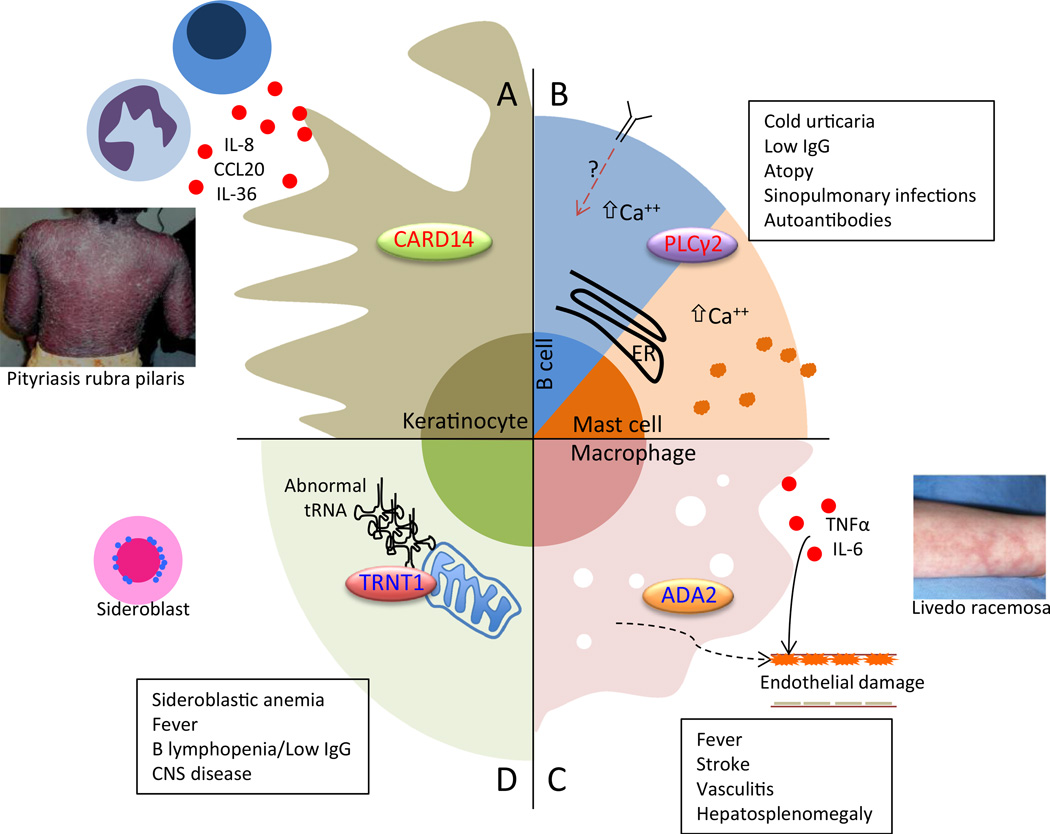
This section includes four disorders that highlight novel mechanisms of autoinflammatory disease and tissue/organ dysfunction (Fig. 2).
Fig. 2. Schematic of pathogenesis of four novel autoinflammatory diseases.
In CARD14-mediated psoriasis (CAMPS), excessive CARD14 activity promotes NF-kB signaling in keratinocytes, which release inflammatory and chemotactic molecules (a). Neutrophils and IL-17 and 23-secreting lymphocytes recruited to the area induce psoriasiform rash. In PLCy2-associated antibody deficiency and immune dysregulation (PLAID), abnormal activity of PLCy2 promotes increased basal and stimulated intracellular calcium, which apparently impairs B cell responses but promotes excessive mast cell degranulation (b). In deficiency of ADA2 (DADA2), the absence of adenosine deaminase 2 in immune cells promotes inflammatory macrophage differentiation (c). This inflammation, as well as a potential extrinsic defect in endothelial cell development, results in widespread vasculitis most critically affecting CNS vessels. Photo reproduced from reference 40. Sideroblastic anemia, immunodeficiency, fevers, developmental delay (SIFD) is a complex fever syndrome with disrupted erythroid and B cell development, innate immune activation, and CNS disease all caused by impairment of (largely) mitochondrial protein synthesis and resultant cellular stress (d). ER endoplasmic reticulum, IgG immunoglobulin gamma, CNS central nervous system
Organ-specific defects that contribute to chronic inflammation
Keratinocyte-specific CARD14 hyperactivity (CAMPS)
In CARD14-mediated psoriasis (CAMPS), a genetic defect in nonhematopoietic cells results in innate cell recruitment and tissue-specific inflammation. CAMPS is caused by gain-of-function mutations in CARD14, which encodes an NF-kB activating scaffold protein downstream of protein kinase C [39, 40]. CARD14 expression is almost exclusive to keratinocytes, and CAMPS mutations result in IL-8, CCL20, and IL-36 secretion. CAMPS manifests as early-onset generalized pustulosis, plaque psoriasis, pityriasis rubra pilaris, and/or nail pitting [39–41]. Recurrent fevers have been reported but may be related to superinfected skin lesions. CAMPS patients may respond to inhibitors of the IL-17/23 pathway, similar to conventional psoriasis [42].
Endothelial cell differentiation defect associated with vasculitis, stroke, fevers, and immune dysregulation due to ADA2 deficiency (DADA2)
Loss-of-function mutations in ADA2 result in deficiency of adenosine deaminase 2 (DADA2), a heritable form of early-onset vasculitis similar to polyarteritis nodosa [43, 44]. Severely affected patients develop early-onset recurrent fevers, livedoid or urticarial rash, low cell counts, hypogammaglobulinemia, hepatosplenomegaly, and small vessel vasculitis that can manifest as strokes but can also affect the coronary arteries, bowel, and kidneys. Systemic inflammation accompanies the fevers, and patient monocytes appear primed for inflammatory differentiation. Inhibition of TNFα or IL-6, or hematopoietic stem cell transplantation has been used to treat patients. Whereas deficiency of ADA1 results in the absence of lymphocytes and severe combined immunodeficiency, ADA2 appears critical for enabling alternative macrophage activation and normal endothelial development [43]; both chronic inflammation and abnormal endothelial differentiation combine to promote the predilection for vasculitis.
Mitochondrial stress affecting innate and organ-specific cells
Sideroblastic anemia, immunodeficiency, fevers, and developmental delay (SIFD)
A syndrome of severe anemia with red blood cell mitochondrial iron deposits (sideroblasts), recurrent noninfectious fevers, B cell lymphopenia, hypogammaglobulinemia, variable immunodeficiency, and a variety of CNS insults (e.g., sensorineural hearing loss, but not basal ganglion calcification), and variable muscle weakness is caused by autosomal recessive mutations in TRNT1, encoding an enzyme critical for mitochondrial and cytosolic transfer-RNA synthesis [45–47]. Like other causes of sideroblastic anemia, TRNT1 deficiency impairs mitochondrial protein synthesis and causes cell stress related to the degree of enzymatic deficiency. This stress seems to impair neural and B cell development, while promoting inflammation, suggesting cell-type-specific effects. As in Hyper IgD Syndrome (mevalonate kinase deficiency) and other metabolic diseases, SIFD patients demonstrate another mechanism by which toxic buildup of enzymatic substrates can cause a complex inflammatory syndrome.
Signaling molecule dysfunction triggers autoinflammation and causes immunodeficiency
Cold-induced urticaria, atopy, and immune dysregulation due to alterations in PLCγ2 (PLAID)
Gain-of-function mutations in PLCγ2, a signaling molecule associated with increased calcium flux, result in PLCγ2-associated antibody deficiency and immune dysregulation (PLAID). All reported patients present with cold-induced urticaria, and many patients developed low immunoglobulins, atopy, granulomatous rash, sinopulmonary infections, autoantibodies, and/or autoimmunity [48]. The mutations appeared to increase the activity of this molecule specifically at cold temperatures, suggesting cold as a unique trigger for mast cell or other innate cell degranulation and inflammation. The inflammation of PLAID may partially involve the mutations’ effects on baseline and stimulated intracellular calcium levels, which can serve as a trigger for the NLRP3 inflammasome [49]; although a complete response to IL-1 inhibition has not been observed, IL-1 may be contributory. Overall, the mutation appeared to promote innate cell responses but blunt those in adaptive cells.
Summary
Novel monogenic autoinflammatory disorders continue to refine the concept of autoinflammation. We describe a new class of autoinflammatory diseases caused by excess IFN signaling that is characterized by vasculopathy, cerebral calcifications, variable pulmonary disease, and variable autoantibody formation. Although the mechanistic pathways that lead to overlapping innate and adaptive immune dysfunction need to be better characterized, there is the clinical need for measurement of IFN responses and clinical trials of drugs blocking the IFN-pathway. An autoinflammatory disease associated with MAS provides unexpected links between the inflammasome products IL-1β and IL-18, NF-kB activity, and cytotoxicity, suggesting novel targets for therapy. Future research must identify environmental and intrinsic sources of variation, identify new inflammatory mediators, and examine the basis for organ-specific inflammation. Rare monogenic autoinflammatory syndromes continue to not only provide insights into more common disorders but also inform how we understand and treat patients with excess inflammation.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the NIH
Footnotes
Conflict of interest None.
Contributor Information
Scott W. Canna, Email: scott.canna@nih.gov.
Raphaela Goldbach-Mansky, Email: goldbacr@mail.nih.gov.
References
- 1.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 2.Goldbach-Mansky R, Kastner DL. Autoinflammation: the prominent role of IL-1 in monogenic autoinflammatory diseases and implications for common illnesses. J Allergy Clin Immunol. 2009;124:1141–1149. doi: 10.1016/j.jaci.2009.11.016. quiz 50-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoggins JW, Wilson SJ, Panis M, et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal AK, Xing C, DeMartino GN, et al. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am J Hum Genet. 2010;87:866–872. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arima K, Kinoshita A, Mishima H, et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc Natl Acad Sci U S A. 2011;108:14914–14919. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura A, Maekawa Y, Uehara H, et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J Clin Invest. 2011;121:4150–4160. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Ramot Y, Torrelo A, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64:895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu W, Zhang Y, McDonald DO, et al. Dual proteolytic pathways govern glycolysis and immune competence. Cell. 2014;159:1578–1590. doi: 10.1016/j.cell.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–518. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeremiah N, Neven B, Gentili M, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–5520. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aicardi J, Goutieres F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol. 1984;15:49–54. doi: 10.1002/ana.410150109. [DOI] [PubMed] [Google Scholar]
- 14.Crow YJ, Chase DS, Lowenstein Schmidt J, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am J Med Genet A. 2015;167A:296–312. doi: 10.1002/ajmg.a.36887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice GI, del Toro Duany Y, Jenkinson EM, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat Genet. 2014;46:503–509. doi: 10.1038/ng.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogunovic D, Byun M, Durfee LA, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Bogunovic D, Payelle-Brogard B, et al. Human intracellular ISG15 prevents interferon-alpha/beta over-amplification and auto-inflammation. Nature. 2015;517:89–93. doi: 10.1038/nature13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briggs TA, Rice GI, Daly S, et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat Genet. 2011;43:127–131. doi: 10.1038/ng.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lausch E, Janecke A, Bros M, et al. Genetic deficiency of tartrate-resistant acid phosphatase associated with skeletal dysplasia, cerebral calcifications and autoimmunity. Nat Genet. 2011;43:132–137. doi: 10.1038/ng.749. [DOI] [PubMed] [Google Scholar]
- 20.Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–1959. [PubMed] [Google Scholar]
- 21.Pachlopnik Schmid J, Cote M, Menager MM, et al. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev. 2010;235:10–23. doi: 10.1111/j.0105-2896.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 22.Villanueva J, Lee S, Giannini EH, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman KM, Linghu B, Szustakowski JD, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2014;66:3486–3495. doi: 10.1002/art.38793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose NR, Hamilton RG, Detrick B. Manual of clinical laboratory immunology. 6th edn. Washington D.C: ASM Press; 2002. [Google Scholar]
- 25.Romberg N, Al Moussawi K, Nelson-Williams C, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46:1135–1139. doi: 10.1038/ng.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canna SW, de Jesus AA, Gouni S, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46:1140–1146. doi: 10.1038/ng.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichida H, Kawaguchi Y, Sugiura T, et al. Clinical manifestations of adult-onset still's disease presenting with erosive arthritis: association with low levels of ferritin and IL-18. Arthritis care & Res. 2013;66:642–646. doi: 10.1002/acr.22194. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu M, Nakagishi Y, Yachie A. Distinct subsets of patients with systemic juvenile idiopathic arthritis based on their cytokine profiles. Cytokine. 2013;61:345–348. doi: 10.1016/j.cyto.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura A, Sasaki Y, Abe T, Kano H, Yasutomo K. An inherited mutation in NLRC4 causes autoinflammation in human and mice. J Exp Med. 2014;211:2385–2396. doi: 10.1084/jem.20141091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rigaud S, Fondaneche MC, Lambert N, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 31.Marsh RA, Madden L, Kitchen BJ, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116:1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aguilar C, Lenoir C, Lambert N, et al. Characterization of Crohn disease in X-linked inhibitor of apoptosis-deficient male patients and female symptomatic carriers. J allergy and Clin Immunol. 2014;134:1131–1141. doi: 10.1016/j.jaci.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Speckmann C, Lehmberg K, Albert MH, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol. 2013;149:133–141. doi: 10.1016/j.clim.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, Kanegane H, Ohta K, et al. Sustained elevation of serum interleukin-18 and its association with hemophagocytic lymphohistiocytosis in XIAP deficiency. Cytokine. 2014;65:74–78. doi: 10.1016/j.cyto.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Damgaard RB, Gyrd-Hansen M. Inhibitor of apoptosis (IAP) proteins in regulation of inflammation and innate immunity. Discovery Med. 2011;11:221–231. [PubMed] [Google Scholar]
- 36.Damgaard RB, Nachbur U, Yabal M, et al. The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell. 2012;46:746–758. doi: 10.1016/j.molcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 37.de Jesus AA, Canna SW, Liu Y, Goldbach-Mansky R. Molecular mechanisms in genetically defined autoinflammatory diseases: disorders of amplified danger signaling. Annu Rev Immunol. 2015;33:823–874. doi: 10.1146/annurev-immunol-032414-112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yabal M, Muller N, Adler H, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Reports. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Jordan CT, Cao L, Roberson ED, et al. Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. Am J Hum Genet. 2012;90:796–808. doi: 10.1016/j.ajhg.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784–795. doi: 10.1016/j.ajhg.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuchs-Telem D, Sarig O, van Steensel MA, et al. Familial pityriasis rubra pilaris is caused by mutations in CARD14. Am J Hum Genet. 2012;91:163–170. doi: 10.1016/j.ajhg.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eytan O, Sarig O, Sprecher E, van Steensel MA. Clinical response to ustekinumab in familial pityriasis rubra pilaris caused by a novel mutation in CARD14. British J Dermatol. 2014;171:420–422. doi: 10.1111/bjd.12952. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. N Engl J Med. 2014;370:911–920. doi: 10.1056/NEJMoa1307361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370:921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 45.Chakraborty PK, Schmitz-Abe K, Kennedy EK, et al. Mutations in TRNT1 cause congenital sideroblastic anemia with immunodeficiency, fevers, and developmental delay (SIFD) Blood. 2014;124:2867–2871. doi: 10.1182/blood-2014-08-591370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiseman DH, May A, Jolles S, et al. A novel syndrome of congenital sideroblastic anemia, B-cell immunodeficiency, periodic fevers, and developmental delay (SIFD) Blood. 2013;122:112–123. doi: 10.1182/blood-2012-08-439083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasarman F, Thiffault I, Weraarpachai W, et al. The 3' addition of CCA to mitochondrial tRNASer(AGY) is specifically impaired in patients with mutations in the tRNA nucleotidyl transferase TRNT1. Human Mol genet. 2015;24:2841–2847. doi: 10.1093/hmg/ddv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ombrello MJ, Remmers EF, Sun G, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chae JJ, Park YH, Park C, et al. Connecting two pathways through Ca signaling: NLRP3 inflammasome activation induced by a hypermorphic PLCG2 mutation. Arthritis & Rheumatol. 2014;67:563–567. doi: 10.1002/art.38961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Livingston JH, Lin JP, Dale RC, et al. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J Med Genet. 2014;51:76–82. doi: 10.1136/jmedgenet-2013-102038. [DOI] [PubMed] [Google Scholar]